Key Points
Contrary to population data, survival of Hispanic AYA ALL patients enrolled on CALGB 10403 was equivalent to non-Hispanic patients.
Geographical alignment between Hispanic patient incidence and trial sites may increase Hispanic patient enrollment onto clinical trials.
Abstract
In this secondary analysis of Hispanic adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL) treated on Cancer and Leukemia Group B (CALGB) 10403, we evaluated outcomes and geographic enrollment patterns relative to US population data. We used demographic, clinical, and survival data on AYAs enrolled on CALGB 10403 (N = 295, 2007-2012). Surveillance, Epidemiology, and End Results registries provided overall survival (OS) for US AYA ALL by ethnicity/race. North American Association of Cancer Registries provided AYA ALL incidence overall and proportion among Hispanics by US state. Of AYAs enrolled on CALGB 10403, 263 (89%) reported ethnicity/race: 45 (17%) Hispanic, 172 (65%) non-Hispanic White (NHW), 25 (10%) non-Hispanic Black (NHB), and 21 (8%) other. Compared with NHWs, Hispanic and NHB patients had lower household income, and Hispanic patients were more likely to harbor high-risk CRLF2 aberrations. Relative to US estimates, where Hispanic patients represented 46% of newly diagnosed AYA ALL patients and experienced inferior OS compared with NHW (P < .001), Hispanic AYAs on CALGB 10403 did as well as NHW patients (3 year OS, 75% vs 74%; P = NS). Hispanic patients also had higher rates of protocol completion (P = .05). Enrollments on CALGB 10403 differed relative to the distribution of Hispanic AYA ALL in the United States: enrollment was highest in the Midwest; t and only 15% of enrollees were from states with a high proportion of Hispanic AYA ALL patients. In summary, Hispanic patients treated on CALGB 10403 did as well as NHWs and better than population estimates. Geographical misalignment between trial sites and disease epidemiology may partially explain the lower-than-expected enrollment of Hispanic AYA ALL patients.
Introduction
There is a growing awareness that the incidence of acute lymphoblastic leukemia (ALL) among Hispanic individuals living in the United States (US) exceeds that of non-Hispanic populations.1-3 This is particularly true in the adolescent and young adult (AYA) population, where the incidence rate of ALL among US Hispanic AYAs has shown striking increases over the last 2 decades, and ∼35% to 40% of AYA ALL patients are currently of Hispanic ethnicity.2 Conversely, population-based studies including childhood,4,5 AYA,6 and admixed age cohorts7 have demonstrated that ALL outcomes among US Hispanic patients are significantly inferior to that of non-Hispanic White (NHW) patients. This survival disparity may be partially explained by recent evidence that ALL in patients of Hispanic ethnicity is more likely to harbor high-risk Philadelphia chromosome (Ph)-like aberrations, including gene translocations involving CRLF2,8,9 IKZF1 deletions, and germline polymorphisms in GATA3.10,11 Additional challenges disproportionately affecting Hispanic patients, such as lower socioeconomic status,12 reduced medication adherence,13 higher rates of obesity,14 altered drug metabolism,15 and low rates of clinical trial enrollment16 also likely contribute.
Although survival improvements among Hispanic ALL patients have been slower than in other groups,1 recent progress has occurred. One of the major advancements in ALL over the last decade was the recognition that AYA patients have superior survival following intensive, pediatric-inspired regimens as opposed to traditional adult ALL regimens. In the United States, CALGB 10403 was the largest prospective, multicenter clinical trial to examine the use of a traditional pediatric ALL regimen delivered to AYAs in the adult cancer care setting and was the first Intergroup trial in adult ALL to be conducted through the US National Clinical Trial Network (NCTN). The trial results demonstrated that this regimen was far superior to historical adult cooperative group approaches and that the presence of CRLF2 aberrations and obesity were independently associated with reduced disease-free survival.17
CALGB 10403 was accessible to clinical sites across the United States who participate in the National Cancer Institute’s NCTN. Although the trial was open between 2007 and 2012 in 57 adult-treating clinical sites across 31 states, enrollees were mostly NHW, and the proportion of Hispanic AYAs (17%) was substantially lower than expected based on US population estimates. In the current analysis, we examined survival outcomes of the Hispanic patients treated on CALGB 10403 compared with NHW and non-Hispanic Black (NHB) trial participants and relative to US AYA ALL survival estimates by race/ethnicity. We then evaluated potential explanations for the Hispanic under-enrollment on CALGB 10403 and hypothesized that Hispanic AYA ALL populations may have had limited access to the trial. To test this broadly, we compared the geographical distribution of CALGB 10403 enrollments with the incidence of AYA ALL among Hispanic patients across the United States.
Methods
Data sources
CALGB 10403 was a prospective phase 2 clinical trial conducted by the US adult cancer cooperative groups to test the feasibility, safety, and efficacy of delivering an intensive pediatric ALL regimen to newly diagnosed AYAs in the adult cancer treatment setting. Between November 2007 and September 2012, 318 AYAs (ages 17-39 years) with newly diagnosed B-cell ALL or T-cell ALL were enrolled, of whom 295 were evaluable. A detailed description of the eligibility, methodology, and treatment regimen administered has been published and informed consent for participation was obtained from each study participant.17 Patient demographic and leukemia variables (including ethnicity and race) were reported by the treating center. Yearly household income was obtained from patient self-report. Protocol treatment nonadherence was defined as termination of trial therapy for a reason other than refractory leukemia, relapse, development of a secondary cancer, or death.
Data from the public use US population-based cancer registries of the Surveillance, Epidemiology, and End Results (SEER) program18 were used to estimate the overall survival (OS) of AYA ALL among US Hispanic, NHW, and NHB patients diagnosed between 2008 and 2012 between the ages of 15 and 39 years. The SEER program of the National Cancer Institute collects cancer incidence and survival data from population-based cancer registries representing ∼48% of the US population (http://seer.cancer.gov). The population covered by SEER is comparable to the general US population; efforts to include minority populations, including Hispanic people, have contributed to the generalizability of these data.19 The Cancer in North America public use dataset from the North American Association of Central Cancer Registries (NAACCR)20 was used to determine the incidence of newly diagnosed ALL between 2013 and 2017 among people aged 20 to 49 across individual US states and the proportion of ALL patients who were Hispanic. For both SEER and NAACCR, ALL was identified by International Classification of Diseases for Oncology histology codes 9826 and 9835-9837.21
Statistical analyses
Descriptive statistics were used to compare baseline patient and leukemia characteristics by ethnicity/race (Hispanic vs NHW vs NHB) among patients enrolled on CALGB 10403. Categorical outcomes were compared using the 1-way analysis of variance test or Kruskal-Wallis test.
OS was defined as either the time from ALL diagnosis (SEER) or from study registration (CALGB 10403) to death from any cause. The distribution of OS was estimated using the Kaplan-Meier method and compared across ethnicity/race groups using log-rank tests. Event-free survival in CALGB 10403 was defined as the time from study registration to the earliest occurrence of any of the following: lack of disease response by day 60, death, relapse, or development of a second malignancy. The incidence rate (per 100 000) of ALL in each state was obtained from NAACCR. In addition, the proportion of ALL patients of Hispanic ethnicity in each state was calculated as the number of newly diagnosed Hispanic ALL patients divided by the total number of newly diagnosed ALL patients. All CALGB 10403 analyses were performed using R version 3.6.2; SEER, and NAACR analyses were performed using SEERStat 8.3.8.
Results
Study population
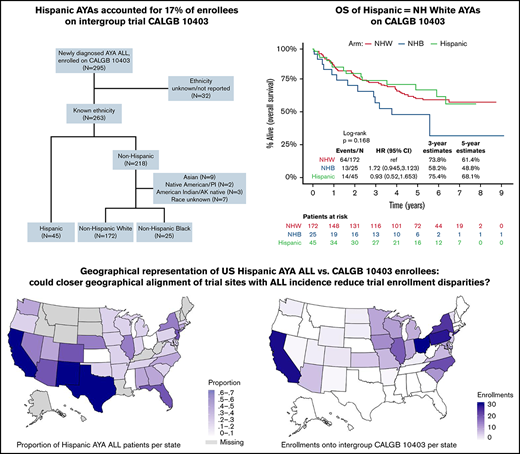
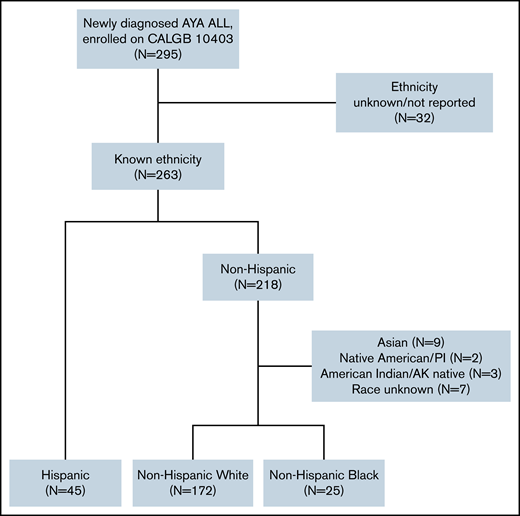
Of the 263 (89%) CALGB 10403 enrollees with known ethnicity, 45 (17%) were Hispanic, 172 (65%) were NHW, and 25 (10%) were NHB; 21 (8%) had another or unknown race (Figure 1). The survival analyses presented in this manuscript focused on the 242 trial participants with known ethnicity (Hispanic or non-Hispanic) and either White or Black race as reported by the treating center. Baseline patient and leukemia characteristics are described in Table 1. Relative to NHW, both Hispanic and NHB patients had significantly lower self-reported yearly household income, with 61% and 69% of Hispanic and NHB patients reporting < $20 000, respectively. Leukemia immunophenotype differed across groups, with B-cell ALL occurring in 98% of Hispanic patients and saT-cell ALL in 56% of NHB patients. Aberrations in CRLF2 were significantly more common among Hispanic patients (41%) relative to NHW (16%) and NHB (20%) patients (P = .03). Hispanic patients trended toward lower rates of protocol completion nonadherence (24%) relative to NHW (44%) and NHB (36%) (P = .05) (Table 2).
Baseline patient and ALL characteristics of Hispanic, NHW, and NHB patients enrolled on CALGB 10403
| . | NHW (n = 172) . | Hispanic (n = 45) . | NHB (n = 25) . | P . |
|---|---|---|---|---|
| Age, years, no. (%) | .45 | |||
| 15-19.9 | 40 (23) | 11 (24) | 9 (36) | |
| 20-24.9 | 47 (27) | 18 (40) | 7 (28) | |
| 25-29.9 | 42 (24) | 9 (20) | 6 (24) | |
| 30-34.9 | 28 (16) | 3 (7) | 1 (4) | |
| 35-39.0 | 15 (9) | 4 (9) | 2 (8) | |
| Sex, no. (%) | .64 | |||
| Male | 102 (59) | 30 (67) | 16 (64) | |
| Female | 70 (41) | 15 (33) | 9 (36) | |
| BMI, no. (%) | .86 | |||
| Underweight | 3 (2) | 0 | 1 (4) | |
| Normal | 66 (38) | 17 (38) | 9 (36) | |
| Overweight | 46 (27) | 11 (24) | 8 (32) | |
| Obese | 57 (33) | 17 (38) | 7 (28) | |
| Yearly household income, no. (%) | .003 | |||
| < $20 000 | 36 (29) | 19 (61) | 11 (69) | |
| $20 000 to $49 000 | 35 (28) | 7 (23) | 3 (19) | |
| $50 000 to $74 999 | 19 (15) | 3 (10) | 2 (13) | |
| $75 000 to $99 000 | 20 (16) | 0 | 0 | |
| ≥ $100 000 | 16 (13) | 2 (7) | 0 | |
| Missing (n) | 46 | 14 | 9 | |
| Immunophenotype, no. (%) | <.001 | |||
| B-cell ALL | 133 (77) | 44 (98) | 11 (44) | |
| T-cell ALL | 39 (23) | 1 (2) | 14 (56) | |
| WBC at Dx, ×106/uL, No. (%) | .09 | |||
| ≤30 | 134 (79) | 29 (64) | 17 (68) | |
| 30 | 36 (21) | 16 (36) | 8 (32) | |
| Missing (n) | 2 | 0 | 0 | |
| Cytogenetics, no. (%) | .69 | |||
| Favorable | 11 (13) | 3 (16) | 1 (8) | |
| Intermediate | 60 (69) | 15 (79) | 10 (83) | |
| Unfavorable | 16 (18) | 1 (5) | 1 (8) | |
| Missing (n) | 85 (49) | 26 (58) | 13 (52) | |
| Ph-like, no. (%) | .09 | |||
| No | 57 (74) | 12 (55) | 5 (50) | |
| Yes | 20 (26) | 10 (46) | 5 (50) | |
| Missing (n) | 95 | 23 | 15 | |
| CRLF2 aberration, no. (%) | .03 | |||
| No | 65 (84) | 13 (59) | 8 (80) | |
| Yes | 12 (16) | 9 (41) | 2 (20) | |
| Missing (n) | 95 | 23 | 15 |
| . | NHW (n = 172) . | Hispanic (n = 45) . | NHB (n = 25) . | P . |
|---|---|---|---|---|
| Age, years, no. (%) | .45 | |||
| 15-19.9 | 40 (23) | 11 (24) | 9 (36) | |
| 20-24.9 | 47 (27) | 18 (40) | 7 (28) | |
| 25-29.9 | 42 (24) | 9 (20) | 6 (24) | |
| 30-34.9 | 28 (16) | 3 (7) | 1 (4) | |
| 35-39.0 | 15 (9) | 4 (9) | 2 (8) | |
| Sex, no. (%) | .64 | |||
| Male | 102 (59) | 30 (67) | 16 (64) | |
| Female | 70 (41) | 15 (33) | 9 (36) | |
| BMI, no. (%) | .86 | |||
| Underweight | 3 (2) | 0 | 1 (4) | |
| Normal | 66 (38) | 17 (38) | 9 (36) | |
| Overweight | 46 (27) | 11 (24) | 8 (32) | |
| Obese | 57 (33) | 17 (38) | 7 (28) | |
| Yearly household income, no. (%) | .003 | |||
| < $20 000 | 36 (29) | 19 (61) | 11 (69) | |
| $20 000 to $49 000 | 35 (28) | 7 (23) | 3 (19) | |
| $50 000 to $74 999 | 19 (15) | 3 (10) | 2 (13) | |
| $75 000 to $99 000 | 20 (16) | 0 | 0 | |
| ≥ $100 000 | 16 (13) | 2 (7) | 0 | |
| Missing (n) | 46 | 14 | 9 | |
| Immunophenotype, no. (%) | <.001 | |||
| B-cell ALL | 133 (77) | 44 (98) | 11 (44) | |
| T-cell ALL | 39 (23) | 1 (2) | 14 (56) | |
| WBC at Dx, ×106/uL, No. (%) | .09 | |||
| ≤30 | 134 (79) | 29 (64) | 17 (68) | |
| 30 | 36 (21) | 16 (36) | 8 (32) | |
| Missing (n) | 2 | 0 | 0 | |
| Cytogenetics, no. (%) | .69 | |||
| Favorable | 11 (13) | 3 (16) | 1 (8) | |
| Intermediate | 60 (69) | 15 (79) | 10 (83) | |
| Unfavorable | 16 (18) | 1 (5) | 1 (8) | |
| Missing (n) | 85 (49) | 26 (58) | 13 (52) | |
| Ph-like, no. (%) | .09 | |||
| No | 57 (74) | 12 (55) | 5 (50) | |
| Yes | 20 (26) | 10 (46) | 5 (50) | |
| Missing (n) | 95 | 23 | 15 | |
| CRLF2 aberration, no. (%) | .03 | |||
| No | 65 (84) | 13 (59) | 8 (80) | |
| Yes | 12 (16) | 9 (41) | 2 (20) | |
| Missing (n) | 95 | 23 | 15 |
BMI, body mass index; No., number; WBC, white blood cell.
CALGB 10403 outcomes of interest, stratified by race/ethnicity
| . | NHW (n = 172) . | Hispanic (n = 45) . | NHB (n = 25) . | P . |
|---|---|---|---|---|
| Days on treatment | .39 | |||
| Median | 469 | 786.5 | 224 | |
| IQR | 89.5-990 | 107-1200 | 104-872 | |
| Protocol completion | .055 | |||
| Nonadherence, no. (%) | 76 (44) | 11 (24) | 9 (36) | |
| 100-day mortality, no. (%) | 4 (2) | 2 (4) | 2 (8) | .18 |
| 3-year EFS (95% CI) | 60% (53-68) | 55% (41-72) | 42% (26-67) | .30 |
| 3-year OS (95% CI) | 74% (67-81) | 75% (63-90) | 58% (41-82) | .17 |
| . | NHW (n = 172) . | Hispanic (n = 45) . | NHB (n = 25) . | P . |
|---|---|---|---|---|
| Days on treatment | .39 | |||
| Median | 469 | 786.5 | 224 | |
| IQR | 89.5-990 | 107-1200 | 104-872 | |
| Protocol completion | .055 | |||
| Nonadherence, no. (%) | 76 (44) | 11 (24) | 9 (36) | |
| 100-day mortality, no. (%) | 4 (2) | 2 (4) | 2 (8) | .18 |
| 3-year EFS (95% CI) | 60% (53-68) | 55% (41-72) | 42% (26-67) | .30 |
| 3-year OS (95% CI) | 74% (67-81) | 75% (63-90) | 58% (41-82) | .17 |
CI, confidence interval; EFS, event-free survival; IQR, interquartile range; No., number.
Survival
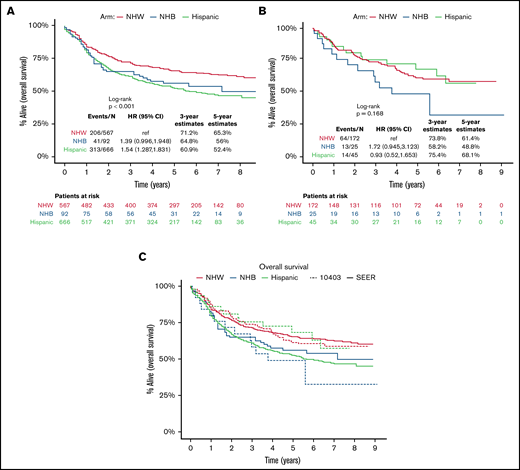
Among the SEER population-based cohort of US AYA ALL patients (N = 1325), the estimated 3-year OS among NHW, Hispanic, and NHB patients was 71% (95% CI, 68% to 75%), 61% (95% CI, 57% to 65%), and 65% (95% CI, 56% to 75%), respectively (Figure 2A). Among the CALGB 10403 cohort (N = 242), the estimated 3-year OS among NHW, Hispanic, and NHB patients was 74% (67% to 81%), 75% (63% to 90%), and 58% (41% to 82%), respectively (Figure 2B). Relative to the population-based cohort, the CALGB 10403 cohort (N = 242) included fewer AYAs <20 years (38% vs 25%), and more AYAs 20 to 29.9 years (36% vs 53%; P < .001) (supplemental Table 1). The population-based cohort also included a significantly higher proportion of Hispanic AYA ALL patients (46%) relative to CALGB 10403 (19%) (P < .001). When comparing outcomes between the population-based cohort and CALGB 10403, the 3-year OS for both NHW and NHB was similar across the 2 cohorts, but the 3-year OS for Hispanic patients trended toward favoring CALGB 10403 (60.9% vs 75.4%) (Figure 2C).
Overall survival of AYA ALL. Kaplan-Meier estimates according to race/ethnicity of OS among newly diagnosed AYA ALL (15-39 years), SEER, 2008-2012 (A); OS among AYA ALL on CALGB 10403 (B); and overlay of AYA ALL OS by SEER vs CALGB 10403 (C).
Overall survival of AYA ALL. Kaplan-Meier estimates according to race/ethnicity of OS among newly diagnosed AYA ALL (15-39 years), SEER, 2008-2012 (A); OS among AYA ALL on CALGB 10403 (B); and overlay of AYA ALL OS by SEER vs CALGB 10403 (C).
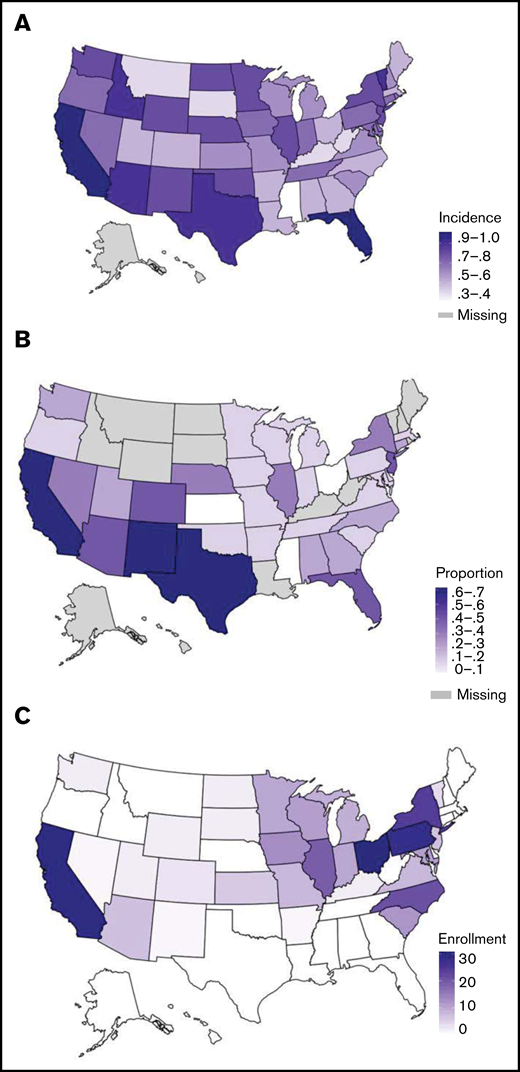
Geographic descriptions of AYA ALL. Geographical distributions of incidence of newly diagnosed AYA ALL (20-49 years) per 100 000, per state, NAACCR, 2013-2017 (A); proportion of AYA ALL (20-49 years) who are Hispanic per state, NAACCR, 2013-2014 (B); and absolute number AYA ALL enrolled on CALGB 10403 per state (C).
Geographic descriptions of AYA ALL. Geographical distributions of incidence of newly diagnosed AYA ALL (20-49 years) per 100 000, per state, NAACCR, 2013-2017 (A); proportion of AYA ALL (20-49 years) who are Hispanic per state, NAACCR, 2013-2014 (B); and absolute number AYA ALL enrolled on CALGB 10403 per state (C).
Population-based incidence and geographic distribution of AYA ALL
To explore potential explanations for the under-enrollment of Hispanic patients on CALGB 10403, we evaluated the geographical distribution of AYA ALL incidence among Hispanic patients across the United States and compared these findings to state-wide enrollments on the clinical trial. The incidence rates (per 100 000) of AYA ALL among US states from NAACCR are shown in Figure 3A and the proportion of AYA ALL patients reported as Hispanic in Figure 3B. The states with the highest incidence rates of AYA ALL were California and Florida, followed by Texas, New Mexico, Idaho, and Vermont. The states with highest proportion of Hispanic AYA ALL patients included California, Texas, and New Mexico, followed by Florida, Arizona, Colorado, and New Jersey. Although CALGB 10403 enrolled patients in 31 US states (Figure 3C; supplemental Table 2), the trial did not open in Texas, Florida, or New Jersey. Trial enrollment numbers were highest across the Midwestern United States, with only 45/295 (15.3%) of enrollments coming from the 7 states with a high proportion of Hispanic AYA ALL patients.
Discussion
In this secondary analysis of CALGB 10403, a large, practice-changing US intergroup clinical trial evaluating the pediatric regimen in AYA ALL, we found that the outcomes of Hispanic trial enrollees were similar to NHW trial enrollees and encouraging relative to US population-based estimates of survival among Hispanic AYAs with ALL. These results are promising, especially as the Hispanic patients on CALGB 10403 were more likely than NHW patients to exhibit high-risk Ph-like genetic profiles, including rearrangements in CRLF2. Disappointingly, when compared with the high proportion of Hispanic AYA ALL patients across the United States, we found that Hispanic AYAs were under-represented on CALGB 10403. While this is in keeping with other reports showing disparities in clinical trial enrollments among Hispanic patients,16,22 -25 we were able to demonstrate that under-enrollment was in part due to a misalignment between the geographical distribution of enrollees and the distribution of Hispanic AYA ALL patients across the United States. These findings imply that strategies to improve clinical trial enrollment among Hispanic ALL patients should include efforts to more closely align study sites and accrual projections with disease incidence patterns.
Although a majority of observational studies have shown inferior outcomes for Hispanic patients with ALL,4 -7,26 the 45 Hispanic enrollees on CALGB 10403 fared relatively well. Examination of baseline risk factors across the Hispanic, NHW, and NHB cohorts demonstrated only that the Hispanic patients were more likely to have CRLF2 rearrangements and that both Hispanic and NHB patients were more likely to have lower socioeconomic status, both features that are associated with inferior ALL outcomes.27,28 Interestingly, we found that protocol nonadherence, which was defined as discontinuation of the study treatment in the absence of lack of response, relapse, or death, was lowest in Hispanic patients. The underlying reasons for this are unclear, but it is conceivable that adherence to the trial therapy was associated with improved outcome, which is in keeping with other ALL studies.29 Although the relatively small subgroups limited the power to reach statistically significant differences between CALGB 10403 and SEER population-based cohorts, the outcomes of NHB AYAs on trial were not particularly favorable (3-year OS, 58.2%). In addition to higher proportion of poverty-level income, NHB trial patients were more likely to have T-cell ALL, an association that has been described in other cohorts.30 Recently, the Children’s Oncology Group reported superior survival associated with the addition of nelarabine to the augmented Berlin-Frankfurt-Muenster regimen in children and AYAs with T-cell ALL,31 an approach that may further improve upon CALGB 10403 outcomes for T-cell ALL patients.
Efforts aimed at providing equitable access to cancer clinical trials and transparent reporting of trial participant demographics have gained traction in recent years.24,32-34 In the current analysis, we observed that representation of Hispanic AYA ALL patients on CALGB 10403 was approximately threefold lower than expected based on population incidence. This disparity has great significance, as ALL is a cancer that is over-represented among Hispanic individuals. Thus, starting from clinical trial development through implementation, studies must make every effort to more closely align Hispanic patient representation on ALL trials in order to close this gap. Based on our results, an initial effort would be to ensure that a proportion of clinical trial sites are located in regions with Hispanic patients. We found that although CALGB 10403 could have opened across all US states, the trial failed to open in several states with high proportions of Hispanic patients, such as Texas and Florida. Whereas our descriptive analyses only provided a high-level state-wide geographical assessment, additional work should be done to identify regional patterns of ALL disease incidence and barriers in accessing and accruing to trials across race/ethnicity on a local level.
Poor enrollment in clinical trials by minority populations has been well described.35 Inequities in enrollment and access to care are complex, and multiple factors outside of geographical proximity to a treating site have been identified. Although our study does not explore the contribution of these important issues, lack of trust in the health care system and research community, perceived harm, cost to the patient, lack of transportation, and lack of education are among the common barriers reported by minority patients.36 Interestingly, multiple studies have reported a high willingness to participate in clinical trials by Hispanic patients, highlighting the need for directed initiatives that ensure inclusion of this vulnerable population.37,38 In the current analysis, we found substantial under-enrollment of Hispanic AYA ALL patients across nearly every state. In California, for example, of the 32 state-wide enrollees, only 34% were Hispanic, which is markedly low when acknowledging that ∼65% of the state’s AYA ALL patients are Hispanic. Similarly, in Pennsylvania, only 7% of the state’s 31 enrollees were Hispanic, which was also well below the proportion of Hispanic patients (∼35%) among the state’s AYA ALL population. Strategies for enhanced recruitment in minority populations have been studied but have yet to become the standard of care in clinical trial design.39 Previously reported interventions span the spectrum and include approaches that mitigate barriers to trial awareness, participation, and acceptance. Our study underscores the need to deviate from the standardapproaches to clinical trial enrollment and to develop a culturally competent, inclusive method to ensure that the study population mirrors the population affected by the disease.
We aimed to draw attention to an important patient subgroup; however, our findings are not without limitations. First, the characterization of ethnicity and race in CALGB 10403 was ascertained through basic demographic data reported from each participating clinical site and does not consider the diversity of ancestry of origin. In contrast, ethnicity definitions abstracted from US population-based datasets are typically based on the NAACCR Hispanic Identification Algorithm, which uses a combination of self-reported ethnicity, surname, and country of origin.40 Although prior attempts to classify Hispanic populations in population-based datasets have been imperfect,41 the NAACCR algorithm is fairly comprehensive and is frequently used as a gold standard to ascertain cancer incidence among US Hispanic populations. It is certainly possible that had we been able to use a similar algorithm, the proportion of Hispanic patients enrolled on CALGB 10403 may have increased. In keeping with this point, the trial also lacked clear ethnicity information on 32 patients, including reports of ethnicity as “other.” Mechanisms to assign race and ethnicity through self-identification should be considered across NCTN clinical trials, which would improve accuracy and allow for greater granularity. As previously mentioned, the Hispanic and NHB patients represented small subgroups on CALGB 10403, thus limiting statistical power to determine definitive differences across groups. Finally, comparisons of clinical trial data to population-based observations are associated with inherent limitations, including the bias of confounding. For example, prior work has demonstrated a lack of consistency when comparing observational studies with randomized controlled trial results.42 In our study, relative to the NAACCR and SEER cohorts, the CALGB 10403 population displayed variation in represented ages, and we were unable to ensure that unaccounted for differences between datasets did not exist. That said, both observational data and clinical trials offer important, and often complementary, insights into specific cancer populations.43
In conclusion, we observed that although Hispanic AYAs under-enrolled on CALGB 10403, Hispanic trial participants experienced favorable survival. Inequity in cancer clinical trial enrollment is a critically important issue that will require a multipronged approach to resolve. As a start, clinical trialists should make every effort to ensure that their clinical trial population aligns with the regional or national epidemiology of the disease they are studying. This is particularly the case in ALL, where there is tremendous opportunity to not only improve enrollment of Hispanic patients but to greatly advance their outcomes.
Acknowledgment
This work was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology).
Authorship
Contribution: L.M., J.Y., T.H.M.K., and W.S. designed the study; J.Y., S.J., and A.W. performed the analyses; L.M., J.Y., and W.S. wrote the paper; and all authors edited the paper.
Conflict-of-interest disclosure: L.M. reports consultancy at Pfizer, Amgen, Jazz, Kite, Medexus, CTI Biopharma, and Astellas; research funding from Jasper, Kite, and Astellas; and honoraria from Adaptive. A.S.A. reports being on an advisory board at Glycomimetics, Amgen, Seattle Genetics, and Kite, and research funding from Glycomimetics, Abbvie, Macrogenics, Pfizer, Amgen, OBI, Immunogen, Seattle Genetics, and Kite. R.A.L. has acted as a consultant or advisor to Amgen, Ariad/Takeda, Astellas, Celgene/BMS, CVS/Caremark, Epizyme, MorphoSys, Servier, and Novartis and has received clinical research support from Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven/Gilead, Novartis, and Rafael Pharmaceuticals, and royalties from UpToDate. Tallman Research funding- Abbvie Orsenix Biosight Glycomimetics Rafael Pharmaceuticals Amgen Adv bds- Abbvie Daiichi-Sankyo Orsenix KAHR Jazz Pharmaceuticals Roche Biosight Innate Pharmaceuticals Kura Syros Ipsen Biopharmaceuticals Royalties- UpToDate Honoraria- Innate Pharmaceuticals Syros Northwell ASH Wolters-Kluwer Abbvie Society for Immunotherapy of Cancer WebMD Japan Society of Hematology Syros. The remaining authors declare no competing financial interests.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Correspondence: Lori Muffly, Division of Blood and Marrow Transplantation and Cellular Therapy, Stanford University, 300 Pasteur Drive, H0144, Stanford, CA 94305; e-mail: lmuffly@stanford.edu.
References
Author notes
Requests for data sharing may be submitted to Lori Muffly (lmuffly@stanford.edu).
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11-14 December 2021.
The full-text version of this article contains a data supplement.