TO THE EDITOR:
Nucleophosmin (NPM) is a ubiquitous nucleocytoplasmic shuttle phosphoprotein with critical physiologic roles in the maintenance of genomic stability, ribosome synthesis, and response to stress stimuli and DNA repair.1 Although NPM1 overexpression and translocation were reported in gastrointestinal cancers and lymphomas respectively, the most prevalent and clinically relevant dysregulation of nucleophosmin is seen in acute myeloid leukemia (AML). In AML, one-third of patients harbor frameshift mutations in exon 12 of the NPM1 gene resulting in altered nuclear localization signals that cause cytoplasmic relocalization of this shuttle protein and its cargo.2 AML cells expressing NPM1mut exhibit (1) differentiation arrest, (2) apoptosis blockage, (3) impaired DNA repair, (4) increased cell proliferation, and (5) often concomitant mutations in FLT3 and RAS. Current data suggest that NPM1 mutations are key transforming events in leukemia models, and AML with these mutations is highly vulnerable to cytotoxic or bcl2 targeted therapy. In this manuscript, we try to reconcile the dichotomous observations of cytoplasmic NPM1 mutations being oncogenic while exhibiting enhanced treatment sensitivity. We postulate that these divergent effects of the mutation may be linked to the binding interactions of the NPM protein.
Clinical observations have established that NPM1 mutations result in enhanced chemosensitivity in AML and confer a low risk of relapse,3,4 particularly in the absence of deleterious concomitant FLT3-ITD mutations. Moreover, the presence of an NPM1 mutation obviated the benefit of allogeneic stem cell transplant in AML.5 The current World Health Organization and European LeukemiaNet guidelines classify these patients as favorable risk with a clinical recommendation to treat them with chemotherapy alone, while advances in detection of minimal residual disease by quantitative polymerase chain reaction allows identification of the subset of patients who are at risk of relapse after intensive chemotherapy.6 Recent data suggest the favorable outcomes of NPM1mut AML also extend to the US Food and Drug Administration–approved bcl2 inhibitor venetoclax, now widely incorporated into first-line treatment for frail patients with AML. Additional Ref: C. D. DiNardo, I. S. Tiong, A. Quaglieri, S. MacRaild, S. Loghavi, F. C. Brown, R. Thijssen, G. Pomilio, A. Ivey, J. M. Salmon, C. Glytsou, S. A. Fleming, Q. Zhang, H. Ma, K. P. Patel, S. M. Kornblau, Z. Xu, C. C. Chua, Xufeng Chen, P. Blombery, C. Flensburg, N. Cummings, I. Aifantis, H. Kantarjian, D. C. S. Huang, A. W. Roberts, I. J. Majewski, M. Konopleva, A. H. Wei; Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020; 135 (11): 791–803. doi: https://doi.org/10.1182/blood.2019003988
Mutant NPM oligomerizes with its wild-type counterpart and, although mutations are heterozygous, there is a dominant negative effect induced by significant nuclear depletion of haploinsufficient NPM. Early studies using RNA inhibition as well as oligomerization inhibitors7 suggest a dependency on residual nuclear wild-type NPM for nucleolar integrity. This in turn leads to a heightened sensitivity to drug therapy due to nucleolar instability compared with cells with a full nuclear complement of wild-type NPM. A phase 2 study demonstrated clinical activity of dactinomycin in relapsed/refractory AML that capitalizes on the lowered threshold for nucleolar stress resulting from the mislocalized mutant NPM1 protein.8
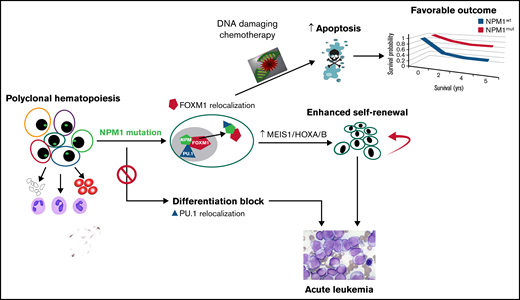
It is also postulated that NPM1 mutant protein exerts its biologic effect by binding and dislocating other oncogenic protein partners into leukemic cell cytoplasm and consequently interfering with their functions. One possible candidate protein to explain the observed effect of chemosensitization conferred by the NPM1 mutation is FOXM1 (Figure 1). The FOXM1 regulatory network is a major predictor of poor prognosis across all human cancers.9 FOXM1 interacts with NPM in all cancer cells and specific to AML, mutant NPM (NPM1c) drives FOXM1 to the cytoplasm where it loses its transcriptional function.10 The functional significance of FOXM1 in chemoresistance in AML was demonstrated using a FOXM1-overexpressing transgenic mouse model and FOXM1 inhibition using RNA interference and pharmacologic inhibitors.11,12 These data provide strong proof of principle that nuclear FOXM1, which is active as a transcription factor, may partially account for resistance to chemotherapy in AML. NPM1 mutations are also associated with high expression of HOXA genes that were reported as markers of venetoclax sensitivity in primary AML samples.13 These findings were recapitulated by FOXM1 knockdown, which resulted in venetoclax sensitization and a very similar pattern of HOXA gene overexpression.14 An association between FOXM1 inactivation, HOXA expression and response to venetoclax suggests that FOXM1 negatively regulates HOXA expression and mediates resistance to this drug (Figure 1). This provides a potential avenue to sensitize NPM1wt AML cells to venetoclax by targeting FOXM1.
The oncogenic function of the NPM1 mutation is well studied and widely accepted. In human AML, NPM1 mutations are found in the more committed myeloid progenitor cells with sparing of the hematopoietic stem cell (HSC) and lymphoid compartments suggesting the oncogenic effects are cell-context specific.15 This may not be accurately depicted in animal models with forced expression of humanized NPM1 mutations in more undifferentiated HSCs. Early zebrafish models showed an expansion of HSCs with expression of the NPM1c mutant protein.16 Murine knock-in models reflect enhanced proliferation in the committed myeloid progenitor cells and increased self-renewal capacity that culminates in the gradual development of leukemia17 that can be accelerated by the introduction of additional mutations. NPM1mut AML cells exhibit higher HOX levels compared with NPM1wt AML and HOX expression in hematopoietic stem and progenitor cells is known to contribute to leukemic transformation.18 NPM1mut AML has a critical dependency on the MLL complex, through which it upregulates MEIS1/HOX expression,19 and this HOX overexpression is critical to maintaining the leukemic stem cell state (Figure 1). Removal of the NPM1 mutant from cytoplasm using CRISPR-Cas9 gene editing elegantly resulted in downregulation of HOXA expression and restoration of myeloid differentiation.20 A recent publication highlighted the MLL-menin complex as a therapeutic vulnerability of NPM1mut AML21 and showed an oral inhibitor of the menin-MLL interaction was able to eradicate NPM1mut AML cells in xenografts and prevent leukemia development in the knock-in murine model.22
Another mechanism underlying the oncogenic effects of the NPM1 mutation is nuclear export of the tumor suppressor p14 (ARF), which is subsequently degraded. Loss of nuclear ARF inhibits its interaction with MDM2 and blunts the p53 response.23
Additionally, proteomic studies demonstrate that NPM1c results in cytoplasmic export of PU.1, a member of the PU.1/CEBPA/RUNX1 transcription factor complex critical for myeloid differentiation. In the absence of PU.1, the transcriptional factors CEBPA and RUNX1 become corepressors, suppressing the expression of more than 300 differentiation genes. PU.1 nuclear reintroduction is sufficient to abort the self-renewal phenotype and promote differentiation of PU.1-null myeloid precursors to terminal monocytic fates.24
Finally, it has recently been shown that long-noncoding RNA (lncRNA) (LONA) is overexpressed in patients with NPM1mut AML and that its intracellular localization inversely reflects that of NPM1. LONA overexpression in NPM1mut AML cells inhibits myeloid differentiation, while it exerts an opposite pro-myeloid differentiation effect in the NPM1wt setting. LONA becomes nuclear as mutant NPM1 relocalizes into the cytoplasm. In vivo, LONA acts as an oncogenic lncRNA reducing the survival of mice transplanted with AML cells. These data suggest that NPM1 mutation in AML leads to activation of oncogenic function of lncRNA, LONA.25
NPM1 mutations in AML contribute to enhanced leukemogenic activity of hematopoietic progenitor cells by facilitating HOX expression and inactivating tumor suppressors such as p53, but paradoxically the patients exhibit superior responses to treatment, which remains poorly understood. Here, we discuss the biologic basis for this paradox and highlight FOXM1 cytoplasmic inactivation as a plausible explanation for sensitizing this AML subset to drug treatment. These data suggest that in AML, and in human cancer generally, oncogenicity and sensitivity to drugs may have distinct pathobiology. Natural selection of aggressive mutations such as NPM1mut that provides favorable treatment responses present instructive insights for developing novel therapies.
Contribution: I.K. and A.L.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irum Khan, Division of Hematology and Oncology, Department of Medicine, 840 South Wood St, Ste 820 E-CSB, University of Illinois, Chicago, IL 60612; e-mail: irumkhan@uic.edu; or Andrei L. Gartel, Division of Hepatology, Department of Medicine, 840 South Wood St, Room 1041, University of Illinois, Chicago, IL; e-mail: agartel@uic.edu.