Key Points
Many patients who are “MRD negative” by standard MFC have clinically significant MRD that is detectable with an ultrasensitive NGS assay.
Early achievement of MRD negativity using a high-sensitivity NGS assay identifies patients who have a very low risk of relapse.
Abstract
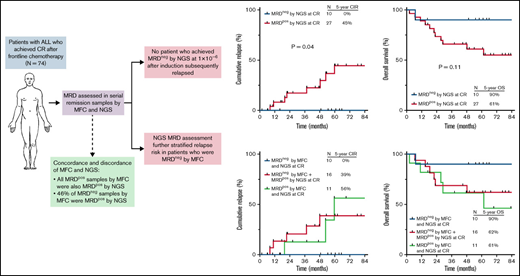
Measurable residual disease (MRD) is highly prognostic for relapse and overall survival (OS) in acute lymphoblastic leukemia (ALL), although many patients with apparent “MRD negativity” by standard assays still relapse. We evaluated the clinical impact of a highly sensitive next-generation sequencing (NGS) MRD assay in 74 adults with ALL undergoing frontline therapy. Among remission samples that were MRD negative by multiparameter flow cytometry (MFC), 46% were MRD+ by the NGS assay. After 1 cycle of induction chemotherapy, MRD negativity by MFC at a sensitivity of 1 × 10−4 and NGS at a sensitivity of 1 × 10−6 was achieved in 66% and 23% of patients, respectively. The 5-year cumulative incidence of relapse (CIR) among patients who achieved MRD negativity by MFC at complete remission (CR) was 29%; in contrast, no patients who achieved early MRD negativity by NGS relapsed, and their 5-year OS was 90%. NGS MRD negativity at CR was associated with significantly decreased risk of relapse compared with MRD positivity (5-year CIR, 0% vs 45%, respectively; P = .04). Among patients who were MRD negative by MFC, detection of low levels of MRD by NGS identified patients who still had a significant risk of relapse (5-year CIR, 39%). Early assessment of MRD using a highly sensitive NGS assay adds clinically relevant prognostic information to standard MFC-based approaches and can identify patients with ALL undergoing frontline therapy who have a very low risk of relapse and excellent long-term survival.
Introduction
Persistent or recurrent measurable residual disease (MRD) after initial therapy for acute lymphoblastic leukemia (ALL) is associated with high rates of relapses and poor overall survival (OS) across many retrospective and prospective studies, including in a large meta-analysis.1-8 Although achievement of MRD negativity is associated with more favorable outcomes, this level of response is not fully protective against relapse. In many reports, patients who are “MRD−” by conventional assays still have relapse rates of 25% and higher.2,8,9 These relapses are presumably driven by very low levels of residual disease that persist below the level of detection of conventional assays and predispose to subsequent relapse. For patients with Philadelphia chromosome (Ph)-negative ALL, the most common MRD assays in clinical practice are a patient-specific quantitative polymerase chain reaction (PCR) assay to identify clonal immunoglobulin heavy chain (IGH) or T-cell receptor (TCR) rearrangements, which is used in many regions of Europe, and multiparameter flow cytometry (MFC), which is widely used in the United States and in other countries.10 Standard MFC assays are capable of detecting residual leukemia cells in 1 out of 10 000 cells (1 × 10−4 or 0.01%), although some ≥8-color MFC assays may achieve a sensitivity of 10−5 with adequate cellular input.11-13
MRD assays that can achieve higher levels of sensitivity than conventional MFC and PCR will be better able to detect and quantify low levels of MRD and may further improve our prognostication in ALL. Highly sensitive next-generation sequencing (NGS)-based MRD assays have been developed that can detect MRD at a sensitivity of 1 × 10−6, which is 1 to 2 logs deeper than standard MFC or PCR assays.10 The clonoSEQ MRD assay is Food and Drug Administration–cleared for MRD detection in ALL, although the clinical utility of this assay compared with other standard MRD assays has not been robustly proven across clinical contexts. Previous studies have shown that this NGS assay can detect low levels of MRD in patients who were considered “MRD−” by less sensitive MFC or PCR technologies.14,15 It has also been shown to outperform conventional MRD assays and improve risk stratification in childhood ALL14,16 and in patients with ALL who have undergone allogeneic stem cell transplantation (alloSCT).15,17 However, there are limited data supporting the use of a high-sensitivity NGS-based MRD assay in adults with ALL undergoing frontline therapy and demonstrating its superiority compared with other standard-of-care MRD assays in this context. We therefore sought to evaluate the clinical utility of this NGS MRD assay in adult patients with newly diagnosed ALL, with the goal of determining whether achievement of MRD negativity with this high-sensitivity assay could identify patients who have a very low risk of relapse. We also sought to determine whether this NGS assay could provide additional prognostic information to standard MFC-based MRD assessment.
Methods
Study design and participants
This is a retrospective study evaluating the prognostic impact of a highly sensitivity NGS MRD assay in patients with Ph− ALL. Eligible patients received frontline ALL therapy at our institution between 2/2012 and 7/2018 using a backbone of either hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) or mini–hyper-CVD (hyperfractionated cyclophosphamide, vincristine and dexamethasone alternating with methotrexate and cytarabine) and achieved complete remission (CR) as best response after 1 induction cycle. Patients were selected for inclusion based on the availability of a banked pretreatment bone marrow sample (for purposes of clonality determination) and at least 1 posttreatment remission bone marrow sample collected between 1 month and 4.5 months from the start of treatment (for purposes of MRD tracking). This study was conducted at a single academic center (The University of Texas MD Anderson Cancer Center). This study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center and was conducted in accordance with the Declaration of Helsinki.
Multiparameter flow cytometry for MRD assessment
MRD assessment was performed on fresh bone marrow aspiration samples using 6-color multiparameter flow cytometry (MFC) as described previously.18 MRD was defined in comparison with known patterns of antigen expression by normal maturing B-cell precursors (hematogones). Analysis was performed on at least 200 000 cells, and a minimum of 20 events was required to constitute a leukemic blast population. The analytic sensitivity of the assay was 0.01%. Per our institutional practice, MFC for MRD was assessed with each remission bone marrow, generally every 1 to 3 months for the first year of treatment. MFC MRD information was available from the clinical laboratory and was available to clinicians for decision making.
NGS assay for MRD assessment
The clonoSEQ MRD assay (Adaptive Biotechnologies Co., Seattle, WA) is an NGS-based immunosequencing assay that was used for this analysis. Pretreatment genomic DNA derived from stored bone marrow specimens was sequenced using multiplex PCR assays composed of primers targeted to the variable genes (forward primers) and joining genes (reverse primers) of the CDR3 region within immunoglobin and TCR genes. For B-cell tumors, sequences of the IGH, IGK, and IGK receptor genes, as well as IGH/BCL1 and IGH/BCL2 translocations, were assessed. For T-cell tumors, separate assays were used to assess the CDR3 regions of TCR-γ (TCRG) and TCR-β. Because 30% to 50% of patients with B-cell ALL have a trackable TCR sequence,19 samples for which a trackable B-cell receptor sequence was not identified were reflexed for analysis of TCRG followed subsequently by TCRB. After the PCR and NGS steps were completed, the data were analyzed via proprietary algorithms to mitigate residual amplification bias that may be introduced by the PCR reactions.20,21 Using high-throughput NGS, the index sequence(s) identified in the diagnostic sample were specifically searched for and quantified, if present, in the remission bone marrow. This assay has an analytic sensitivity of 0.0001% (1 × 10−6), with a sensitivity that is dependent on the cellular input. To achieve 95% confidence that the frequency of the clone of interest is <10−6 in a remission sample, an input of 1.9 million cells was required. NGS-based MRD assessment was performed retrospectively, and this information was therefore not available to clinicians for decision-making.
Response and outcome definitions
CR was defined as 5% or fewer blasts in the bone marrow with an absolute neutrophil ≥1 × 109/L and a platelet count of ≥100 × 109/L. Relapse was defined as recurrence of bone marrow blasts >5% or extramedullary ALL. Cumulative incidence of relapse (CIR) was calculated from the time of CR until relapse, censored for death in CR or if the patient was alive at last follow-up. OS was calculated from the time of treatment initiation until death from any cause, censored if the patient was alive at last follow-up. Survival estimates were not censored at the time of alloSCT.
Statistical methods
Patient characteristics were summarized using median (range) for continuous variables and frequencies (percentages) for categorical variables. To compare 2 groups, Fisher’s exact test was performed for categorical variables, and Wilcoxon rank-sum test was performed for continuous variables. The Kaplan-Meier method was used to estimate the probabilities for RFS and OS, and differences between groups were evaluated with the log-rank test. The Gray’s test was used to compare cumulative incidence probabilities between groups. All statistical analyses were performed using GraphPad Prism 9.
Results
Calibration of ID samples
We identified 90 patients with Ph− ALL who underwent frontline ALL therapy at our institution and had adequate banked pretreatment samples for NGS MRD analysis. Clonality of the baseline sample was able to be determined in 81 patients (overall calibration rate, 90%). Among these 81 baseline samples, 76 calibrated on BCR, 0 calibrated on TCRB, and 5 calibrated on TCRG. The median number of dominant sequences per baseline sample was 2 (range, 1-9). The characteristics for patients who underwent successful identification calibration and those who did not are shown in supplemental Table 1. The ID calibration rate was higher in patients with B-cell ALL than in those with T-cell ALL (96% vs 60%, respectively; P < .001). Among the 6 patients with early T-cell precursor ALL, only 3 (50%) calibrated, which may be reflective of the earlier stage of development of these blasts, which may manifest with absence of a clonal TCR.22,23
Study population characteristics
Among the 81 patients who underwent successful identification calibration, 74 had at least 1 remission sample for NGS MRD assessment. These 74 patients with complete ID and MRD information are the primary subjects of this analysis, and their baseline characteristics are shown in Table 1. Thirty-four patients (46%) had at least 1 poor-risk baseline feature, defined as either poor-risk cytogenetics (ie, low hypodiploidy/near triploidy, complex cytogenetics, or KMT2A rearrangement), CRLF2 positivity by MFC, or a TP53 mutation. Fifty-one patients (69%) received a hyper-CVAD–based regimen, and 23 patients (31%) received a lower-intensity mini–hyper-CVD-based regimen as frontline ALL therapy. Specific treatment regimens are shown in supplemental Table 2. Four patients who received hyper-CVAD (8% of hyper-CVAD–treated patients) and 19 patients who received mini–hyper-CVD (83% of mini-hyper-CVD–treated patients) also received inotuzumab ozogamicin as part of their frontline regimen; no patients received blinatumomab as part of their frontline regimen. The median duration of follow-up for the entire study population is 64 months (range, 17-103 months). Twenty-three patients (31%) underwent alloSCT in first remission. At last follow-up, 18 patients (25%) had relapsed, and 26 patients (35%) had died. Of note, there was no significant difference in CIR or OS in the patients evaluated in this study compared with a cohort of 89 patients with ALL who received frontline therapy during the same time period at our institution and in whom banked samples were not available.
Baseline characteristics of study population (n = 74)
| Characteristic . | Value . |
|---|---|
| Age (years) | 45 [19-83] |
| ALL subtype | |
| B-cell | 65 (88) |
| T-cell | 9 (12) |
| WBC (× 109/L) | 4.1 [0.5-309.2] |
| Bone marrow blasts (%) | 81 [24-99] |
| Karyotype | |
| Diploid | 27 (36) |
| High hyperdiploidy | 5 (7) |
| Low hypodiploidy / near triploidy | 13 (18) |
| KMT2A rearranged | 2 (3) |
| Complex | 4 (5) |
| Miscellaneous | 21 (29) |
| Insufficient metaphases | 2 (3) |
| Poor-risk cytogenetics | 19/74 (26) |
| CRLF2 overexpression by flow cytometry | 9/48 (19) |
| Ph-like ALL (CRLF2 overexpression or Ph-like fusion) | 12/53 (23) |
| TP53-mutated | 20/69 (29) |
| Frontline therapy | |
| Hyper-CVAD–based | 51 (69) |
| Hyper-CVD–based | 23 (31) |
| Characteristic . | Value . |
|---|---|
| Age (years) | 45 [19-83] |
| ALL subtype | |
| B-cell | 65 (88) |
| T-cell | 9 (12) |
| WBC (× 109/L) | 4.1 [0.5-309.2] |
| Bone marrow blasts (%) | 81 [24-99] |
| Karyotype | |
| Diploid | 27 (36) |
| High hyperdiploidy | 5 (7) |
| Low hypodiploidy / near triploidy | 13 (18) |
| KMT2A rearranged | 2 (3) |
| Complex | 4 (5) |
| Miscellaneous | 21 (29) |
| Insufficient metaphases | 2 (3) |
| Poor-risk cytogenetics | 19/74 (26) |
| CRLF2 overexpression by flow cytometry | 9/48 (19) |
| Ph-like ALL (CRLF2 overexpression or Ph-like fusion) | 12/53 (23) |
| TP53-mutated | 20/69 (29) |
| Frontline therapy | |
| Hyper-CVAD–based | 51 (69) |
| Hyper-CVD–based | 23 (31) |
Continuous variables are listed as median [range] and categorical variables as n (%) or n/N (%).
WBC, white blood cell.
Correlation of MFC MRD and NGS MRD
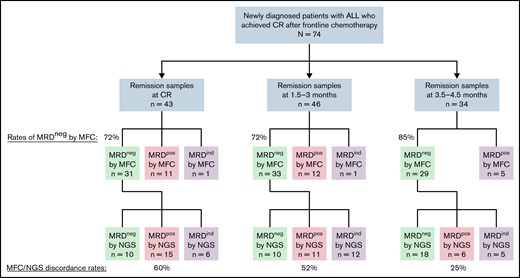
The rates of MRD negativity at 3 different time points (at CR [ie, after the first induction course, or 3 to 4 weeks into therapy], at 1.5-3 months from the start of treatment, and at 3.5 to 4.5 months from the start of treatment) are shown in Figure 1. Forty-three patients had an MFC and NGS MRD sample available at CR, 46 patients had samples available at 1.5 to 3 months, and 34 patients had samples available at 3.5 to 4.5 months. The rate of MRD negativity by MFC was 72%, 72%, and 85%, respectively, at these 3 time points. The NGS MRD assay was performed on a total of 121 remission samples. Among 28 samples that were MRD+ by MFC, all were confirmed to also be MRD+ by NGS. Among 93 samples that were MRD− by MFC, 23 were indeterminate for NGS MRD at 10−6 due to inadequate DNA/cellular input, and 70 were able to be classified as either MRD+ or MRD negativity by the NGS assay. Among these 70 samples that were MRD− by MFC and were evaluable for MRD assessment by NGS at 10−6, 32 (46%) were MRD+ by NGS. The median level of detectable MRD by the NGS assay among these discordant cases (ie, MRD− by MFC and MRD+ by NGS) was 0.0027% and was significantly lower than in the concordant cases (ie, MRD+ by both MFC and NGS) where the median level of MRD was 0.125% (P < .0001). The discordance rates between MFC and NGS were higher at earlier timepoints than at later timepoints (60% at CR, 52% at 1.5 to 3 months, and 25% at 3.5 to 4.5 months), suggesting that NGS MRD assessment may have more utility when performed earlier in the treatment course.
Flowchart of MRD response by MFC and NGS at various time points. Discordance rates were calculated including only patients who were assessed as either MRD-positive or -negative by NGS at a sensitivity of 10−6 and excluding patients who were MRD indeterminate by NGS at 10−6. One MRD sample at time of CR and at 1.5 to 3 months was indeterminate by MFC; in both cases, MRD was detected by the NGS assay. MRDpos, MRD+; MRDneg, MRD−; MRDind, MRD indeterminate.
Flowchart of MRD response by MFC and NGS at various time points. Discordance rates were calculated including only patients who were assessed as either MRD-positive or -negative by NGS at a sensitivity of 10−6 and excluding patients who were MRD indeterminate by NGS at 10−6. One MRD sample at time of CR and at 1.5 to 3 months was indeterminate by MFC; in both cases, MRD was detected by the NGS assay. MRDpos, MRD+; MRDneg, MRD−; MRDind, MRD indeterminate.
Association of baseline characteristics and likelihood of MRD response
The baseline characteristics of patients who were MRD− (n = 10), MRD+ (n = 27), or MRD indeterminate (n = 6) at 10−6 by NGS at the time of CR are shown in supplemental Table 3. Interestingly, the rates of NGS MRD negativity were similar in patients who had at least 1 poor-risk disease feature and those who did not (24% vs 22%, respectively; P = .89). Among the 4 patients with a poor-risk baseline feature who achieved MRD negativity by NGS, 1 patient had complex cytogenetics, 1 patient had low hypodiploid karyotype with a TP53 mutation, 1 patient had CRLF2 overexpression by MFC and a TP53 mutation, and 1 patient had CRLF2 overexpression by MFC and a JAK2 mutation. Similar rates of MRD negativity by NGS at CR were also observed between patients who received a hyper-CVAD or mini-hyper-CVD–based regimen (23% for both groups).
Impact of MRD status by MFC at CR
MRD negativity by MFC at the time of CR (ie, after 1 induction course) was achieved in 49 of the 74 (66%) evaluable patients with NGS MRD information. Eleven (22%) of these MRD− patients underwent alloSCT in first remission. Despite achieving early MRD negativity by MFC, 12 patients still relapsed, with a median time to relapse of 16.3 months (range, 6.2 to 48.2 months). The 5-year CIR and OS rates for patients who were MRD− by MFC at CR were 29% and 68%, respectively (supplemental Figure 1). Similar rates of relapse were observed in patients treated with either hyper-CVAD or mini–hyper-CVD regimens who were MRD− by MFC (5-year CIR rates, 30% and 24%, respectively).
Impact of MRD status by NGS at CR
Ten patients of the 43 patients (23%) with an evaluable CR sample for NGS MRD analysis achieved NGS MRD negativity at 10−6. Achieving MRD negativity by NGS at the time of CR better predicted the likelihood of durable remission than did achieving MRD negativity by MFC. None of the 10 patients who achieved NGS MRD negativity at 10−6 at the time of CR subsequently relapsed (CIR rate, 0%), and their 5-year OS rate was 90%. Of note, 1 patient who was 69 years of age achieved NGS MRD negativity at CR but died in remission after 7 months. All other patients who achieved NGS MRD negativity at CR were alive and in ongoing remission at last follow-up. Outcomes were superior for patients who achieved NGS MRD negativity at CR compared with those who were MRD+ (5-year CIR rate, 0% vs 45%, respectively, P = .04; 5-year OS rate, 90% vs 61%, respectively, P = .11; Figure 2A-B). Achievement of NGS MRD negativity at cutoffs above 10−6 provided less discrimination for favorable outcomes. The 5-year CIR rates for patients who were MRD− at CR using a cutoff 10−4 and 10−5 were 16% and 21%, respectively (supplemental Figure 2). Two of the 6 patients who were MRD− at 10−5 but were indeterminate for MRD at 10−6 subsequently relapsed, and the outcomes of these patients with indeterminate MRD were similar to those who were MRD+ at 10−6 (supplemental Figure 3).
Outcomes by MRD status by NGS at 10−6 sensitivity at the time of CR. (A) Cumulative incidence of relapse and (B) OS. MRDpos, MRD+; MRDneg, MRD−.
Outcomes by MRD status by NGS at 10−6 sensitivity at the time of CR. (A) Cumulative incidence of relapse and (B) OS. MRDpos, MRD+; MRDneg, MRD−.
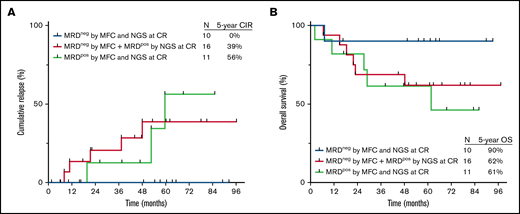
Assessment of NGS MRD added useful prognostic information to assessment of MRD by MFC alone. Importantly, relapses rates were similar between patients who were MRD+ by both MFC and NGS at the time of CR, as compared with those who were MRD− by MFC but positive by NGS (5-year CIR rate, 56% and 39%, respectively; Figure 3A). Five-year OS rates were also similar between these 2 groups (61% and 62%, respectively; Figure 3B). Among patients who were MRD− by MFC at the time of CR, detection of MRD by a high-sensitivity NGS identified patients at significantly higher risk of relapse (39% vs 0% among those who were MRD− by NGS; P = .05).
Outcomes by MRD status by NGS at 10−6 sensitivity and by MFC at the time of CR. (A) Cumulative incidence of relapse and (B) OS. MRDpos, MRD+; MRDneg, MRD−.
Outcomes by MRD status by NGS at 10−6 sensitivity and by MFC at the time of CR. (A) Cumulative incidence of relapse and (B) OS. MRDpos, MRD+; MRDneg, MRD−.
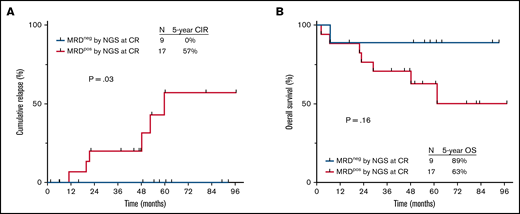
Among the patients who achieved MRD negativity by NGS at CR, only 1 patient (10%) underwent alloSCT in first remission; in contrast, 10 of the 27 patients (37%) who were MRD+ by NGS at CR underwent alloSCT. As receipt of alloSCT could confound the impact of MRD on relapse rates, we repeated these analyses in the subgroup of patients who did not undergo alloSCT in first remission. Similar to the findings in global population, MRD negativity by NGS at CR was associated with significantly reduced risk of relapse among nontransplanted patients (5-year CIR rate, 0% vs 57% in MRD+ patients; P = .03; Figure 4A) and a trend toward better long-term survival (5-year OS rates, 89% vs 63%, respectively; P = .16; Figure 4B). Among nontransplanted patients who were MRD− by MFC at CR, assessment of NGS MRD status provided additional prognostic information. The 5-year CIR rates among nontransplanted patients who were MRD− by both assays and those who were MRD− by MFC but positive by NGS were 0% and 36%, respectively (supplemental Figure 4).
Outcomes by MRD status by NGS at 10−6 sensitivity at the time of CR in nontransplanted patients. (A) Cumulative incidence of relapse and (B) OS. MRDpos, MRD+; MRDneg, MRD−.
Outcomes by MRD status by NGS at 10−6 sensitivity at the time of CR in nontransplanted patients. (A) Cumulative incidence of relapse and (B) OS. MRDpos, MRD+; MRDneg, MRD−.
Impact of MRD status by NGS at late time points
We also evaluated the impact of NGS MRD status at later time points (ie, assessed between 1.5 months and 4.5 months from the start of therapy). Fifty-three patients had NGS MRD assessed in at least 1 “post-CR” time point: 41 patients at 1 time point, 8 patients at 2 time points, and 4 patients at 3 time points. The median time of post-CR MRD assessment was 3.0 months (range, 1.5 to 4.5 months). Overall, 31 of these 53 evaluable patients (58%) achieved MRD negativity at 1 or more post-CR time points. Interestingly, achievement of MRD negativity by NGS at this later time point did not significantly impact relapse rates or survival. The 5-year CIR rates for patients who were MRD− vs MRD+ by NGS at a post-CR time point were 28% and 16%, respectively (P = .48), and the 5-year OS rates were 74% and 58%, respectively (P = .23) (supplemental Figure 5). Notably, patients who were MRD+ by NGS were more likely to be transplanted in first remission than were those who were MRD− by NGS (alloSCT rates, 68% and 19%, respectively; P = .001). However, when the analysis was repeated in only those patients who did not undergo alloSCT in first remission, post-CR MRD status still did not significantly impact relapse or survival (supplemental Figure 6). Among the 25 nontransplanted patients who achieved NGS MRD negativity at a post-CR time point, the 5-year CIR rate was 30%, suggesting that assessment of MRD at these later time points may not be adequate to identify patients at very low risk of relapse.
Discussion
Assessment of MRD is imperative for proper risk stratification in ALL and can be used to identify high-risk patients who may be candidates for alternative therapies (eg, blinatumomab or alloSCT for MRD+ disease) or low-risk patients for whom treatment de-escalation may be considered.24 Current consensus recommendations advise that patients without poor-risk pretreatment disease features who rapidly achieve MRD negativity with conventional MRD assays using a sensitivity of at least 1 × 10−4 do not generally require alloSCT in first remission.10 For those patients with B-cell ALL who are MRD+ at a level of ≥0.1% after initial therapy, MRD-directed therapy with blinatumomab is recommended, with or without subsequent alloSCT.25 However, in this study, we observed that these MRD thresholds may not be adequate to optimally stratify patients according to risk of relapse. We observed that 46% of bone marrow samples that were considered “MRD−” by MFC had detectable MRD using a highly sensitive NGS-based MRD assay with a sensitivity of 1 × 10−6. Importantly, these patients who were MRD− by MFC but MRD+ by NGS had similarly poor outcomes to those who were MRD+ by both assays, with a 5-year CIR rate of 39% in the former group. These data suggest that early assessment of MRD using a high-sensitivity NGS assay may provide better assessment of relapse risk in ALL than conventional MRD assays with lesser sensitivity.
We also observed that rapid achievement of MRD negativity by NGS identified patients at extremely low risk of relapse; in fact, none of the 10 patients who achieved NGS MRD negativity at a level of 10−6 after 1 course of induction chemotherapy subsequently relapsed. This translated to a 5-year OS rate of 90% (due to 1 death in remission). When less sensitive cutoffs were used for the NGS assay, the test lost some discrimination for outcomes, with relapses rates of 16% to 21% observed with cutoffs of 10−4 or 10−5. These relapses rates were similar to, albeit numerically lower than, the 29% relapse rate observed in patients who were MRD− by MFC. Overall, these data suggest that MRD assessment after 1 cycle of induction chemotherapy using a cutoff of 10−6 identifies a subgroup of patients undergoing frontline ALL therapy (<25% in our study) who have excellent outcomes and extremely low risk of relapse. Importantly, no relapses were observed in this group of patients despite the vast majority (9/10, 90%) not undergoing alloSCT in first remission. However, these data should be interpreted with cautious optimism as only 10 patients achieved this MRD milestone in our study. Larger studies will be needed to more definitively quantify the expected rate of relapse in this group of patients, particularly for the patients with poor-risk baseline disease characteristics (eg, adverse risk cytogenetics). Current recommendations are that these patients should undergo alloSCT in first remission, regardless of MRD status10 ; however, it is possible that alloSCT may not be required for such patients with rapid and deep MRD response to frontline therapy. In fact, in our cohort, 4 patients with adverse-risk ALL achieved NGS MRD negativity at the time of CR, and these patients had excellent outcomes, despite their historically poor-risk features. It is also notable that the rates of early NGS MRD negativity were the same (23%) with hyper-CVAD and mini-hyper-CVD–based regimens (the latter of which predominantly consisted of mini-hyper–CVD plus inotuzumab ozogamicin). These results suggest that lower-intensity chemotherapy in combination with novel monoclonal antibodies such as inotuzumab ozogamicin may lead to similar depths of remission as conventional, intensive chemotherapy without immunotherapy. If confirmed in larger studies, this could have implications for the use of these less intensive immunochemotherapy-based regimens even in younger, fit patients.
Interestingly, assessment of NGS MRD at later time points had less prognostic significance than assessment at the time of CR. Whereas none of the patients who achieved NGS MRD negativity (using a cutoff of 10−6) at the time of CR subsequently relapsed, the 5-year CIR rate was 28% for patients who were MRD− by the NGS assay at one of these later time points (ie, 1.5 to 4.5 months from the start of therapy). These findings are in line with previous MRD analyses that show that earlier achievement of MRD negativity is associated with superior outcomes.9,26 It should be noted that we did not have consecutive remission samples on enough patients in order to evaluate the optimal time of achieving MRD negativity using this NGS assay, nor to determine whether conversion from MRD positivity to MRD negativity resulted in superior outcomes compared with persistent MRD positivity, as has been shown in some previous studies.9 Although it remains possible that achievement of MRD negativity at a level of 10−6 after 1 or 2 consolidation cycles may still confer a favorable outcome, our data suggest that MRD negativity immediately after induction appears to provide the greatest protection against subsequent relapse. Thus, caution is required when using NGS-based MRD information obtained beyond the time of initial achievement of CR for clinical decision making or prognostication.
In addition to providing important prognostic information, assessment of MRD using a high-sensitivity NGS assay has potential therapeutic implications in the management of patients with ALL undergoing frontline therapy. Given their very low risk of relapse, patients who rapidly achieve MRD negativity at a threshold of 10−6 may be candidates for treatment de-escalation, perhaps with shortening of the intensity or duration of chemotherapy. Such approaches have already been employed in some pediatric protocols, albeit using less sensitive MRD assays.27-29 Additionally, the relatively high rate of relapse for patients who are MRD− by conventional MFC but have low-level MRD detectable by a highly sensitive NGS assay (5-year CIR of 39% when assessed at the time of CR) suggests that these patients should be considered for alternative therapies. Although blinatumomab is approved for patients with B-cell ALL with MRD at a level of ≥0.1%, our data suggest that use of this agent and other MRD-directed therapies in patients with lower levels of MRD should be explored.
This study has several limitations. First, it is a retrospective study in a relatively heterogeneous population, consisting of patients with B-cell or T-cell ALL and who received intensive hyper-CVAD or lower-intensity mini-hyper-CVD–based regimens. The applicability of our results to patients treated with pediatric-inspired regimens remains unknown, and studies to evaluate the clinical utility of NGS MRD assessment and the optimal timing of MRD negativity in patients treated with these regimens are warranted. Additionally, only 10 patients in our cohort achieved NGS MRD negativity at a sensitivity of 10−6. Although none of these 10 patients relapsed, our results should be interpreted with caution, given the relatively small number of NGS MRD− patients in our analysis. Finally, the MFC MRD assay in our study had a sensitivity of only 10−4, although 8-color next-generation MFC assays with sensitivities as low as 2 × 10−6 have been reported. In one analysis by the PETHEMA (Programa de Estudio y Tratamiento de las Hemopatías Malignas) group, early MRD response using this more sensitive MFC assay was associated with relatively favorable outcomes in patients with high-risk ALL, even when alloSCT was not performed in first remission.30 It is unclear to what extent NGS MRD assessment would provide additional prognostic information when compared with such high-sensitivity MFC assays.
Future studies evaluating the clinical impact of high-sensitivity NGS-based MRD assays in patients with ALL across other clinical contexts will be imperative to clarify the optimal role of this assay. A recent study comparing the same clonoSEQ assay in the peripheral blood and bone marrow in patients with ALL showed strong correlation between these 2 sources (r = 0.87; P < .001).31 Prospective studies are needed to clarify the role of peripheral blood NGS-based MRD assessment in the frontline setting and how it correlates with relapse and survival outcomes. It will also be important to evaluate the role of MRD negativity at a threshold of 10−6 in patients with Ph+ ALL and in relapsed/refractory ALL. In patients with Ph+ ALL, achievement of a complete molecular response by PCR is associated with superior OS and can identify patients who may not require alloSCT in first remission.32 However, ∼20% to 25% of patients with Ph+ ALL who achieve MRD negativity by PCR still relapse, and more sensitive MRD assays may identify those patients in complete molecular response who are at higher risk of relapse. Conversely, low-level BCR-ABL1 transcripts can be detected for years in some patients with Ph+ ALL in ongoing CR, suggesting that this may not represent true MRD of Ph+ ALL but rather a more chronic myeloid leukemia–like state.33 Additionally, the advent of blinatumomab, inotuzumab ozogamicin, and chimeric antigen receptor T-cell therapies has dramatically improved the outcomes of patients with relapsed/refractory B-cell ALL.24 Whereas relapsed/refractory ALL was historically considered uncurable without alloSCT, the combination of low-intensity chemotherapy with mini–hyper-CVD with inotuzumab and blinatumomab in patients with Ph− B-cell ALL in first salvage results in a 2-year OS rate of 64% in nontransplanted patients, suggesting that many patients have durable remissions even without alloSCT.34 Evaluation of MRD negativity using high-sensitivity assays may help to better identify those patients expected to have durable responses, and perhaps cure, with chemoimmunotherapy alone.
In conclusion, we have shown that the use of a highly sensitive NGS-based MRD assay can identify a significant proportion of patients with ALL undergoing frontline therapy who are apparently “MRD−” by conventional MFC but who still have an unacceptably high rate of relapse. In contrast, no patients in our cohort who achieved MRD negativity at a sensitivity of 1 × 10−6 after 1 course of induction chemotherapy subsequently relapsed, and this early NGS-based MRD endpoint was the most prognostic for outcomes. Together, these results suggest that NGS MRD assessment provides clinically relevant prognostic information, beyond that achieved with standard MFC, and can be used to identify patients at very low risk of relapse.
Acknowledgments
The authors thank Lik Wee Lee for their assistance with the bioinformatic analysis. Adaptive Biotechnologies Co. performed the NGS MRD assay at no cost to the authors.
Supported by an Anderson Cancer Center Support Grant (CA016672) and SPORE. N.J.S. is supported by the K12 Paul Calabresi Clinical Oncology Scholar Award and the American Society of Hematology Junior Faculty Scholar Award in Clinical Research.
Authorship
Contribution: N.J.S. designed the study, collected and analyzed the data, treated patients, and wrote the first draft of the manuscript; H.K., M.K., N.J., F.R., T.M.K., M.Y., P.K., and K.T. treated patients; R.K.-S., K.P.P., S.W., and J.L.J. performed and interpreted standard of care MRD analyses; W.M. and R.G. collected and analyzed the data and created the tables and figures; L.W.L. performed the bioinformatic analysis; S.M.K., S.M.P., W.F., and J.M. collected and distributed banked samples; E.J. designed the study and treated patients; and all authors critically reviewed the manuscript and approved of the final version.
Conflict-of-interest disclosure: E.J. has received research funding and has served as a consultant for Adaptive Biotechnologies Co. The remaining authors declare no competing financial interests.
Correspondence: Nicholas Short, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail: nshort@mdanderson.org; and Elias Jabbour, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail: ejabbour@mdanderson.org.
References
Author notes
E-mail the corresponding author for data sharing: nshort@mdanderson.org.
The full-text version of this article contains a data supplement.