TO THE EDITOR:
Three recent randomized controlled trials (RCTs) reported results comparing CD19-directed chimeric antigen receptor (CAR) T-cell therapy to standard of care (SOC) in patients with relapsed or refractory large B cell lymphoma (R/R LBCL) after frontline chemoimmunotherapy (CIT).1 The ZUMA-7 (NCT03391466) and TRANSFORM (NCT03575351) trials, using axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel), respectively, reported meeting the primary endpoint of event-free survival (EFS) favoring CAR T-cell therapy, while the BELINDA (NCT03570892) trial using tisagenlecleucel (tisa-cel) did not meet the primary EFS endpoint.2-4 In all 3 trials, the comparator arm was SOC second-line CIT followed by, in chemosensitive patients, autologous stem cell transplant (ASCT). The 3 trials only included patients that did not achieve a response to frontline CIT or who relapsed within 12 months of CIT, a high-risk group for SOC treatment failure.5 However, it is not known whether the pattern of response to initial CIT also associates with inferior outcomes in patients treated with CAR T-cell therapy. Therefore, we performed a retrospective study in patients receiving CD19 CAR T-cell therapy after ≥2 prior lines of therapy and sought to determine whether their pattern of response to frontline CIT was associated with subsequent CAR T-cell therapy outcomes.
We identified 173 patients with LBCL treated with either axi-cel or tisa-cel as a third or later line of therapy at Moffitt Cancer Center per the current US Food and Drug Administration label as of March 2021, or who were treated in the expanded access programs for out-of-specification axi-cel (NCT03153462) and tisa-cel (NCT03601442) (supplemental Figure 1). We retrospectively assigned each patient to 1 of 4 categories based on response to frontline anthracycline-based CIT, adapted from6 : primary progression with progressive disease during frontline CIT (PP); residual disease at the end of frontline CIT (partial response or stable disease) (RD); early relapse within 12 months of achieving a CR to frontline CIT (ER); or late relapse occurring >12 months later (LR). Kaplan-Meier survival curves with the log-rank test were used to compare progression-free survival (PFS) and overall survival (OS) starting from the date of CAR T-cell infusion. We investigated the predictors of OS and PFS by the Cox proportional hazards univariate and multivariate regression analysis (MVA) to report hazard ratio (HR) and 95% confidence intervals (CIs). The MVA models were adjusted by sex, cell of origin, double-hit lymphoma/triple-hit lymphoma, revised International Prognostic Index (R-IPI), and pattern of failure, all established clinical variables affecting frontline LBCL outcomes.7-11 All analyses were conducted at the significance level of 0.05. The Moffitt Cancer Center Institutional Review Board approved the study, which was conducted according to the Declaration of Helsinki.
Table 1 shows the baseline patient characteristics at the time of CAR T-cell therapy according to the patients’ historical category of failure to frontline CIT. A higher proportion of patients in the PP group had poor risk by R-IPI score and had not undergone ASCT.
Baseline patient characteristics
| . | PP (n = 50) . | RD (n = 33) . | ER (n = 48) . | LR (n = 42) . | P value* . |
|---|---|---|---|---|---|
| Age (y), median (interquartile range) | 64.8 (57.5-71.9) | 65.4 (61.7-71.0) | 65.5 (59.5-70.3) | 65.5 (60.0-69.6) | .97† |
| Sex, n (%) | |||||
| Male | 34 (68.0) | 21 (63.6) | 24 (50.0) | 18 (42.9) | .06 |
| Female | 16 (32.0) | 12 (36.4) | 24 (50.0) | 24 (57.1) | |
| Stage, n (%) | |||||
| Unknown | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .53 |
| I/II | 7 (14.0) | 5 (15.2) | 12 (25.0) | 10 (23.8) | |
| III/IV | 42 (84.0) | 28 (84.8) | 36 (75.0) | 32 (76.2) | |
| Cell of origin by Hans algorithm, n (%) | |||||
| Unknown | 6 (12.0) | 2 (6.1) | 8 (16.7) | 12 (28.6) | .06 |
| GCB | 35 (70) | 23 (69.7) | 26 (54.2) | 18 (42.8) | |
| non-GCB | 9 (18.0) | 8 (24.2) | 14 (29.1) | 12 (28.6) | |
| DHL/THL, n (%) | |||||
| Unknown | 13 (26.0) | 6 (18.2) | 12 (25.0) | 12 (28.6) | .58 |
| Yes (DHL/THL) | 8 (16) | 10 (30.3) | 7 (14.6) | 6 (14.3) | |
| No | 29 (58.0) | 17 (51.5) | 29 (60.4) | 24 (57.1) | |
| R-IPI, n (%) | |||||
| Unknown | 6 (12.0) | 0 (0.0) | 2 (4.2) | 0 (0.0) | .008 |
| Very good (IPI = 0) | 0 (0.0) | 1 (3.0) | 4 (8.3) | 0 (0.0) | |
| Good (IPI = 1,2) | 13 (26.0) | 10 (30.3) | 15 (31.3) | 21 (50.0) | |
| Poor (IPI = 3,4,5) | 31 (62.0) | 22 (66.7) | 27 (56.3) | 21 (50.0) | |
| Prior lines of therapy, n (%) | |||||
| Unknown | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0 (0.0) | .23 |
| 2 | 17 (34.0) | 12 (36.4) | 18 (37.5) | 11 (26.2) | |
| 3 | 14 (28.0) | 12 (36.4) | 9 (18.8) | 7 (16.7) | |
| 4+ | 19 (38.0) | 9 (27.2) | 20 (41.7) | 24 (57.1) | |
| Prior SCT, n (%) | |||||
| Unknown | 0 (0.0) | 0 (0.0) | 1 (2.1) | 1 (2.4) | .03 |
| Yes | 3 (6.0) | 7 (21.2) | 10 (20.8) | 14 (33.3) | |
| No | 47 (94.0) | 26 (78.8) | 37 (77.1) | 27 (64.3) | |
| CAR-T product, n (%) | |||||
| Axi-cel | 41 (82.0) | 31 (93.9) | 42 (87.5) | 33 (78.6) | .26 |
| Tisa-cel | 9 (18.0) | 2 (6.1) | 6 (12.5) | 9 (21.4) | |
| . | PP (n = 50) . | RD (n = 33) . | ER (n = 48) . | LR (n = 42) . | P value* . |
|---|---|---|---|---|---|
| Age (y), median (interquartile range) | 64.8 (57.5-71.9) | 65.4 (61.7-71.0) | 65.5 (59.5-70.3) | 65.5 (60.0-69.6) | .97† |
| Sex, n (%) | |||||
| Male | 34 (68.0) | 21 (63.6) | 24 (50.0) | 18 (42.9) | .06 |
| Female | 16 (32.0) | 12 (36.4) | 24 (50.0) | 24 (57.1) | |
| Stage, n (%) | |||||
| Unknown | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .53 |
| I/II | 7 (14.0) | 5 (15.2) | 12 (25.0) | 10 (23.8) | |
| III/IV | 42 (84.0) | 28 (84.8) | 36 (75.0) | 32 (76.2) | |
| Cell of origin by Hans algorithm, n (%) | |||||
| Unknown | 6 (12.0) | 2 (6.1) | 8 (16.7) | 12 (28.6) | .06 |
| GCB | 35 (70) | 23 (69.7) | 26 (54.2) | 18 (42.8) | |
| non-GCB | 9 (18.0) | 8 (24.2) | 14 (29.1) | 12 (28.6) | |
| DHL/THL, n (%) | |||||
| Unknown | 13 (26.0) | 6 (18.2) | 12 (25.0) | 12 (28.6) | .58 |
| Yes (DHL/THL) | 8 (16) | 10 (30.3) | 7 (14.6) | 6 (14.3) | |
| No | 29 (58.0) | 17 (51.5) | 29 (60.4) | 24 (57.1) | |
| R-IPI, n (%) | |||||
| Unknown | 6 (12.0) | 0 (0.0) | 2 (4.2) | 0 (0.0) | .008 |
| Very good (IPI = 0) | 0 (0.0) | 1 (3.0) | 4 (8.3) | 0 (0.0) | |
| Good (IPI = 1,2) | 13 (26.0) | 10 (30.3) | 15 (31.3) | 21 (50.0) | |
| Poor (IPI = 3,4,5) | 31 (62.0) | 22 (66.7) | 27 (56.3) | 21 (50.0) | |
| Prior lines of therapy, n (%) | |||||
| Unknown | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0 (0.0) | .23 |
| 2 | 17 (34.0) | 12 (36.4) | 18 (37.5) | 11 (26.2) | |
| 3 | 14 (28.0) | 12 (36.4) | 9 (18.8) | 7 (16.7) | |
| 4+ | 19 (38.0) | 9 (27.2) | 20 (41.7) | 24 (57.1) | |
| Prior SCT, n (%) | |||||
| Unknown | 0 (0.0) | 0 (0.0) | 1 (2.1) | 1 (2.4) | .03 |
| Yes | 3 (6.0) | 7 (21.2) | 10 (20.8) | 14 (33.3) | |
| No | 47 (94.0) | 26 (78.8) | 37 (77.1) | 27 (64.3) | |
| CAR-T product, n (%) | |||||
| Axi-cel | 41 (82.0) | 31 (93.9) | 42 (87.5) | 33 (78.6) | .26 |
| Tisa-cel | 9 (18.0) | 2 (6.1) | 6 (12.5) | 9 (21.4) | |
GCB, germinal center B-cell.
Pearson chi-square test.
Kruskal-Wallis test.
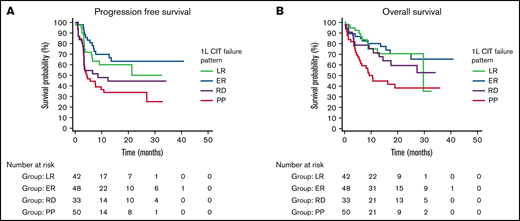
In all patients after CAR T-cell therapy, the best overall response rate (ORR) was 76.3%, the best CR rate was 57.8%, the median PFS was 21.3 months (95% CI, 7.5-26.8), and the median OS was not reached. We compared outcomes after CAR T-cell therapy based on the retrospective categorization of the initial response to frontline therapy (PP, RD, ER, or LR). By category of failure of frontline therapy, the best ORR after CAR T-cell therapy was PP, 70.0%; RD, 69.7%; ER, 81.2%; and LR, 83.3%. The best CR rate after CAR T-cell therapy was PP, 42.0%; RD, 54.5%; ER, 70.8%; and LR, 64.3%. The median PFS after CAR T-cell therapy was PP, 4.4 months (95% CI, 3.2-10.7); RD, 8.5 months (95% CI, 3.3-12.4); ER, not reached; and LR, not reached. Median OS after CAR T-cell therapy was PP, 10.6 months (95% CI, 2.9-18.2); not reached for RD and ER; and LR, 29.6 months (95% CI, 5.5-53.7).
Figure 1 depicts Kaplan-Meier curves demonstrating differences in OS (P = .005) and PFS (P = .003) based on the pattern of failure to frontline therapy. Cox proportional hazard analysis demonstrated that patients with PP had significantly worse outcomes than patients with RD, ER, or LR on MVA for PFS (HR, 1.9; 95% CI, 1.03-3.6) and OS (HR, 2.3; 95% CI, 1.2-4.4) (supplemental Table 1). Other significant baseline characteristics of MVA included the R-IPI (PFS HR, 1.9; 95% CI, 1.02-3.4; OS HR, 3.4; 95% CI, 1.6-7.3), and male sex (OS only HR, 2.1; 95% CI, 1.04-4.2).
Outcomes of CAR T-cell–treated patients by the pattern of failure of previous frontline therapy. PFS (A) and OS (B) estimates according to a pattern of failure. P = .003; P = .005.
Outcomes of CAR T-cell–treated patients by the pattern of failure of previous frontline therapy. PFS (A) and OS (B) estimates according to a pattern of failure. P = .003; P = .005.
In this study, we found that patients who received CAR T-cell therapy for LBCL had poor outcomes if they had PP during their initial frontline CIT. This has several possible implications. First, it suggests that some LBCL have crossresistance to both CIT and CAR T-cell therapy, despite differing mechanisms of action of these therapies. Speculatively, this could be due to tumor biology. For example, Rushton and colleagues recently found that TP53 is mutated in 51% of R/R LBCL cases after CIT, with the mutations remaining clonally persistent between diagnostic and relapse samples, suggesting that TP53 mutations contribute to primary treatment resistance.12 Similarly, for CAR T-cell therapy, Shouval and colleagues found that pretreatment TP53 mutations associated with a poorer outcome.13 Second, one may speculate that this is in part why efficacy outcomes on the CAR T-cell arms of the ZUMA-7, TRANSFORM, and BELINDA trials treating patients after frontline CIT were, on the whole, similar to efficacy outcomes after CAR T-cell therapy on the single-arm ZUMA-1 (NCT02348216), TRANSCEND (NCT02631044), and JULIET (NCT02445248) trials treating heavily pretreated patients.2-4,14-16 Refractoriness, selected for in the RCTs, is associated with poorer outcomes after CAR T-cell therapy in our data. The enrichment of patients with refractory disease on ZUMA-7 may have counterbalanced the benefits of treating patients with fewer prior lines of therapy compared with ZUMA-1. Third, a much lower proportion of patients with PP had ASCT before coming to CAR T-cell therapy, confirming other studies that indicated a low success of second-line CIT in this group.6 Finally, it suggests that patients who are primary progressors to frontline CIT are a high-risk population in need of a different approach. In ZUMA-12 (NCT03761056), a hypothesis-generating single-arm study, patients received axi-cel if they had positron emission tomography-avid disease after 2 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and showed a 73% 12-month EFS.17 It may be possible to overcome adverse tumor biology by intervening earlier when disease burden is low. However, intrinsic resistance may remain, highlighting the need to better understand the interplay between patient factors, CAR T cells, and tumor biology. One such interacting factor could be male sex, which has previously been observed to associate with poorer lymphoma outcomes,11 and that we have found here to be associated with poorer OS after CAR T-cell therapy.
There are limitations of this study that may affect its generalizability: we only included patients who were able to receive CD19 CAR T-cell infusion, representing a survivorship bias of R/R patients who made it through subsequent lines of therapy to receive CAR T cells under the SOC label. By contrast, the RCTs treated all patients relapsing within 12 months, and the relative proportions of PP, RD, and ER may be different than those treated in our standard of care population.
In summary, patients with LBCL with primary progression to frontline chemotherapy have poor outcomes despite subsequent CAR T-cell therapy in the third line setting. This represents a high-risk group in need of novel treatment approaches that could overcome this potential crossresistance.
Contribution: A.P.P., S.G., and M.D.J. designed the study; J.C.C., B.D.S., F.K., T.N., A.L., M.L.D., F.L.L., S.G., and M.D.J. contributed patients and/or materials; A.P.P., G.J., K.P., B.A., A.W., C.A.B., R.M., and M.D.J. performed data collection and analysis; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: J.C.C. is on the advisory board for Kite/Gilead, Novartis, Bayer, and Genentech and on the Speaker Bureau for Genentech. A.L. is on the scientific advisory board for EUSA Pharma US LLC. M.L.D. has received research funding from Celgene, Novartis, and Atara; received other financial support from Novartis, Precision Biosciences, Celyad, Bellicum, and GlaxoSmithKline; and holds stock options from Precision Biosciences, Adaptive Biotechnologies, and Anixa Biosciences. C.A.B. is a scientific advisor for Kite/Gilead and part of the speaker's bureau for Novartis. T.N. has received research support to the institution from Novartis and Karyopharm. M.D.J. provides consultancy/advisory for Kite/Gilead, BMS, Takeda, and Novartis; receives research funding from Incyte and Kite/Gilead. S.G. provides consultancy for AbbVie, BeiGene, Epizyme, TG Therapeutics, ADC Therapeutics; receives speaking funding from TG Therapeutics and ADC Therapeutics; receives research funding from Adaptive Biotechnology and Epizyme. B.D.S. provides consultancy/advisory for Adaptive, BMS/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, Kite, Pharmacyclics/Janssen, Acrotech/Spectrum, BeiGene, Incyte, Jazz, Seattle Genetics, and Stemline Therapeutics. F.L.L. provides consultancy/advisory for Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, Gammadelta Therapeutics, Iovance, Kite, Janssen, Legend Biotech, Novartis, Takeda, Wugen, Umoja, Cowen, Eco R1, Emerging Therapy Solutions, and Gerson Leherman Group. The remaining authors declare no competing financial interests.
Correspondence: Michael D. Jain, Department of Blood and Marrow Transplant and Cellular Immunotherapy, Moffitt Cancer Center, CSB-7, 12902 Magnolia Drive, Tampa, FL 33612; e-mail: michael.jain@moffitt.org.
References
Author notes
S.G. and M.D.J. contributed equally to this study.
Requests for data sharing may be submitted to Michael D. Jain (michael.jain@moffitt.org).
The full-text version of this article contains a data supplement.