TO THE EDITOR:
Obinutuzumab (G) in combination with chlorambucil (Chl) has been approved in Italy in 2017 as frontline treatment for patients with chronic lymphocytic leukemia (CLL) and comorbidities, following results of the CLL11 trial1 and real-life studies.2-4 Few studies have focused on reduction of relative dose intensity (RDI) in CLL.5,6 The aim of this study was to evaluate the impact of reduced RDI of G-Chl on the overall response rate (ORR), progression-free survival (PFS), time to next treatment (TTNT), and overall survival (OS); we also wanted to identify patients at higher risk for dose reductions. We collected and retrospectively analyzed data of 130 patients diagnosed with CLL and comorbidities from the Italian centers with the highest use of the G-Chl regimen outside clinical trials between 2017 and 2020. Patients’ characteristics are shown in supplemental Table 1. The study was conducted according to the Helsinki Declaration, Good Clinical Practice, and the applicable national regulations; all patients provided written informed consent, and the study was approved by the Institutional Ethical Committee of the Fondazione Policlinico Agostino Gemelli IRCCS. The RDI was calculated as the ratio between the dose actually delivered over time and the expected correct dose: a dose reduction of 20% was considered the best cutoff according to previous studies4,5 and was confirmed by a receiver-operating characteristic analysis in this study. For each patient, data were collected from the medical records of each center in order to assess clinical and laboratory characteristics, focusing in particular on the different categories of comorbidities. Specifically, we investigated the impact on RDI of each parameter of the cumulative illness rating scale (CIRS) taken individually and also the impact of CIRS >6, CIRS >8, or CIRS with at least 1 component with grading ≥3 (CIRS 3+).
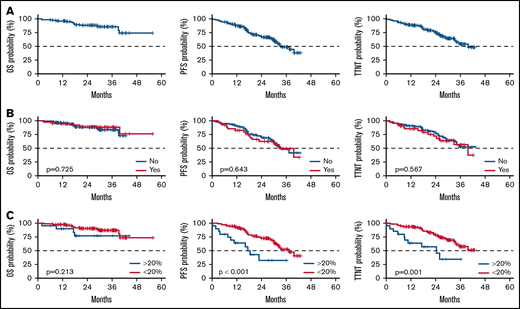
The median age was 76 years (range, 42-88); 91% of patients were over 65 years. The median CIRS score was 7 (range, 1-18), 72% of patients had a creatinine clearance <70 mL/min, and Eastern Cooperative Oncology Group Performance Status (ECOG PS) was ≥2 in 28% of patients. The median follow-up was 29.1 months (range, 1.8-55.7). Overall, the ORR was 88% (26% clinical complete response, 62% partial response), median PFS was 33 months, median TTNT was 40 months, and 24-month and 36-month OS were 88% and 85%, respectively (Figure 1). Collectively, these findings ar e aligned with those reported in the literature.1-3 In multivariate analysis, the only factor that independently impacted outcome in terms of PFS and TTNT was a reduction of obinutuzumab RDI >20%: hazard ratios were 3.03 (range, 1.49-6.25; P = .002) and 2.94 (range, 1.37-6.25; P = .006), respectively.
Outcome of the treatment chlorambucil-obinutuzumab. (A) PFS, TTNT, and OS curves of the entire cohort. (B) PFS, TTNT, and OS curves by change in dose of chlorambucil. (C) PFS, TTNT, and OS curves by obinutuzumab RDI reduction >20%.
Outcome of the treatment chlorambucil-obinutuzumab. (A) PFS, TTNT, and OS curves of the entire cohort. (B) PFS, TTNT, and OS curves by change in dose of chlorambucil. (C) PFS, TTNT, and OS curves by obinutuzumab RDI reduction >20%.
More than half of the patients (58.5%) received the standard G-Chl regimen without change in dose of either obinutuzumab or chlorambucil. Reduction of obinutuzumab dose occurred in 24% (n = 31) of patients; a reduction of >20% occurred in 15% (n = 20); chlorambucil dose was reduced in 32% (n = 42) of patients (extensive analysis in supplemental Tables 2-9 and supplemental Figures 1-3). Although dose modifications of chlorambucil did not have an impact, a decrease of >20% of RDI in obinutuzumab negatively impacted outcome in terms of ORR, PFS, and TTNT but not OS (Figure 1). ORR was significantly lower in the group with an RDI reduction of >20% (93% vs 61%, P = .001). We then compared the median PFS and TTNT in patients who did or did not receive a reduced RDI >20% (Table 1); there was a significant decrease in both PFS and TTNT in patients with a reduced RDI of >20% (PFS 17.2 vs 37.3 months; TTNT 24.4 months vs NR, respectively; P = .001). The difference was maintained at 2 and 3 years (Table 1), even when considering the subgroup with 1% to 20% reduction (supplemental Figures 1-3). Considering the causes of dose reduction, ≤20% reduction was due to 2 infusion-related reactions (IRRs), grade 1 to 2; 6 hematologic toxicities; and 3 extrahematologic toxicities. Patients who reduced >20% experienced: 4 IRRs, grade 3 to 4; 1 IRR, grade 1 to 2; 10 extrahematologic toxicities (5 infections in neutropenic patients, 2 atrial fibrillation, 1 acute renal failure, 1 transaminitis, and 1 gastrointestinal toxicity); and 5 hematologic toxicities (all grade 3-4). In no patient was the administered dose of obinutuzumab reduced per se; overall dose reduction was due to missed doses or treatment discontinuation.
Association of RDI with treatment outcome and patients' characteristics
| Impact of obinutuzumab RDI reduction >20% on outcome . | ||||
|---|---|---|---|---|
| Characteristic . | Overall (n = 130) . | RDI reduction of obinutuzumab dose . | P value* . | |
| >20% (n = 20) . | ≤20% (n = 110) . | |||
| ORR, n (%) | 111 (88%) | 11 (61%) | 100 (93%) | .001 |
| Median PFS | 33 mo | 17.2 mo | 37.3 mo | .001 |
| 24-mo PFS | 68% | 32% | 74% | <.001 |
| 36-mo PFS | 49% | 32% | 52% | <.001 |
| Median TTNT | 40 mo | 24.4 mo | NR | .001 |
| 24-mo TTNT | 75% | 57% | 79% | .001 |
| 36-mo TTNT | 54% | 34% | 58% | .001 |
| Impact of obinutuzumab RDI reduction >20% on outcome . | ||||
|---|---|---|---|---|
| Characteristic . | Overall (n = 130) . | RDI reduction of obinutuzumab dose . | P value* . | |
| >20% (n = 20) . | ≤20% (n = 110) . | |||
| ORR, n (%) | 111 (88%) | 11 (61%) | 100 (93%) | .001 |
| Median PFS | 33 mo | 17.2 mo | 37.3 mo | .001 |
| 24-mo PFS | 68% | 32% | 74% | <.001 |
| 36-mo PFS | 49% | 32% | 52% | <.001 |
| Median TTNT | 40 mo | 24.4 mo | NR | .001 |
| 24-mo TTNT | 75% | 57% | 79% | .001 |
| 36-mo TTNT | 54% | 34% | 58% | .001 |
| Impact of patients’ characteristic on obinutuzumab RDI reduction > 20% . | |||||
|---|---|---|---|---|---|
| Characteristic . | Overall (n = 130) . | RDI reduction of obinutuzumab dose . | |||
| >20% (n = 20) . | ≤20% (n = 110) . | P value* . | Q value† . | ||
| ECOG PS in class, n (%) | .027 | .027 | |||
| 0-1 | 94 (72) | 10 (50) | 84 (76) | ||
| ≥2 | 36 (28) | 10 (50) | 26 (24) | ||
| ANC before treatment, median (range) | 3920 (313, 16 000) | 3200 (313, 9290) | 4100 (580, 16 000) | .018 | .29 |
| CIRS in class, n (%) | .085 | .56 | |||
| ≤6 | 49 (38) | 4 (20) | 45 (41) | ||
| >6 | 81 (62) | 16 (80) | 65 (59) | ||
| Impact of patients’ characteristic on obinutuzumab RDI reduction > 20% . | |||||
|---|---|---|---|---|---|
| Characteristic . | Overall (n = 130) . | RDI reduction of obinutuzumab dose . | |||
| >20% (n = 20) . | ≤20% (n = 110) . | P value* . | Q value† . | ||
| ECOG PS in class, n (%) | .027 | .027 | |||
| 0-1 | 94 (72) | 10 (50) | 84 (76) | ||
| ≥2 | 36 (28) | 10 (50) | 26 (24) | ||
| ANC before treatment, median (range) | 3920 (313, 16 000) | 3200 (313, 9290) | 4100 (580, 16 000) | .018 | .29 |
| CIRS in class, n (%) | .085 | .56 | |||
| ≤6 | 49 (38) | 4 (20) | 45 (41) | ||
| >6 | 81 (62) | 16 (80) | 65 (59) | ||
ANC, absolute neutrophil count; NR, not reached.
Fisher's exact test.
False discovery rate correction for multiple testing.
We then looked at factors predicting obinutuzumab dose reduction, and we found 2 significant predictors: a lower absolute neutrophil count at the start of treatment (P = .018) and an ECOG performance status ≥2 (P = .027). Notably, neither neutropenia nor ECOG showed an impact on OS, PFS, and TTNT per se (supplemental Tables 7 and 9), confirming the impact of G-reduction on survivals.
Dose modification of chlorambucil was linked to a higher comorbidity burden, expressed both as CIRS >8 (43% vs 25%, P = .045) and CIRS 3+ (67% vs 45%, P = .026).
To date, only 1 study, conducted by European Research Initiative on CLL and the Israeli group, has reported the impact on outcome of RDI G-Chl. They showed an impact on PFS and OS of any obinutuzumab dose reduction.3 Our choice to evaluate the impact of a 20% RDI reduction is based on 2 main considerations: first, previous studies on CLL have evaluated a reduction in chemoimmunotherapy with a 20% cutoff based on the clinical need to tailor dosing on patient tolerability.5,6 Secondly, obinutuzumab is associated with side effects (eg, IRR,1,2 nonovert disseminated intravascular coagulopathy with thrombocytopenia,7 prolonged neutropenia, etc), which frequently causes delayed or missed administration of obinutuzumab, especially on the second and/or eighth day of the first course. As a result, patients on treatment often skip at least 1 dose. This study suggests that patients treated with at least 80% RDI achieve comparable response and survival when compared with patients given the full dose of obinutuzumab. Of note is the fact that, despite G-Chl having one of the lowest doses among several possible chlorambucil regimens, the reduction in chlorambucil is not associated with adverse prognosis, confirming the greater significance of obinutuzumab RDI in outcome.
The impact of the performance status in CLL patients has been investigated in several studies, mostly for chemoimmunotherapy8-12 and ibrutinib.13-15 Studies in which ECOG PS showed a negative impact included more unfit and, on average, older patients, whereas a higher CIRS showed an impact in younger and fit patients, independently of the treatment received.16 Our results align with this observation, considering that our cohort includes exclusively unfit patients, almost all of whom are older than 65 years.
In conclusion, a decrease >20% of obinutuzumab RDI results in a worse outcome in terms of ORR, PFS, and TTNT; a smaller dose reduction (≤20%), in contrast, showed no difference when compared with 100% RDI. ECOG PS ≥2 could be considered a predictor of dose reduction, although it does not affect prognosis per se. On the other hand, the need for multiple dose reductions in unfit patients should prompt reevaluation of performance status as well; frail patients may have a worse outcome if treatment is not optimized. These results need further investigation in the coming years, when obinutuzumab will be increasingly used in combination with venetoclax,17,18 to see if the survival impact of RDI reduction could be overcome by a more effective oral agent.
Acknowledgments: This work was supported by a grant for secondary data use from Roche S.p.A.
Contribution: F.A., G.C., A.V., A.T., M.M., C.V., A. Chiarenza, F.M., P.S., R.M., G.S., A. Cuccaro, R.M., A.S., C.P., I.A., M.C., L.T., D.P., and I.I. provided the data; A.P. performed the statistical analysis; A.F. and L.L. wrote the manuscript; and L.L. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luca Laurenti, Fondazione Policlinico Universitario A. Gemelli IRCCS, Istituto di Ematologia, Largo A. Gemelli 8, 00168 Rome, Italy; e-mail: luca.laurenti@unicatt.it.
References
Author notes
Presented in abstract form at the 48th Congress of the Italian Society of Hematology in October 2021.
Requests for data sharing may be submitted to Luca Laurenti (luca.laurenti@unicatt.it).
The full-text version of this article contains a data supplement.