Key Points
Our mobile health exercise intervention adapted for older patients with myeloid neoplasms is feasible, usable, and safe.
Geriatric assessment domain impairments are highly prevalent; there is a need for supportive care interventions to mitigate toxicities.
Abstract
Many older patients with myeloid neoplasms experience treatment-related toxicities. We previously demonstrated that a home-based, progressive aerobic walking and resistance exercise program (EXCAP) improved physical and psychological outcomes in patients with cancer. However, older patients have more difficulty adhering to exercise than younger patients. Reasons may include low motivation, difficulty with transportation, and limited access to exercise professionals. To improve exercise adherence, we integrated a mobile app with EXCAP (GO-EXCAP) and assessed its feasibility and usability in a single-arm pilot study among older patients with myeloid neoplasms undergoing outpatient chemotherapy. GO-EXCAP intervention lasts for 2 cycles of treatment, and the primary feasibility metric was data reporting on the app. Usability was evaluated via the system usability scale (SUS). Patients were interviewed at mid and postintervention to elicit their feedback, and deductive thematic analysis was applied to the transcripts. Twenty-five patients (mean age, 72 years) were recruited. Recruitment and retention rates were 64% and 88%, respectively. Eighty-two percent (18/22) of patients entered some exercise data on the app at least half of the study days, excluding hospitalization (a priori, we considered 70% as feasible). Averaged daily steps were 2848 and 3184 at baseline and after intervention, respectively. Patients also performed resistance exercises 26.2 minutes per day, 2.9 days per week at low intensity (rate of perceived exertion 3.8/10). Usability was above average (SUS, 70.3). In qualitative analyses, 3 themes were identified, including positive experience with the intervention, social interactions, and flexibility. The GO-EXCAP intervention is feasible and usable for older patients with myeloid neoplasms undergoing outpatient chemotherapy. This trial was registered at www.clinicaltrials.gov as #NCT04035499.
Introduction
Most cases of myeloid neoplasms (eg, acute myeloid leukemia [AML], myelodysplastic syndrome [MDS], MDS/myeloproliferative neoplasm [MPN] overlap) are diagnosed in adults aged ≥60 years.1,2 With the increasing availability of outpatient chemotherapy regimens, a greater number of older adults are able to receive cancer-directed treatments.3,4 Older adults with cancer are at risk for toxicities due to concomitant aging-related conditions such as physical and cognitive impairments.5-7 Behavioral and supportive care interventions, including exercise, have the potential to prevent or mitigate impairments and toxicities related to cancer and its treatment (eg, physical function impairments, fatigue, mood disturbances, and worse quality of life).8,9
Previous studies have evaluated the feasibility and preliminary efficacy of various structured exercise programs in patients receiving chemotherapy for myeloid neoplasms; 2 focused exclusively on older adults.8,10-12 Results demonstrated the feasibility of exercise interventions conducted in older patients with myeloid neoplasms receiving cancer treatment.8,10 , -14 However, most of these older patients were receiving inpatient intensive treatment. There is a lack of data on the feasibility and efficacy of exercise intervention conducted in older patients receiving outpatient treatment for myeloid neoplasms. Compared with older patients receiving intensive inpatient therapy, those receiving outpatient chemotherapy are more likely to have physical function impairments (because they are not candidates for intensive inpatient therapy). Also, their treatment in the outpatient setting offers less health care supervision and structured ways for assessing and improving physical function.15
Common exercise barriers faced by older adults, in general, include low motivation, difficulty with transportation, and limited access to exercise professionals, which lead to lower exercise adherence.16-20 Mobile health (mHealth) technologies integrated with an exercise program permit monitoring of exercise adherence and identification of barriers to exercise and recognition of symptoms during treatment. Furthermore, mHealth technologies provide reminders to exercise and can facilitate the delivery of feedback from their clinicians and exercise professionals to patients.21-23
Exercise for Cancer Patients (EXCAP) is an established home-based progressive aerobic walking and resistance exercise program that has been shown to improve physical and psychological outcomes among patients with cancer.16,24 -27 However, older patients have more difficulty adhering to exercise than younger patients.16 We have previously tested a mobile app to deliver supportive care interventions (without a structured exercise program) and found it to be feasible and usable.23 Based on these data, we integrated the mobile app with the EXCAP intervention (Geriatric Oncology-EXCAP [GO-EXCAP]). Considering feedback provided by 13 older patients with myeloid neoplasms recruited for a qualitative study, we adapted the GO-EXCAP intervention and procedures (eg, initiating the intervention at the second or subsequent cycles of chemotherapy, personalizing exercise goals).21 In order to ultimately prepare for a phase 2 randomized clinical trial in patients with myeloid neoplasms, we conducted the first single-arm pilot study (GO-EXCAP 1) to primarily assess the feasibility and usability of the GO-EXCAP intervention and collect participant feedback to further refine the intervention that combined an exercise intervention with mHealth technologies. We also assessed changes in outcomes (physical function, fatigue, mood, and quality of life) from baseline to postintervention, and their correlations with exercise data.
Methods
Study design, setting, and participants
We conducted a single-arm pilot trial and recruited older patients with myeloid neoplasms from the University of Rochester Medical Center Wilmot Cancer Institute (WCI) in New York (ClinicalTrials.gov identifier: NCT04035499). Eligible patients were (1) aged ≥60 years; (2) diagnosed with a myeloid neoplasm (eg, AML, MDS, chronic neutrophilic leukemia, and MDS/MPN overlap syndrome, which includes chronic myelomonocytic leukemia and atypical chronic myeloid leukemia); (3) receiving outpatient chemotherapy; (4) had a physician-verified Eastern Cooperative Oncology Group (ECOG) performance status (PS) between 0 and 2; (5) had no medical contraindications to exercise per the treating oncologist; (6) Able to walk 4 meters; (7) English speaking; and (8) capable of providing informed consent. We initially limited enrollment to patients starting cycle 2 of hypomethylating agent (HMA)-based chemotherapy (the most common outpatient treatment regimen). However, due to recruitment challenges during the COVID-19 pandemic, we expanded the eligibility criteria in December 2020 to include patients starting any cycle of outpatient chemotherapy. Patients with a platelet count of ≤10 000 per microliter on the most recent complete blood count and who did not receive platelet transfusion (eg, platelet refractoriness, hospice) were excluded.
Patients with myeloid neoplasm who were seen at WCI from February 2020 to July 2021 were screened. After confirming with the treating oncology team, those who met the aforementioned eligibility criteria were approached in person or via telephone. The treating physician and principal investigator (K.P.L.) confirmed the eligibility for study participation. This study received approval from The University of Rochester Research Subjects Review Board. Specifically for the qualitative data, we followed the Standards for Reporting Qualitative Research checklist.28
Intervention description
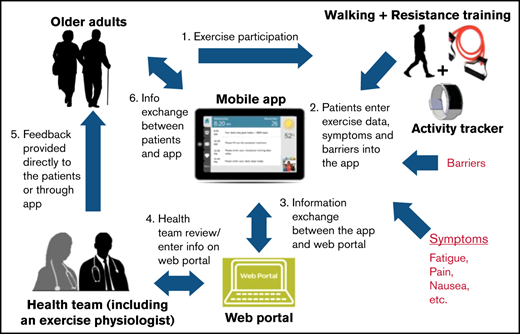
The proposed intervention has been described in detail previously (Figure 1).21 Briefly, the GO-EXCAP intervention consists of 2 components: (1) The EXCAP exercise program is an individually tailored, low to moderate intensity, home-based exercise program consisting of progressive walking and resistance-band exercises. Individualized exercise prescriptions are provided by an American College of Sports Medicine (ACSM)-certified exercise physiologist either in an in-person or virtual session. In addition, an EXCAP kit that includes a bag, a printed manual, an activity tracker, and 3 different resistance-level therapeutic bands is provided to patients; (2) a mobile app with a patient interface for data entering and an online dashboard for data monitoring by study personnel and exercise physiologist. The mobile app has previously been shown to be feasible for use in older patients with myeloid neoplasms.23
Study procedures
Baseline.
After obtaining informed consent (verbal or written), patients completed demographics and baseline measures. A geriatric assessment (eg, physical function, comorbidity, social support, nutritional status, cognition, and psychological health) was performed using self-administered questionnaires or objective tests to characterize vulnerabilities in this population (Table 1).23,29 ,,,,,,, -39 Patients also completed questionnaires to assess fatigue and quality of life. Clinical data (eg, number of medications) and treatment characteristics were collected or extracted by study staff from the electronic medical record.
Geriatric assessment domains
| Geriatric assessment domain and measure . | Description of domain and measure . | Mean (SD) or median (IQR) . | Definitions of impairment . | n = 25, n with impairment (%) . |
|---|---|---|---|---|
| Medications or polypharmacy | Number of scheduled and as-needed medications, excluding chemotherapy | 9.5 (SD, 4.9) | ≥5 scheduled and as-needed medications, excluding chemotherapy | 22 (88.0) |
| Physical function | ≥1 impairment in SPPB, ADL, IADL, and fall | 21 (84.0) | ||
| In-person SPPB | Three tasks that include balance, gait speed, chair stands; evaluates lower extremity function; scores range from 0 to 12; lower score is worst32 | 9.2 (SD, 1.7) | ≤9 | 13 (76.5) |
| Virtual SPPB* | Assesses patient’s perceived ability to perform the 3 tasks on SPPB; scores range from 0 to 12; lower score is worst34 | 8.5 (SD, 3.0) | ≤9 | 14 (77.7) |
| ADL | Kats ADL; assesses independence in 6 self-care activities (e.g., bathing, ambulating); scores range from 0 to 6; lower score is worst30 | 6.0 (IQR, 0) | <6 | 3 (12.0) |
| IADL | OARS IADL; assesses independence in 7 self-care activities that are more complex (e.g., preparing meals, managing finances); scores range from 0 to 14; lower score is worst31 | 13.0 (IQR, 2.0) | <14 | 13 (52.0) |
| Fall† | Fall history over the past year | 0 (IQR, 1) | ≥1 | 8 (33.3) |
| Comorbidities | OARS Comorbidity Scale; patients report the presence or absence of 13 comorbidities and how the comorbidities affect them31 | 3.6 (SD, 1.8) | ≥1 comorbidity that affects them a “great deal” or ≥3 comorbidities | 19 (76.0) |
| Instrumental social support) | MOS Social Support survey; patients indicate support in 4 questions (if they had someone to help if they were confined to bed, take them to the doctor if needed, prepare their meals if they were unable to do it, and help with daily chores if they were sick), scores range from 4 to 20; higher score is better35 | 17.0 (IQR, 6.0) | Patients selected some of the time, a little of the time, or none of the time for any of the 4 questions | 7 (28.0) |
| Nutritional status | BMI and self-reported weight loss in the previous year | Mean BMI = 29.0 (SD, 5.2)Mean weight loss in kg = 5.2 (SD, 7.4) | A BMI <21 or >10% weight loss in the previous year | 7 (28.0) |
| Cognition | MOCA or MOCA-blind‡ (if in-person assessment was not possible), scores range from 0 to 30 and 0 to 22, respectively; lower score is worst36-38 | MOCA = 26.0 (IQR, 4.5) | MOCA <26 or MOCA-blind <19 | 12 (48.0) |
| Psychological health | CES-D; 20 items and assesses depressive symptoms, scores range from 0 to 60; higher score is worse39 | Mean = 11.7 (SD, 7.7) | CES-D ≥16 | 7 (28.0) |
| Geriatric assessment domain and measure . | Description of domain and measure . | Mean (SD) or median (IQR) . | Definitions of impairment . | n = 25, n with impairment (%) . |
|---|---|---|---|---|
| Medications or polypharmacy | Number of scheduled and as-needed medications, excluding chemotherapy | 9.5 (SD, 4.9) | ≥5 scheduled and as-needed medications, excluding chemotherapy | 22 (88.0) |
| Physical function | ≥1 impairment in SPPB, ADL, IADL, and fall | 21 (84.0) | ||
| In-person SPPB | Three tasks that include balance, gait speed, chair stands; evaluates lower extremity function; scores range from 0 to 12; lower score is worst32 | 9.2 (SD, 1.7) | ≤9 | 13 (76.5) |
| Virtual SPPB* | Assesses patient’s perceived ability to perform the 3 tasks on SPPB; scores range from 0 to 12; lower score is worst34 | 8.5 (SD, 3.0) | ≤9 | 14 (77.7) |
| ADL | Kats ADL; assesses independence in 6 self-care activities (e.g., bathing, ambulating); scores range from 0 to 6; lower score is worst30 | 6.0 (IQR, 0) | <6 | 3 (12.0) |
| IADL | OARS IADL; assesses independence in 7 self-care activities that are more complex (e.g., preparing meals, managing finances); scores range from 0 to 14; lower score is worst31 | 13.0 (IQR, 2.0) | <14 | 13 (52.0) |
| Fall† | Fall history over the past year | 0 (IQR, 1) | ≥1 | 8 (33.3) |
| Comorbidities | OARS Comorbidity Scale; patients report the presence or absence of 13 comorbidities and how the comorbidities affect them31 | 3.6 (SD, 1.8) | ≥1 comorbidity that affects them a “great deal” or ≥3 comorbidities | 19 (76.0) |
| Instrumental social support) | MOS Social Support survey; patients indicate support in 4 questions (if they had someone to help if they were confined to bed, take them to the doctor if needed, prepare their meals if they were unable to do it, and help with daily chores if they were sick), scores range from 4 to 20; higher score is better35 | 17.0 (IQR, 6.0) | Patients selected some of the time, a little of the time, or none of the time for any of the 4 questions | 7 (28.0) |
| Nutritional status | BMI and self-reported weight loss in the previous year | Mean BMI = 29.0 (SD, 5.2)Mean weight loss in kg = 5.2 (SD, 7.4) | A BMI <21 or >10% weight loss in the previous year | 7 (28.0) |
| Cognition | MOCA or MOCA-blind‡ (if in-person assessment was not possible), scores range from 0 to 30 and 0 to 22, respectively; lower score is worst36-38 | MOCA = 26.0 (IQR, 4.5) | MOCA <26 or MOCA-blind <19 | 12 (48.0) |
| Psychological health | CES-D; 20 items and assesses depressive symptoms, scores range from 0 to 60; higher score is worse39 | Mean = 11.7 (SD, 7.7) | CES-D ≥16 | 7 (28.0) |
ADL, activities of daily living; BMI, body mass index; CES-D, Center for Epidemiological Studies Depression Scale; IADL, instrumental activities of daily living; MOCA, Montreal Cognitive Assessment; MOS, Medical Outcomes Survey; OARS, older American resources and services; SPPB, short physical performance battery.
7 patients had missing data.
1 patient had missing data.
1 patient had MOCA-blind administered.
To obtain baseline step count, patients wore an activity tracker (Garmin Forerunner 35) for 4 to 7 days before the start of the intervention. Within 3 days of intervention initiation, study participants met with an ACSM-certified exercise physiologist who delivered the EXCAP exercise program and provided individualized exercise prescriptions and another study staff who demonstrated how to use the mobile app. Progressive step goals were generated from baseline step data and shared with the participant before the start of the intervention to encourage increasing step counts by 5% to 20% weekly over the study period (5% to 20% weekly increase in steps would allow a patient in the sedentary category [ie, <5000 steps] to progress toward or achieve the active category [ie, ≥10 000 steps] during the intervention period).40 Participants were then provided with an EXCAP kit and a tablet preloaded with the mobile app.
Intervention period.
Participants completed a safety questionnaire that included questions on symptoms (chest pain or extreme fatigue, anemia, or dizziness), recent blood count, and whether they had received a transfusion if their blood count was low before exercising every day. Participants were encouraged to reach a prescribed daily step goal and perform resistance-band exercises every day during the intervention period. At the end of each day, participants entered the number of steps walked (gathered from the activity tracker), minutes of resistance-band exercise performed, as well as the rate of perceived exertion (the intensity of exercise, 0-10, with increasing intensity) on the mobile app.41 Participants also completed symptom surveys that were conveyed to their treating oncology team via e-mails on a weekly basis. The exercise physiologist monitored the online dashboard twice per week during the intervention, provided individualized feedback, including motivational messages via the app, and adjusted exercise prescriptions as needed. The study team spoke with each participant once per week (either in-person or via phone) to address any issues and provide encouragement. At midintervention (weeks 4-6), a 15- to 30-minute virtual or in-person audio-recorded interview was conducted (by C.S. or E.E.W. [study coordinators trained by K.P.L., a geriatric hematologist]; see supplemental Figure 1 for interview script which was pilot tested) to elicit their experience, barriers to adherence to the intervention, and app functionality.
Postintervention.
At postintervention, data on step counts and resistance training were collected from the app for 4 to 7 days. Patients also completed postintervention measures (same as baseline) as well as the SUS and had another 15- to 30-minute virtual or in-person audio-recorded interview, similar to the one conducted at midintervention.
Intervention duration
The intervention period was initially specified to be 8 weeks, or 2 cycles of chemotherapy, with each cycle repeated every 4 weeks. Due to frequent treatment delays, treatment-related complications such as cytopenias and hospitalizations, the intervention period was modified to be ≥8 weeks and up to 12 weeks (but still 2 cycles of chemotherapy), whichever was longer, with the exception of those who underwent hematopoietic stem cell transplantation (HSCT) before 8 weeks. One patient underwent HSCT at week 6 and therefore participated in the study intervention for only 6 weeks.
Intervention feasibility, usability, and safety assessments
The primary feasibility metric was the percentage of patients entering exercise data into the mobile app. We also collected recruitment (percentage of patients approached and consented) and retention (percentage of patients who completed the baseline assessment and subsequently completed postintervention assessment). The reasons patients declined to enroll as well as reasons for dropout were collected. We also used a 10-item questionnaire, the SUS, rating responses from “strongly agree” to “strongly disagree” to assess intervention usability postintervention (supplemental Figure 2).42 An SUS >68 is considered above average.42 The safety of the EXCAP exercise program was also assessed using the Common Terminology Criteria for Adverse Events.
Outcomes
Outcomes of interest were physical function, fatigue, mood, and quality of life. Physical function was assessed using the Short Physical Performance Battery (SPPB), which is a valid 3-component objective assessment used to evaluate physical function in older adults.32 It ranges from 0 to 12; a higher score indicates a greater physical function, and a score of ≤9 is considered impaired.33 Fatigue was measured using the Brief Fatigue Inventory (BFI). The BFI is a reliable and validated 9-item questionnaire that assesses fatigue in patients with cancer.43 Scores range from 0 to 11, with higher scores indicating greater patient-reported fatigue. Mood was assessed using the Center for Epidemiological Studies Depression Scale (CES-D), a validated 20-item questionnaire with scores ranging from 0 to 60. A score of ≥16 is considered impaired.39 Quality of life was assessed using the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu), a reliable and valid measure of health-related quality of life (HRQoL) in patients with acute or chronic leukemia. Each question is rated on a 5-point Likert scale, with higher scores relating to a better HRQoL.44
Qualitative interviews
All interviews were uploaded for transcription by a professional transcription service and subsequently deleted from the audio recorder. Transcripts were not provided to participants for comments, correction, or feedback. An audit trail was kept to enhance trustworthiness.
Patient enrollment and reasons for refusal to consent and withdrawal.
Statistical analyses
The SAS v.9.4 (SAS Institute Inc., Cary, NC) was used to perform all quantitative analyses. We used descriptive statistics to summarize our study sample, feasibility metrics, and geriatric assessment domain impairments. We calculated a 95% exact confidence interval (CI) using the Clopper-Pearson method.45 A priori, we considered the GO-EXCAP mobile app as feasible if ≥70% of patients entered exercise data into the mobile app ≥50% of the study period days, excluding hospitalization, based on our previous app study and published exercise studies in older patients with AML.8,23,46 We anticipated that about 20% to 30% of the participants would withdraw before postintervention assessment due to rapid disease progression or death. These patients are not included in the feasibility analysis because their withdrawals from the study were not related to the intervention. With 25 patients enrolled, we anticipated ≥17 patients would be evaluable for our primary feasibility metric. We did not prespecify a goal for recruitment and retention rates. Paired t tests or Wilcoxon tests were used to assess exercise data and outcomes from baseline to postintervention. Spearman’s correlation was used to assess relationships between exercise data and outcomes from baseline to postintervention. Because this was a small single-arm pilot study, we prespecified α = 0.10 (2-tailed) for hypothesis testing.47-49
We analyzed the interview transcripts using deductive thematic analysis. Two independent coders (C.S., E.E.W., or K.T.; K.T. is a medical student trained by C.S. in coding) analyzed all transcripts using MAXQDA (VERBI Software GmbH, Berlin, Germany). An initial coding scheme was created, which was revised and finalized using the first 2 transcripts. Any discrepancies were resolved through consensus with a third investigator (K.P.L.). Data saturation was achieved (ie, no new themes have emerged) as determined by the coders.50,51
Results
Recruitment and retention rates
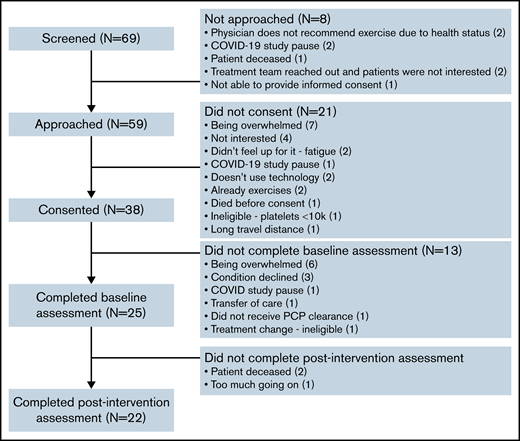
We approached 59 patients, and 38 consented to study participation, yielding a recruitment rate of 64%. Of these 38 patients, 25 completed baseline assessments, and 22 completed postintervention assessments, with a retention rate of 88% (22/25). Reasons for refusal to consent and for withdrawal are shown in Figure 2; supplemental Table 1 shows the demographics of the various groups for comparison.
Demographics and clinical characteristics
The mean age of the 25 participants who completed baseline assessments was 72 (standard deviation [SD], 4.9; range, 62-81), 32% (8/25) were females, 92% (23/25) were white, 96% (24/25) were non-Hispanic or Latino, and 64% (16/25) were married (Table 2). Sixty percent (15/25) had AML, and 44% (11/25) were receiving HMA-based combination treatment.
Demographics and clinical characteristics
| Variables . | n = 25, n (%) . |
|---|---|
| Age (y), mean (SD, range) | 72 (4.9, 62-81) |
| Gender | |
| Male | 17 (68.0) |
| Female | 8 (32.0) |
| Race | |
| White | 23 (92.0) |
| Black or African American | 1 (4.0) |
| Prefer not to say | 1 (4.0) |
| Ethnicity | |
| Not Hispanic or Latino | 24 (96.0) |
| Prefer not to say | 1 (4.0) |
| Marital status | |
| Married | 16 (64.0) |
| Divorced or widowed | 4 (16.0) |
| Single | 5 (20.0) |
| Education | |
| High school or below | 4 (16.0) |
| At least some college | 6 (24.0) |
| College graduate | 5 (20.0) |
| Postgraduate level | 9 (36.0) |
| Prefer not to say | 1 (4.0) |
| Living arrangement | |
| Partner (spouse/significant other) | 18 (72.0) |
| Alone | 7 (28.0) |
| Karnofsky performance status | |
| 90-100 | 4 (16.0) |
| 70-80 | 15 (60.0) |
| 50-60 | 6 (24.0) |
| Diagnosis | |
| AML | 15 (60.0) |
| MDS | 8 (32.0) |
| MDS/MPN overlap | 1 (4.0) |
| Chronic neutrophilic leukemia | 1 (4.0) |
| AML risk group, n=15 | |
| Low | 2 (13.3) |
| Intermediate | 5 (33.3) |
| Adverse | 8 (53.3) |
| MDS risk group, n=8 | |
| Low | 4 (50.0) |
| Intermediate | 3 (37.5) |
| Very high | 1 (12.5) |
| Treatment | |
| HMA combination treatment (e.g., venetoclax) | 11 (44.0) |
| HMA only | 11 (44.0) |
| Other | 3 (12) |
| Chemotherapy cycle at initiation of intervention, n=24* | |
| 1 | 3 (12.5) |
| 2 | 13 (54.2) |
| 3 | 4 (16.7) |
| ≥4 | 4 (16.7) |
| Variables . | n = 25, n (%) . |
|---|---|
| Age (y), mean (SD, range) | 72 (4.9, 62-81) |
| Gender | |
| Male | 17 (68.0) |
| Female | 8 (32.0) |
| Race | |
| White | 23 (92.0) |
| Black or African American | 1 (4.0) |
| Prefer not to say | 1 (4.0) |
| Ethnicity | |
| Not Hispanic or Latino | 24 (96.0) |
| Prefer not to say | 1 (4.0) |
| Marital status | |
| Married | 16 (64.0) |
| Divorced or widowed | 4 (16.0) |
| Single | 5 (20.0) |
| Education | |
| High school or below | 4 (16.0) |
| At least some college | 6 (24.0) |
| College graduate | 5 (20.0) |
| Postgraduate level | 9 (36.0) |
| Prefer not to say | 1 (4.0) |
| Living arrangement | |
| Partner (spouse/significant other) | 18 (72.0) |
| Alone | 7 (28.0) |
| Karnofsky performance status | |
| 90-100 | 4 (16.0) |
| 70-80 | 15 (60.0) |
| 50-60 | 6 (24.0) |
| Diagnosis | |
| AML | 15 (60.0) |
| MDS | 8 (32.0) |
| MDS/MPN overlap | 1 (4.0) |
| Chronic neutrophilic leukemia | 1 (4.0) |
| AML risk group, n=15 | |
| Low | 2 (13.3) |
| Intermediate | 5 (33.3) |
| Adverse | 8 (53.3) |
| MDS risk group, n=8 | |
| Low | 4 (50.0) |
| Intermediate | 3 (37.5) |
| Very high | 1 (12.5) |
| Treatment | |
| HMA combination treatment (e.g., venetoclax) | 11 (44.0) |
| HMA only | 11 (44.0) |
| Other | 3 (12) |
| Chemotherapy cycle at initiation of intervention, n=24* | |
| 1 | 3 (12.5) |
| 2 | 13 (54.2) |
| 3 | 4 (16.7) |
| ≥4 | 4 (16.7) |
HMA, hypomethylating agent.
HMA was planned but switched to hydroxyurea following enrollment.
Geriatric assessment domains
Impairments in geriatric assessment domains were highly prevalent, ranging from 28% to 88% (Table 1). On average, patients had impairment in 4 geriatric assessment domains; 12%, 56%, and 32% of patients had ≤2, 3 to 4, and ≥5 impairments, respectively.
Primary feasibility metric, usability, and safety assessments
The average study period days in the 22 patients were 62.7 days, or 8.9 weeks (SD, 11.7 days; range, 38-84 days); 4/22 (18%) were hospitalized during the study period. After excluding days hospitalized, the average study period days were 60.7 days, or 8.7 weeks (SD, 11 days; range 38-84 days). In the assessment of our primary feasibility metric, 82% (18/22; 95% CI, 0.60-0.95) of patients entered any exercise data into the mobile app ≥50% of the study period days, excluding hospitalization. Among those patients, 82% (18/22; 95% CI, 0.60-0.95) entered steps data, and 64% (14/22; 95% CI, 0.41-0.83) entered resistance data into the mobile app ≥50% of the study period days, excluding hospitalization. Supplemental Table 2 shows the differences in characteristics between those who met vs did not meet the primary feasibility metric.
At baseline, patients walked on average 3123 (SD, 2012) daily steps, which increased to 3442 (SD, 2629) daily steps at postintervention (mean difference = +319 [SD, 1962] steps; P = .48). For resistance-band exercises, patients reported performing a mean duration of 27.1 (SD, 10.36) minutes per day, 2.9 (SD, 2.2) days per week (individual paired t test comparing mean duration per day and days per week from baseline to postintervention; both P ≤ .0001), and rated their perceived exertion at 3.3 (SD, 1.1) on a 1 to 10 Likert scale indicating low intensity. Weekly steps and resistance exercise data are shown in supplemental Table 3. Patients reported an average SUS of 70.3 (SD, 20.8) (supplemental Table 4). The intervention was safe, and no adverse events were reported.
Outcomes
Overall, postintervention scores for SPPB, BFI, CES-D, and FACT-Leu were better compared with baseline, but none of them was statistically significant (Table 3). Change in SPPB correlated with both change in steps (r = 0.43; P = .06) and change in total resistance minutes (r = 0.37; P = .09) (Table 4). Change in BFI correlated with change in total resistance minutes (r = 0.43; P = .05).
Outcomes at baseline and postintervention
| n = 22 . | Baseline . | Postintervention . | Change in mean from pre to post (SD) . | P value . |
|---|---|---|---|---|
| Short Physical Performance Battery, mean (SD)* | 9.0 (1.7) | 9.2 (2.4) | +0.2 (1.6) | .61 |
| Brief Fatigue Inventory, mean (SD)† | 29.4 (20.8) | 23.2 (18.8) | −6.2 (17.9) | .12 |
| Center for Epidemiologic Studies Depression, mean (SD)† | 11.7 (7.7) | 10.6 (7.5) | −0.6 (6.1) | .65 |
| Functional Assessment of Cancer Therapy-Leukemia, mean (SD)* | 123.9 (24.7) | 127.1 (22.9) | +3.2 (18.1) | .42 |
| n = 22 . | Baseline . | Postintervention . | Change in mean from pre to post (SD) . | P value . |
|---|---|---|---|---|
| Short Physical Performance Battery, mean (SD)* | 9.0 (1.7) | 9.2 (2.4) | +0.2 (1.6) | .61 |
| Brief Fatigue Inventory, mean (SD)† | 29.4 (20.8) | 23.2 (18.8) | −6.2 (17.9) | .12 |
| Center for Epidemiologic Studies Depression, mean (SD)† | 11.7 (7.7) | 10.6 (7.5) | −0.6 (6.1) | .65 |
| Functional Assessment of Cancer Therapy-Leukemia, mean (SD)* | 123.9 (24.7) | 127.1 (22.9) | +3.2 (18.1) | .42 |
Higher is better.
Higher is worse.
Spearman correlation between change in exercise data and change in outcomes from baseline to postintervention
| Change in outcomes from baseline to postintervention . | Change in steps baseline to postintervention . | Change in total resistance minutes baseline to postintervention . |
|---|---|---|
| Change in in-person Short Physical Performance Battery | r = 0.43; P = .06 (n = 20) | r = 0.37; P = .09 (n = 21) |
| Change in Brief Fatigue Inventory | r = 0.13; P = .59 (n = 20) | r = −0.43; P = .05 (n = 21) |
| Change in Center for Epidemiologic Studies Depression | r = 0.07; P = .76 (n = 20) | r = −0.27; P = .23 (n = 21) |
| Change in Functional Assessment of Cancer Therapy-Leukemia | r = −0.02; P = .92 (n = 20) | r = 0.28; P = .21 (n = 21) |
| Change in outcomes from baseline to postintervention . | Change in steps baseline to postintervention . | Change in total resistance minutes baseline to postintervention . |
|---|---|---|
| Change in in-person Short Physical Performance Battery | r = 0.43; P = .06 (n = 20) | r = 0.37; P = .09 (n = 21) |
| Change in Brief Fatigue Inventory | r = 0.13; P = .59 (n = 20) | r = −0.43; P = .05 (n = 21) |
| Change in Center for Epidemiologic Studies Depression | r = 0.07; P = .76 (n = 20) | r = −0.27; P = .23 (n = 21) |
| Change in Functional Assessment of Cancer Therapy-Leukemia | r = −0.02; P = .92 (n = 20) | r = 0.28; P = .21 (n = 21) |
Examples of feedback provided by participants
| Domains . | Feedback . | Future modifications based on feedback . |
|---|---|---|
| Exercises | Participant wanted to know options on how to modify exercises | Exercise physiologist will reinforce ways to modify exercises during initial teaching and weekly check-ins |
| Communication | Participant wanted an option on the app to explain the challenge with exercising and to be able to communicate to the exercise physiologist | - Include open-ended field on the app when assessing exercise barriers and symptoms - Create video and chat features within the app to allow participants to communicate directly with the exercise physiologist |
| Symptom survey | - Participants felt that some of the questions on symptoms were repetitive (eg, all patients answered a set number of questions on symptoms), and they did not want to answer the symptom surveys if they did not experience the symptoms | - Create algorithms on the back end where if patients respond “no” to certain symptoms, they will not have to answer questions on “severity” - Reinforce the importance of completing the survey questions even though they did not experience certain symptoms |
| - Participant felt differently throughout the day and would like a way of conveying this | - Set daily required questionnaires but include the option of completing questionnaires more frequently if patients wish | |
| Wearable device | Participant had difficulty securing the tracker with a silicone strap | Use a tracker with a Velcro strap |
| Other | Participant stated that having a “buddy” would serve as motivation | Develop a scoreboard and gaming features on the app where participants can view and compare exercise data with other participants with rewards |
| Domains . | Feedback . | Future modifications based on feedback . |
|---|---|---|
| Exercises | Participant wanted to know options on how to modify exercises | Exercise physiologist will reinforce ways to modify exercises during initial teaching and weekly check-ins |
| Communication | Participant wanted an option on the app to explain the challenge with exercising and to be able to communicate to the exercise physiologist | - Include open-ended field on the app when assessing exercise barriers and symptoms - Create video and chat features within the app to allow participants to communicate directly with the exercise physiologist |
| Symptom survey | - Participants felt that some of the questions on symptoms were repetitive (eg, all patients answered a set number of questions on symptoms), and they did not want to answer the symptom surveys if they did not experience the symptoms | - Create algorithms on the back end where if patients respond “no” to certain symptoms, they will not have to answer questions on “severity” - Reinforce the importance of completing the survey questions even though they did not experience certain symptoms |
| - Participant felt differently throughout the day and would like a way of conveying this | - Set daily required questionnaires but include the option of completing questionnaires more frequently if patients wish | |
| Wearable device | Participant had difficulty securing the tracker with a silicone strap | Use a tracker with a Velcro strap |
| Other | Participant stated that having a “buddy” would serve as motivation | Develop a scoreboard and gaming features on the app where participants can view and compare exercise data with other participants with rewards |
Qualitative interviews
We analyzed both mid and postintervention interviews, which revealed 3 themes (see supporting quotes in supplemental Table 5):
- 1.
Positive experience with the intervention
Overall, most participants had positive experiences with the study. The intervention made participants aware of how much they were walking and provided them with a goal to achieve in a progressive fashion. While some participants were not able to meet their exercise goals due to several health or environmental reasons (eg, health deterioration, dizziness, pain, fatigue, unable to walk due to cold or hot weather, not having space for resistance exercises), subjectively they felt that the exercises strengthened their muscles and kept them moving, which made them feel good. One participant felt that the mental benefit was more than the physical benefit. Some expressed reluctance in the beginning but were able to progress through the exercises with time. Participants also enjoyed the “daily accountability” either from the app or the study team and exercise physiologist.
- 2.
Importance of social interactions
The ability to interact with various individuals during the study was valued and appreciated by many participants. Many participants wanted additional interactions with the exercise physiologist or other participants in the program. For example, a participant suggested a virtual session to exercise with others. Participants wanted the ability to communicate directly with the study team and exercise physiologist, as well as more in-person interactions and check-ins. One participant also suggested that communication from the oncologist could serve as a motivation for the participant.
- 3.
Flexibility
Participants highlighted the importance of flexible study procedures and intervention and appreciated that they could do some of the exercises in parts throughout the day. One participant wanted to see modified resistance-band exercises that they could perform at their comfort level, while another wanted to be challenged to do more on certain days. While specific exercise goals were provided to participants, they wanted the study team to emphasize the flexibility of the program and reassure them that they could do as many or as few of the exercises as they were able to. Some participants were concerned that exercises made them feel worse (eg, worsening existing aches and pains) and questioned the appropriateness of exercise when their counts were low, so participants felt that additional reassurance would be helpful. In terms of symptoms, some patients felt that reporting every day was burdensome, while others wanted to enter multiple times in a day because they might experience different symptoms or that severity may be different throughout the day. In addition, they wanted to enter other symptoms not included on our preselected list.
Other examples of feedback specifically on the various intervention domains (eg, exercises, app, and surveys) are shown in Table 5.
Discussion
In this single-arm pilot study, we demonstrated the feasibility of our intervention with >80% of patients entering any exercise data into the mobile app ≥50% of the study period days, excluding hospitalization. In addition, we were able to recruit and retain (64% and 88%, respectively) older patients with myeloid neoplasms receiving outpatient chemotherapy to a supportive care trial evaluating a mHealth exercise intervention. Despite a high prevalence of impairments shown from the geriatric assessment, participants were able to use the mobile app and safely participate in an exercise program. In addition, they perceived the intervention as being usable. Qualitative analyses supported findings from the quantitative analysis, and participants generally had a positive experience. In addition, they pointed out the importance of social interactions as well as the need for flexibility in study procedures and intervention components. Changes in SPPB and BFI correlated with exercise data.
Myeloid neoplasms can have a rapid onset, requiring initiation of treatment within a few days to weeks.52 During treatment, especially within the first few months following diagnosis, older patients often experience serious toxicities requiring hospitalization.53 Impairments in geriatric assessment domains further complicate the course of treatment. Patients with impairments are at higher risk of treatment-related toxicities that require treatment delays or discontinuation, which can compromise survival.7,54 Concerns have been raised regarding the ability of older adults with myeloid neoplasms who are receiving outpatient chemotherapy from participating in an exercise program as well as in their ability to use mHealth technologies, as they are generally more vulnerable than those receiving intensive inpatient therapy. Our study addresses these issues by demonstrating that this highly vulnerable population can still participate in a mHealth exercise intervention successfully and safely. These findings are consistent with previous studies evaluating exercise programs among older patients with AML receiving chemotherapy in the inpatient setting, which showed that these individuals are able to exercise safely.8,9 While the recruitment (64%) and retention (88%) were reasonable in the context of a behavioral intervention,7,46 it is important to note that several patients did drop out between consent and baseline assessments.
Descriptively, participants reported an increase of 319 daily steps on average over ∼8 weeks (from 3123 to 3442). For comparison, based on the National Health and Nutrition Examination Survey, daily average steps in community-dwelling older adults aged 65 to 69, 70 to 74, and 75 to 79 were 3303 to 5269, 3142 to 4422, and 2025 to 3011, respectively.55 Participants also performed resistance-band exercises approximately 3 days a week for 27 minutes each time at a low-intensity level (perceived exertion of 3.3/10). Two previous studies evaluated the EXCAP exercise program specifically in older adults.16,56 In older patients with breast cancer receiving chemotherapy (mean age, 67.7 years), participants increased their daily steps by 269 steps over 6 weeks compared with controls who decreased daily steps by 284 steps. In addition, EXCAP participants performed resistance-band exercises 2.3 days per week for 22 minutes each time at a low-intensity level (perceived exertion of 2.6/10).16 In older patients with prostate cancer receiving androgen deprivation therapy (mean age, 75.7 years), participants increased their daily steps by 1951 steps over 6 weeks compared with controls, who decreased their daily steps by 383 steps.56 While direct comparison of exercise data across studies and populations is challenging, and despite the lack of a control group, patients in our study demonstrated increases in physical activity from baseline to postintervention. In addition, outcomes were stable from baseline to postintervention, and changes in exercise data correlated with changes in SP, PB, and BFI. Our ongoing pilot randomized controlled trial (RCT; ClinicalTrials.gov identifier: NCT04981821) will allow us to compare data between intervention and control groups.
Based on feedback from participants in this single-arm pilot study and a previous qualitative study21 and in response to the COVID-19 pandemic, we have refined our study procedures and the intervention for our ongoing pilot RCT. Many changes were made to accommodate new circumstances and the possible need for treatment modifications. These changes include (1) extending the intervention period to 3 cycles of chemotherapy, or approximately 12 weeks, as several patients continued to engage in the intervention beyond the original study period; (2) allowing patients receiving outpatient high-dose cytarabine to enroll, given its increasing use in our institution; (3) allowing patients to continue enrolling despite changes in initial treatment regimen after consent (eg, treatment changed to hydroxyurea only from an HMA-based regimen); patients will also continue to participate in the exercise program for 3 cycles, or ∼12 weeks, even if their treatment regimen changes during this time; (4) shortening the study duration if patients proceeded to HSCT before the planned 3 cycles of chemotherapy; (5) providing options to conduct assessments and intervention delivery virtually; and (6) increasing involvement of the oncology team. For example, when possible, we will encourage the oncology team to discuss the benefits of participating in the study to improve the consent rate and reduce dropout between consent and baseline assessment. After baseline assessment, if patients are not engaged in the mHealth exercise intervention, the study team will have the oncology team reach out to patients and provide additional encouragement and motivation. While maximal efforts will be made to complete all in-person assessments, patients will be allowed to enroll despite an inability to complete these assessments. Examples of modifications to our study intervention are illustrated in Table 4.
Our study has several strengths. First, we included a vulnerable population that is usually excluded in behavioral intervention trials and studies in the mHealth field. Second, our inclusion criteria were fairly broad, and patients with myeloid neoplasms were allowed to enroll if they were receiving ≥2 cycles of any outpatient chemotherapy regimen. Third, many of the study procedures were adapted to include virtual options, thereby facilitating accrual of patients who resided far away (eg, several patients in this study were comanaged by oncologists at URMC/WCI and a community oncology practice). We also provided all patients with a tablet, thereby including patients who did not own an electronic device. Fourth, we used both quantitative and qualitative methods to evaluate the feasibility and usability of our intervention.
Our study also has several limitations. Participants had to be English-speaking, and most were White and had educational attainment above a high school diploma. Therefore, results may not be generalizable to non-English speaking, non-White, and less educated individuals. We included patients with ECOG PS 0 to 2 as we wanted to ensure the intervention was safe, and we plan to expand to ECOG PS 0 to 3 in our future studies. Approximately one-third of patients withdrew before the completion of baseline assessment, reflecting their vulnerabilities and rapid progression in the disease and related toxicities for this population, which may have been exacerbated during the COVID-19 pandemic when this study was conducted. In addition, we relied on patient self-reported exercise data, which may be subject to under or overreporting.57 Due to the small sample size, we did not perform subgroup analyses. We also did not test the theoretical model when analyzing the qualitative data and we did not construct a theoretical model for testing.
In conclusion, we demonstrated that the GO-EXCAP intervention is feasible and usable for older patients with myeloid neoplasms undergoing outpatient chemotherapy. Our findings allowed us to tailor our study procedures and intervention to this vulnerable population. We will further evaluate the preliminary efficacy of the GO-EXCAP intervention in mitigating treatment-related toxicities in an ongoing pilot RCT. The high prevalence of impairments in geriatric assessment domains in older patients with myeloid neoplasm reinforces the need to incorporate behavioral and supportive care interventions such as our mHealth exercise intervention to prevent or mitigate impairments and toxicities related to cancer and its treatment.
Acknowledgments
The authors thank Susan Rosenthal for her editorial assistance.
This work was supported by the National Cancer Institute at the National Institutes of Health (NIH) (UG1CA189961; K99CA237744, and R00CA237744 to K.P.L.), the National Institute of Aging at the NIH (K24AG056589 and R33AG059206 to S.G.M.), the National Institute of Nursing Research at NIH (K24NR018621 to R.S.), and the Wilmot Research Fellowship Award (to K.P.L.). The authors would like to thank those who provided feedback as part of the Transdisciplinary Research in Energetics and Cancer Research Education Program (TREC Training Workshop; R33CA203650). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: K.P.L. contributed conception and design, data collection, analysis and interpretation of data, manuscript writing, and approval of the final manuscript; C.S. contributed data collection, analysis and interpretation of data, and writing of the manuscript; E.E.W. collected data; M.J.-B. and K.T. analyzed and interpreted data; M.C.J., H.D.K., R.S., E.C., P.V., M.S., S.G.M., and K.M. conceptualized and designed the study and approved the final manuscript; and P.-J.L., J.H.M., J.L.L., and E.J.H. approved the final manuscript.
Conflict-of-interest disclosure: K.P.L. has served as a consultant to Pfizer and Seattle Genetics and has received honoraria from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Kah Poh Loh, Division of Hematology/Oncology, Department of Medicine, University of Rochester Medical Center, James P. Wilmot Cancer Institute, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: kahpoh_loh@urmc.rochester.edu.
References
Author notes
For original data, please contact Kah Poh Loh (kahpoh_loh@urmc.rochester.edu).
The full-text version of this article contains a data supplement.