TO THE EDITOR:
Astolfi et al1 recently described the case of a 6-year-old child with a type of leukemia that shows clinical and morphologic characteristics of acute promyelocytic leukemia (APL), but no evidence of t(15;17) or PML-RARA cryptic fusion. They performed whole-transcriptome sequencing on the leukemic sample and uncovered a chimeric torque teno minivirus (TTMV)-RARA fusion. The TTMV was integrated into RARA intron 2, the same intron involved in the PML-RARA fusion, and through the incorporation of a small intronic sequence, the chimeric transcript remained in-frame with RARA exon 3. A retrospective search of other cases of pediatric leukemia with a normal karyotype found a second case with an equivalent TTMV viral integration and fusion.
We report a third case, this time in an adult. Our patient was a 39-year-old man with leukemia who presented with thrombocytopenia, easy bleeding, and laboratory values consistent with diffuse intravascular coagulation (elevated D-dimer concentration, decreased fibrinogen, and prolonged prothrombin time). By morphology, the blasts were APL-like (Figure 1A) with increased promyelocytes. Karyotype at diagnosis was 46, XY, i(17)(q10)[18]/47, XY, +8, i(17)(q10)[2]; and STAT fluorescence in situ hybridization for t(15;17) was negative. Blasts were CD33+ and MPO+ and CD34−, HLA-DR− by flow cytometry. The patient was initially treated with the standard 7 + 3 regimen of cytarabine+daunorubicin. On day 15, he showed induction failure. His treatment was modified on day 31, when he received mitoxantrone, etoposide, and cytarabine with decitabine and venetoclax with a response. The Fred Hutchinson Cancer Research Center Institutional Review Board approved the study, which was conducted in accordance with the Declaration of Helsinki.
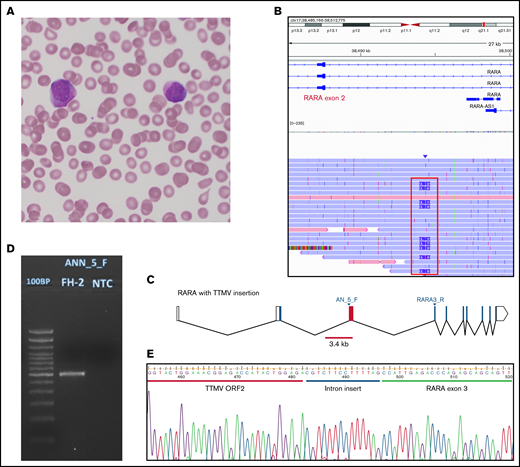
Morphology and TTMV integration structure of our case. (A) A peripheral blood (PB) smear from our patient, stained with Wright-Giemsa, showing circulating promyelocytic blasts with abundant azurophilic granules. (B) Alignment of unique sequencing reads from PB from our patient. Reads shown originated in RARA exon 3, where our CRISPR guide is designed to cut. Nine of 26 unique reads (35%) covering the region of interest showed an insertion of ∼2.4 kb (framed in red). (C) The RARA gene with the TTMV insertion between exons 2 and 3. Blocks represent exons, joined by lines showing introns. Empty blocks represent untranslated regions. The red bar (3.4 kb) is positioned to mark the whole size of the integrated TTMV; the red block is the fragment of the insertion that we could prove was transcribed in our patient. The locations of primers used in panels D and E are marked (AN_5_F and RARA3_R). (D) Agarose gel 2% stained with ethidium bromide showing the product of amplification of the patient’s PB RNA after reverse transcription and amplification with primers designed to target the insertion and RARA exon 3. First lane: 100-bp DNA ladder; second lane: our patient; third lane: the no-template control. The expected product size was 654 bp. The actual product size was closer to 600 bp, as an 84-bp fragment of the insertion was not in the transcribed product. (E) A Sanger sequencing chromatograph, showing the sequence of TTMV ORF2 adjoining RARA exon 3 with a small (14 bp) intron sequence preserved. The cartoon in panel C was generated with Worm-web (http://wormweb.org/exonintron).
Morphology and TTMV integration structure of our case. (A) A peripheral blood (PB) smear from our patient, stained with Wright-Giemsa, showing circulating promyelocytic blasts with abundant azurophilic granules. (B) Alignment of unique sequencing reads from PB from our patient. Reads shown originated in RARA exon 3, where our CRISPR guide is designed to cut. Nine of 26 unique reads (35%) covering the region of interest showed an insertion of ∼2.4 kb (framed in red). (C) The RARA gene with the TTMV insertion between exons 2 and 3. Blocks represent exons, joined by lines showing introns. Empty blocks represent untranslated regions. The red bar (3.4 kb) is positioned to mark the whole size of the integrated TTMV; the red block is the fragment of the insertion that we could prove was transcribed in our patient. The locations of primers used in panels D and E are marked (AN_5_F and RARA3_R). (D) Agarose gel 2% stained with ethidium bromide showing the product of amplification of the patient’s PB RNA after reverse transcription and amplification with primers designed to target the insertion and RARA exon 3. First lane: 100-bp DNA ladder; second lane: our patient; third lane: the no-template control. The expected product size was 654 bp. The actual product size was closer to 600 bp, as an 84-bp fragment of the insertion was not in the transcribed product. (E) A Sanger sequencing chromatograph, showing the sequence of TTMV ORF2 adjoining RARA exon 3 with a small (14 bp) intron sequence preserved. The cartoon in panel C was generated with Worm-web (http://wormweb.org/exonintron).
In our case, the TTMV integration was uncovered when we sequenced the region of RARA involved in PML-RARA translocation. We used an amplification-free enrichment strategy with CRISPR guides targeting RARA and long-range sequencing with nanopore2 (supplemental Material). The specimen was sequenced overnight, to allow for an increased number of reads, with a total time from sample processing to sequence alignment visualization of ∼24 hours. The peripheral blood specimen from the patient at diagnosis showed an insertion at chr17:40 338 090 (∼2.4 kb), in ∼35% of the reads covering the area (Figure 1B-C). The sequence of the insertion aligned to multiple TTV-like minivirus isolates, and the one with the highest query coverage aligned to 99% of the insertion, with 93.04% identity (MN769771.1; E value = 0.0). The same insertion was detected in a bone marrow specimen drawn from the patient several days later.
We designed primers tiled along the integrated TTMV sequence and paired them with a reverse primer on RARA exon 3 in reverse-transcription polymerase chain reaction. We confirmed the transcription of up to 498 bp of the insertion by Sanger sequencing. Analysis of the sequence showed a chimeric construct equivalent to that reported by Astolfi et al,1 with TTMV ORF2 fused to RARA exon 3, linked by a 14-bp intron fragment (Figure 1D-E; Table 1). In our case, the transcript was missing 84 bp of the viral sequence present in the inserted DNA.
TTMV insertion characteristics in the 3 reported cases
| Case . | Insertion size, bp . | Insertion site (hg38) . | TTMV transcribed fragment, bp . | Intronic fragment, bp . |
|---|---|---|---|---|
| Astolfi 1 | 1045 (estimated) | Chr17:40 334 196 | 209 | 38 |
| Astolfi 2 | NA | Chr17:40 333 779 | 328 | 45 |
| Current report | 2450 | Chr17:40 338 090 | 498 | 14 |
| Case . | Insertion size, bp . | Insertion site (hg38) . | TTMV transcribed fragment, bp . | Intronic fragment, bp . |
|---|---|---|---|---|
| Astolfi 1 | 1045 (estimated) | Chr17:40 334 196 | 209 | 38 |
| Astolfi 2 | NA | Chr17:40 333 779 | 328 | 45 |
| Current report | 2450 | Chr17:40 338 090 | 498 | 14 |
We also report a case of acute myeloid leukemia, which was clinically compatible with APL, but without the characteristic PML-RARA fusion and with a TTMV integrated in the genome in the same region where chromosome 17 breaks in the translocation 15;17. Consistent with the findings of Astolfi et al,1 we identified the resulting chimeric transcript that fuses TTMV ORF2 and RARA exon 3. The findings in these cases suggest that TTMV-RARA is a driver of APL-like leukemia. Further screening for this fusion in leukemias with APL characteristics where other RARA rearrangements cannot be identified would be of interest, as proposed by Rau in her commentary.3 Retrospective analysis may be difficult if discrepancies between morphology/clinical presentation and cytogenetics and molecular findings are not recorded, which may make necessary a broader selection of AMLs for screening. We used the same strategy on a second patient with isochromosome 17 but did not detect any insertion in RARA intron 2. Our data demonstrate a role for rapid long-range sequencing in the discovery of novel oncogenic mechanism. The insertion in our patient was ∼10 kb away from the CRISPR/Cas9 cut site designed to target RARA, and we achieved a 20× full read coverage of RARA intron 2 with unique DNA molecules. Our approach enabled us to evaluate the whole sequence of the insertion, including modifications between the DNA and RNA sequences and the precise site of the integration. This information may be relevant to understanding viral integration site preferences and oncogenic mechanisms.
Acknowledgments: The authors thank Phillip E. Starshak, Kaiser Permanente Oakland, for procuring the specimens described in this report.
Funding support for this article was partially provided by the NCCN Young Investigator Award (C.S.Y).
Contribution: O.S.-T. designed the experiments, performed the research, analyzed the data, and drafted, reviewed, and edited the manuscript; L.W.B. performed the research and reviewed and edited the manuscript; F.A.A. performed the background research, and drafted, reviewed, and edited the manuscript; C.C.S.Y. designed the experiments, drafted, reviewed, and edited the manuscript, and funded the work; and J.P.R. funded the work, reviewed and edited the manuscript, and was the senior advisor.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olga Sala-Torra, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D4-100, Seattle, WA 98109; e-mail: osala@fredhutch.org.
References
Author notes
For original data, please contact osala@fredhutch.org.
The full-text version of this article contains a data supplement.