Key Points
Chemoimmunotherapy is an effective treatment strategy for patients with FL who relapse after frontline R2.
SIRPα+ and CSF1R+macrophages are increased in FL patients who relapse after frontline R2.
Abstract
Limited data exist regarding the outcome of patients with follicular lymphoma (FL) who relapse or progress after frontline lenalidomide and rituximab (R2). Moreover, mechanisms of resistance to R2 in FL remain unclear, with increased protumoral macrophages suspected as a major contributory culprit to this phenomenon. This retrospective study analyzed the outcome of patients with advanced-stage FL grade 1 to 3A who relapsed or progressed after frontline R2. A multiplex immunofluorescence macrophage panel, including CD47, CD14, CD68, CD115 (also known as colony-stimulating factor 1 receptor [CSF1R]), CD163, CD172a (also known as signal regulatory protein α [SIRPα]), and CD274 (also known as programmed cell death-ligand 1 [PDL1]), was used to stain tissue biopsy specimens collected before initiation of R2 and at the time of progression. Among 156 patients with advanced-stage FL treated with frontline R2, 33 (21%) relapsed or progressed and required second-line therapy, after a median of 33 months (range, 1-122 months). Second-line therapy was chemoimmunotherapy in 16 (48%) patients and other therapy in 17 (52%). The overall response rate was 78%, and complete response rate was 72%. Median progression-free survival was significantly longer in patients who received chemoimmunotherapy compared with other therapy (99 vs 25 months; P = .004). Three macrophage populations were significantly increased in tissue samples collected at progression compared with before frontline treatment: CD68+CD115+ (P = .02), CD68+CD115+CD172a+ (P = .02), and CD68+CD163+CD172a+ (P = .01). Chemoimmunotherapy is an effective treatment strategy for patients with FL who relapse after frontline R2. Therapies targeting specific macrophage populations may yield novel approaches for improving outcomes with frontline R2.
Introduction
The combination of lenalidomide and rituximab, also referred to as R2, has been shown to be an effective frontline regimen for the treatment of patients with low-grade, advanced-stage follicular lymphoma (FL) with high tumor burden. These data are based on multiple phase 1/2 studies and a randomized phase 3 trial (the RELEVANCE [Combined Rituximab and Lenalidomide Treatment for Untreated Patients with Follicular Lymphoma] study) that compared R2 vs standard chemoimmunotherapy (CIT).1-4 Due to the negative preliminary results of the latter, R2 is not yet approved by the US Food and Drug Administration as a frontline regimen in FL. However, its inclusion in the National Comprehensive Cancer Network guidelines has led to increasing use as a chemotherapy-free frontline option for patients with FL.5
Long-term follow-up data of R2 as initial treatment for FL have shown promising efficacy, with a 8-year progression-free survival (PFS) rate of 65%.6 However, due to its recent implementation, limited data exist regarding the management and outcome of patients who relapse and/or progress after frontline R2, and mechanisms of resistance to this regimen in FL remain unclear.
Interestingly, 2 main mechanisms of resistance to lenalidomide have been described in multiple myeloma, a condition for which this agent has been more extensively used: acquired gene mutations, leading to downregulation of cereblon, and an increase in protumoral macrophages, also referred to as M2 macrophages.7,8 Although the former is a relevant target for lenalidomide in multiple myeloma, the latter may play a crucial role in FL, as highlighted by the fact that FL cell lines cannot be propagated in their absence, and even short-term growth in vitro requires survival signals derived from nurse-like cells.9 In addition, gene expression profiling studies have shown that macrophage-related gene signatures are associated with poor outcomes in patients with FL, although more recent data show that this may vary based on treatment type.10-12 Although conventional immunohistochemistry (IHC) is a widely used diagnostic technique for macrophage characterization, it is associated with a number of limitations, including high interobserver variability and the capacity to label only one marker per tissue section. Multiplex imaging platforms have recently been developed that allow the simultaneous detection of multiple epitopes in a single tissue section. Multiplex immunofluorescence is one of the most used and can simultaneously detect up to 8 markers using a tyramide signal amplification technique, thereby increasing detection sensitivity compared with conventional IHC.13
Materials and methods
Patient selection
This retrospective analysis included patients with advanced-stage FL grade 1 to 3A who received frontline R2 at the MD Anderson Cancer Center between August 2008 and January 2020 and who subsequently progressed or relapsed. The clinical and laboratory features were confirmed by review of the medical records. All patients required therapy as determined by the treating physician.14,15 The Follicular Lymphoma International Prognostic Index score16 was calculated, and the maximum standardized uptake value17 was recorded. R2 was administered2,4 as previously described. The 2014 Lugano classification was retrospectively applied to determine treatment response in all patients.18
The study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center and was conducted in accordance with the principles of the Declaration of Helsinki.
Multiplex imaging assay for macrophage characterization
Incisional core biopsy specimens were used for macrophage characterization. We optimized and validated a multiplex immunofluorescence panel using CD47, CD14, CD68, CD115 (also known as colony-stimulating factor 1 receptor [CSF1R]), CD163, CD172a (also known as signal regulatory protein α [SIRPα]), and programmed cell death-ligand 1 (PDL1). CD14 and CD68 were selected as general monocyte/macrophage markers, and CD115, CD163, CD172a, and PDL1 were selected as markers of protumoral phenotype, based on available FL literature.11,19 , -23 Each antibody was assessed by a uniplex immunofluorescence panel using the Opal 9 kit (catalog #NEL797001KT; Akoya Biosciences), according to the following clones and dilutions: CD47 (clone D3O7P, CST, 1:25), CD14 (clone SP192, Abcam, 1:100), CD68 (clone PG-M1, Agilent, 1:50), CD115 (CSF1R) (clone EPR20754, Abcam, 1:25), CD163 (clone 10D6, Leica, 1:100), CD172a (SIRPα) (clone EPR22930-163, Abcam, 1:50), and CD274 (PDL1) (clone E1L3N, CST, 1:400). The slides were imaged by using the Vectra Polaris spectral imaging system (Akoya Biosciences) using the fluorescence protocol at 10 nm λ from 420 nm to 720 nm. Both germinal center and interfollicular areas from lymph nodes with reactive lymphoid hyperplasia were used as a control. Six regions of interest were selected in each case. Each marker was analyzed at a single-cell level, and a supervised algorithm for phenotyping was built for each marker. Cell density for each marker and all possible combinations were consolidated by using Spotfire software (TIBCO Spotfire).
Statistical analysis
Association between categorical variables was evaluated by using χ2 or Fisher’s exact tests, as appropriate. The difference in a continuous variable between patient groups was evaluated by using the Mann-Whitney test, and the multivariate analysis of variance was used to simultaneously compare means for multiple continuous variables across categorical groups; they were performed separately for single-marker, 2-marker, and 3-marker macrophage combinations. PFS was defined as time interval from start of therapy to progression of disease or death, whichever occurred first, and patients without progression or death were censored at time of stem cell transplantation or last follow-up. Overall survival (OS) time was defined as time interval from start of therapy to death or last follow-up. PFS and OS were calculated for all patients in the study and for subgroups of patients by using Kaplan-Meier estimates, and subgroups were compared by using the log-rank test. A P value ≤.05 (two-tailed) was considered statistically significant. Statistical analyses were completed by using SPSS 21 (IBM SPSS Statistics, IBM Corporation) and GraphPad Prism 8 (GraphPad Software).
Results
Patient baseline characteristics
A total of 156 patients with advanced-stage FL grade 1 to 3A treated with frontline R2 were included in the study. Of these, 33 (21%) relapsed or progressed, with a median time to second-line therapy of 33 months (range, 1-122 months); 12 (8%) patients relapsed and/or progressed within 24 months from initiation of frontline R2. All patients were re-biopsied at time of first relapse and/or progression, but none showed biopsy-proven transformation.
At time of first relapse and/or progression, 12 (36%) patients were aged >60 years, 19 (57%) were male, 9 (27%) had a high-risk Follicular Lymphoma International Prognostic Index score, 13 (39.5%) had a maximum standardized uptake value on positron emission tomography/computed tomography scan >10, and 1 relapsed with localized disease. Remaining characteristics at time of first relapse and/or progression for the 33 patients included in the final analysis are shown in Table 1.
Patient baseline characteristics at time of first relapse (N = 33)
| Characteristic . | N (%) . |
|---|---|
| Age | |
| ≤60 y | 21 (64) |
| >60 y | 12 (36) |
| Sex | |
| Female | 14 (43) |
| Male | 19 (57) |
| Race | |
| Caucasian | 27 (82) |
| Not Caucasian | 6 (18) |
| Hemoglobin | |
| ≥12 g/dL | 28 (85) |
| <12 g/dL | 5 (15) |
| β2-microglobulin | |
| Normal | 9 (27) |
| Elevated | 6 (22) |
| Not done | 18 (51) |
| Lactate dehydrogenase | |
| Normal | 25 (76) |
| Elevated | 8 (24) |
| Bone marrow | |
| Not involved | 15 (45) |
| Involved | 6 (22) |
| Not done | 12 (33) |
| Grade | |
| 1-2 | 23 (70) |
| 3A | 2 (9) |
| Not assessed | 7 (21) |
| Ki-67 | |
| <40% | 19 (55) |
| ≥40% | 2 (9) |
| Not assessed on biopsy | 12 (36) |
| B-symptoms | |
| Absent | 31 (94) |
| Present | 2 (6) |
| Ann Arbor stage | |
| I-II | 1 (3) |
| III-IV | 32 (97) |
| Involved nodal areas | |
| ≤4 | 23 (70) |
| >4 | 10 (30) |
| FLIPI score | |
| Low | 11 (33.5) |
| Intermediate | 13 (39.5) |
| High | 9 (27) |
| SUVmax | |
| ≤10 | 13 (39.5) |
| >10 | 13 (39.5) |
| Not assessed | 7 (21) |
| Characteristic . | N (%) . |
|---|---|
| Age | |
| ≤60 y | 21 (64) |
| >60 y | 12 (36) |
| Sex | |
| Female | 14 (43) |
| Male | 19 (57) |
| Race | |
| Caucasian | 27 (82) |
| Not Caucasian | 6 (18) |
| Hemoglobin | |
| ≥12 g/dL | 28 (85) |
| <12 g/dL | 5 (15) |
| β2-microglobulin | |
| Normal | 9 (27) |
| Elevated | 6 (22) |
| Not done | 18 (51) |
| Lactate dehydrogenase | |
| Normal | 25 (76) |
| Elevated | 8 (24) |
| Bone marrow | |
| Not involved | 15 (45) |
| Involved | 6 (22) |
| Not done | 12 (33) |
| Grade | |
| 1-2 | 23 (70) |
| 3A | 2 (9) |
| Not assessed | 7 (21) |
| Ki-67 | |
| <40% | 19 (55) |
| ≥40% | 2 (9) |
| Not assessed on biopsy | 12 (36) |
| B-symptoms | |
| Absent | 31 (94) |
| Present | 2 (6) |
| Ann Arbor stage | |
| I-II | 1 (3) |
| III-IV | 32 (97) |
| Involved nodal areas | |
| ≤4 | 23 (70) |
| >4 | 10 (30) |
| FLIPI score | |
| Low | 11 (33.5) |
| Intermediate | 13 (39.5) |
| High | 9 (27) |
| SUVmax | |
| ≤10 | 13 (39.5) |
| >10 | 13 (39.5) |
| Not assessed | 7 (21) |
The median age was 56 years (range, 32-85 years). FLIPI, Follicular Lymphoma International Prognostic Index; SUVmax, maximum standardized uptake.
Second-line therapy and response to treatment
Overall, the median number of second-line therapies after R2 was 1 (range, 0-4), and maintenance after any second-line therapy was used in 7 (21%) patients (including 4 patients treated with second-line CIT, 2 patients treated with second-line other systemic therapy, and 1 patient treated with second-line radiotherapy); 1 (3%) patient each had autologous or allogeneic stem cell transplantation, and both had progressed within 24 months of frontline R2. Second-line therapies included: bendamustine with an anti-CD20 monoclonal antibody in 8 (24%) patients; rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in 8 (24%), an anti-CD20 monoclonal antibody alone in 8 (24%), a clinical trial with biological agents in 6 (18%; this included a programmed cell death protein 1 [PD1] inhibitor in 4 patients, a Bruton’s tyrosine kinase [BTK] inhibitor in 1 patient, and an anti-CD74 monoclonal antibody in 1 patient), repeated R2 in 2 (7%), and radiotherapy in 1 patient (with localized disease). Thirty-two patients were evaluable for response after second-line therapy: the overall response rate (ORR) was 78%, and the complete response (CR) rate was 72%. Among patients who received second-line CIT, the ORR and CR rate were both 93% (100% with bendamustine in combination with a monoclonal anti-CD20 antibody, 88% with R-CHOP); among patients who received second-line other therapy, ORR was 63%, and the CR rate was 50%.
PFS and OS after second-line therapy
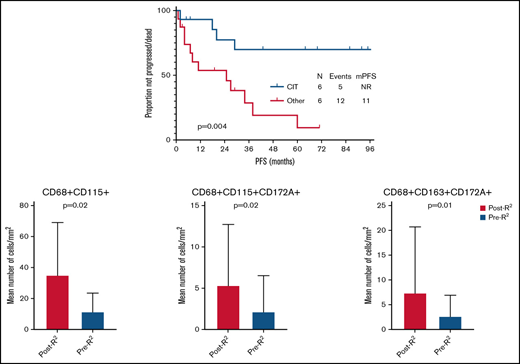
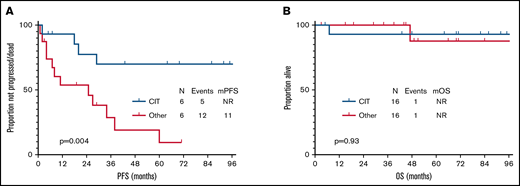
After a median follow-up of 51 months (95% confidence interval, 27-75) from time of second-line therapy initiation, 17 patients progressed and/or died, and median PFS was 38 months (95% confidence interval, 1-82). None of the clinical characteristics collected at time of second-line therapy and shown in Table 1 was significantly associated with PFS. Median PFS was significantly longer in patients who received CIT compared with other therapy at relapse (not reached vs 25 months, P = .004) (Figure 1A). Transformation was identified in 2 (7%) patients, 2 and 20 months after initiation of second-line therapy.
PFS and OS after second-line therapy. (A) PFS after second-line therapy according to treatment type. (B) OS after second-line therapy according to treatment type. mOS, median OS; mPFS, median PFS; NR, not reached.
PFS and OS after second-line therapy. (A) PFS after second-line therapy according to treatment type. (B) OS after second-line therapy according to treatment type. mOS, median OS; mPFS, median PFS; NR, not reached.
At the most recent follow-up, 2 (7%) patients had died (1 of unknown cause, 1 of transformed FL), and median OS has not been reached either for patients treated with second-line CIT or those treated with second-line other therapy (Figure 1B). Second cancers (excluding transformation) were diagnosed in 1 (2%) patient with pancreatic adenocarcinoma at 74 months after second-line chemotherapy.
Macrophage characterization and association with outcome
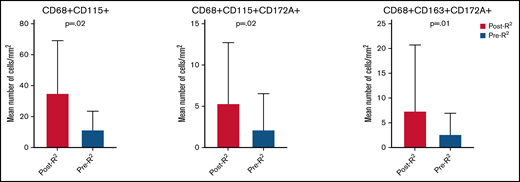
Ten patients were identified with available tissue biopsy specimens before treatment (pre-R2) and after frontline progression (post-R2). Baseline characteristics were similar to those of the larger study population and are shown in supplemental Table 1. A mean tissue area of 0.16 mm2 (range, 0.02-0.16 mm2) and a mean number of 30 178 cells (range, 12 315-51 649 cells) were analyzed by using a customized multiplex immunofluorescence panel. Not including single-marker outputs, 58 combinations were identified for macrophage markers. Among these, only 2-marker and 3-marker combinations with a median of >1 cell/mm2 were included in the final analysis (overall, 6 single-marker and 36 multimarker combinations). When comparing single-marker outputs between the 2 groups, post-R2 cases showed a significantly higher number of CD68+ (P < .001) and PDL1+ (P = .05) cells. On multivariate analysis, the association was maintained only for CD68+ cells (mean, 195 cells/mm2vs 70 cells/mm2; F = 5.4; P = .03). No significant difference in the mean number of CD47+ cells was observed when comparing post-R2 vs pre-R2 samples (mean, 297 cells/mm2vs 814 cells/mm2; P = .25). When comparing 2-marker macrophage combination outputs between the 2 groups, post-R2 cases showed a significantly higher mean number of CD68+CD115+ (P = .02) (Figure 2A-B), CD68+CD172a+ (P = .002), and CD68+PDL1+ (P = .004) cells. When comparing 3-marker macrophage combination outputs between the 2 groups, post-R2 cases showed a significantly higher mean number of CD14+CD68+PDL1+ (P = .003), CD14+CD115+PDL1+ (P = .04), CD68+CD115+CD172a+ (P = .02) (Figure 2C-D), CD68+CD115+PDL1+ (P = .02), CD68+CD163+CD172a+ (P = .01) (Figure 2E-F), CD68+CD163+PDL1+ (P = .04), and CD68+CD172a+PDL1+ (P = .001) cells. On multivariate analysis, the association was maintained only for CD68+CD115+ (mean, 35 cells/mm2vs 11 cells/mm2; F = 4.1; P = .05), CD68+CD115+CD172a+ (mean, 5 cells/mm2vs 3 cells/mm2; F = 7.5; P = .02), and CD68+CD163+CD172a+ (mean, 8 cells/mm2vs 2 cells/mm2; F = 5.2; P = .04) cells (Figure 3).
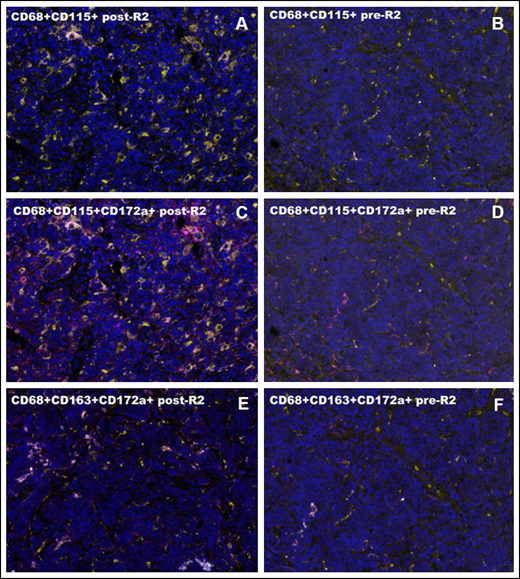
Macrophage characterization by multiplex immunofluorescence. (A-B) CD68+CD115+ cells after and before R2. (C-D) CD68+CD115+CD172a+ cells after and before R2. (E-F) CD68+CD163+CD172a+ cells after and before R2. High magnification areas (40×) are shown for all cases. Intrapatient comparisons are reported. In panel A, the post-R2 FL sample shows a high concentration of CD68+CD115+ cell population (CD68, yellow; CD115, pink). In panel B, the pre-R2 FL sample shows a lower concentration of CD68+CD115+ cell population (CD68, yellow; CD115, pink). In panel C, the post-R2 FL sample shows a high concentration of CD68+CD115+CD172a+ cell population (CD68, yellow; CD115, pink; CD172a/SIRPα, magenta). In panel D, the pre-R2 FL sample shows a lower concentration of CD68+CD115+CD172a+ cell population (CD68, yellow; CD115, pink; CD172a/SIRPα, magenta). In panel E, the post-R2 FL sample shows a high concentration of CD68+CD163+CD172a+ cell population (CD68, yellow; CD163, cyan; CD172a/SIRPα, magenta). In panel F, pre-R2 FL sample shows A lower concentration of CD68+CD163+CD172a+ cell population (CD68, yellow; CD163, cyan; CD172a/SIRPα, magenta).
Macrophage characterization by multiplex immunofluorescence. (A-B) CD68+CD115+ cells after and before R2. (C-D) CD68+CD115+CD172a+ cells after and before R2. (E-F) CD68+CD163+CD172a+ cells after and before R2. High magnification areas (40×) are shown for all cases. Intrapatient comparisons are reported. In panel A, the post-R2 FL sample shows a high concentration of CD68+CD115+ cell population (CD68, yellow; CD115, pink). In panel B, the pre-R2 FL sample shows a lower concentration of CD68+CD115+ cell population (CD68, yellow; CD115, pink). In panel C, the post-R2 FL sample shows a high concentration of CD68+CD115+CD172a+ cell population (CD68, yellow; CD115, pink; CD172a/SIRPα, magenta). In panel D, the pre-R2 FL sample shows a lower concentration of CD68+CD115+CD172a+ cell population (CD68, yellow; CD115, pink; CD172a/SIRPα, magenta). In panel E, the post-R2 FL sample shows a high concentration of CD68+CD163+CD172a+ cell population (CD68, yellow; CD163, cyan; CD172a/SIRPα, magenta). In panel F, pre-R2 FL sample shows A lower concentration of CD68+CD163+CD172a+ cell population (CD68, yellow; CD163, cyan; CD172a/SIRPα, magenta).
Tissue macrophage populations increased at time of progression after frontline R2 in patients with FL. Only populations whose association was maintained on multivariate analysis are shown in the graphic.
Tissue macrophage populations increased at time of progression after frontline R2 in patients with FL. Only populations whose association was maintained on multivariate analysis are shown in the graphic.
Discussion
In this study, we report for the first time the outcome of patients with FL who relapse or progress after frontline R2 and show that CIT is effective as a second-line strategy. We also show that an increase in protumoral macrophages could potentially be a mechanism of resistance to R2 in these patients.
Although CIT is the standard frontline treatment for patients with advanced-stage and high tumor burden FL, R2 has increasingly been used in this setting due to its high efficacy and tolerability.1-5 However, data regarding the activity of CIT in patients who relapse or progress after frontline R2 are lacking. Bendamustine has been compared with the combination of bendamustine and obinutuzumab in a randomized phase 3 study (GADOLIN [An Open-Label, Multicenter, Randomized, Phase III Study to Investigate the Efficacy and Safety of Bendamustine Compared With Bendamustine + RO5072759 (GA101) in Patients With Rituximab-Refractory, Indolent Non-Hodgkin’s Lymphoma]) that included 335 patients with relapsed FL. Patients treated with a combination of bendamustine and obinutuzumab achieved an ORR of 67.7% and a median PFS of 25.3 months.24 Of interest, in our analysis, patients treated with second-line CIT experienced a superior PFS (99 months). Despite the excellent second-line outcomes observed in patients treated with frontline R2, the right sequencing of treatments in FL remains to be validated prospectively.25-27 It is important to note that the aforementioned randomized phase 3 studies also included patients who had received >1 previous line of systemic therapy and that survival is known to decrease after second-line and later therapy.28
Despite the favorable outcome observed with the use of second-line CIT in patients with FL who relapsed after frontline R2, a chemotherapy-free approach remains a desirable end point for patients with indolent B-cell lymphoma. Therefore, the identification of novel molecular targets, favoring the development of second-line biological strategies for patients with FL who relapse after R2, are needed. In our study, the association between single macrophage markers and progression after frontline R2 was not maintained when multiple combinations and more specific macrophage populations were analyzed.
The prognostic role of macrophages has been previously evaluated in 186 patients with FL treated with frontline CIT using CD163 by IHC as a marker of protumoral phenotype.11 Although an increased number of CD163+ cells associated with poor outcomes in patients treated with a rituximab, cyclophosphamide, vincristine sulfate, and prednisone regimen (R-CVP), it conversely associated with a favorable outcome in patients treated with R-CHOP, highlighting the limitations of single-marker analysis. Highly multiplexed techniques may overcome these limitations, and they have been successfully applied to tissue biopsy samples obtained from patients with lymphoma treated with immunotherapy, identifying protumoral macrophage clusters enriched in nonresponders.29
In the current analysis, despite comparable levels of CD47, two SIRPα+ macrophage populations (CD68+CD115+CD172a+ and CD68+CD163+CD172a+) were significantly increased at time of progression in patients with FL previously treated with frontline R2. CD47, an ubiquitously expressed cell surface protein that is overexpressed in lymphoma, belongs to the immunoglobulin superfamily. It interacts in trans with SIRPα, an immunoglobulin superfamily receptor expressed on the surface of macrophage and dendritic cells.30 CD47 delivers to SIRPα a “don’t eat me” signal, inhibiting the phagocytic activity of macrophages, and thus representing a therapeutically appealing novel immune checkpoint.31 Antibodies targeting CD47 have shown significant clinical activity in patients with relapsed or refractory FL, including magrolimab, TTI-621, and ALX-148.32-34 Given the significant increase in SIRPα+ macrophages, our data suggest that antibodies targeting SIRPα, such as CC-95251, may be an effective treatment for patients with FL who relapse after frontline R2.35 In fact, SIRPα+ macrophage subsets have been identified in tissue biopsy samples derived from patients with FL, exhibiting characteristic protumoral features, which were successfully reverted through the use of an SIRPα-blocking agent.20
In our analysis, CSF1R+ macrophages also were significantly increased at time of progression in patients with FL previously treated with frontline R2. CSF1R plays a major role in favoring macrophage proliferation, and its inhibition can significantly decrease macrophage survival.36 Ex vivo primary FL-macrophage cocultures and in vivo mouse co-xenografts have shown that CSF1R is a crucial mediator of the crosstalk between FL cells and macrophages, promoting monocyte recruitment, differentiation, and polarization toward a protumoral phenotype.23 Multiple CSF1R inhibitors are currently under investigation, primarily in solid tumors,37 and their safety and efficacy for the treatment of patients with FL who progress or relapse after lenalidomide, either as a single agent or in combination with anti-SIRPα antibodies, remain to be evaluated.
Finally, the observed increase in protumoral macrophages at time of relapse after frontline R2 may also explain the high efficacy of anthracycline-based regimens such as R-CHOP. Macrophages obtained from mouse xenograft models treated with doxorubicin, in fact, show remarkable antitumoral activity and nonspecifically inhibit growth and DNA synthesis of lymphoma cells in vitro.38
We acknowledge multiple limitations of the current study, including its single-center and retrospective nature, its small population sample size, and the lack of functional analysis to corroborate translational findings. It is also important to note that similar analyses need to be performed in patients treated with other regimens, including CIT, anti-CD20 monoclonal antibodies, and radiotherapy, to determine whether this phenomenon is specific to patients treated with R2.
In conclusion, CIT is an effective treatment strategy for patients with FL who relapse after frontline R2. Although the optimal second-line therapy for these patients needs to be prospectively evaluated, the investigation of SIRPα and/or CSF1R inhibitors for the treatment of patients with FL who relapse after R2 or in combination with frontline R2 is warranted and may result in improved outcomes.
Acknowledgments
The authors thank the technical support of the following Translational Molecular Pathology–Immunoprofiling Laboratory members: Maria Neus Bota Rabassedas, Beatriz Sanchez-Espiridion, Mei Jiang, Auriole Tamegnon, Saxon Rodriguez, Wei Lu, Khaja Khan, and Jianling Zhou.
The study was partially supported by Anderson Cancer Center Support grant CA016672. The salary of P.S. is supported by the Lymphoma Research Foundation Career Development Award and by an NIH R21 grant.
Authorship
Contribution: P.S. and M.L.M.-P. designed the study, analyzed the data, and wrote the manuscript; L.F. performed the statistical analysis and coauthored the paper; M.G., S.G., and M.N. collected data and coauthored the paper; M.G., F.S., F.B.H., J.R.W., H.J.L., M.A.R., S.S.N., J.R.G., N.H.F., C.R.F., and L.J.N. provided clinical care to patients and coauthored the paper; and E.R.P., L.S.S., I.I.W., and F.V. performed multiplex staining and analysis and coauthored the paper.
Conflict-of-interest disclosure: P.S. is a consultant for Genentech-Roche and for Hutchinson MediPharma; and has received research support from AstraZeneca. L.J.N. reports honoraria from Celgene, Genentech, Gilead, Janssen, Juno, Novartis, Spectrum, and TG Therapeutics; and research support from Celgene, Genentech, Janssen, Karus Therapeutics, and Merck. F.S. reports honoraria from Celgene. M.G. reports stock ownership interest in KDAc Therapeutics. S.S.N. served as consultant to Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, and Unum Therapeutics; received research support from Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics, Allogene Therapeutics, Precision Biosciences, and Acerta; received royalties from Takeda Pharmaceuticals; and has intellectual property related to cell therapy. F.V. received research funding from CRISPR Therapeutics and Geron; honoraria from i3Health, Elsevier, America Registry of Pathology, Congressionally Directed Medical Research Program, and the Society of Hematology Oncology. The remaining authors declare no competing financial interests.
Correspondence: Paolo Strati, Department of Lymphoma and Myeloma, Department of Translational Molecular Pathology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: pstrati@mdanderson.org.
References
Author notes
M.L.M.-P., E.R.P., L.J.N., F.V., and P.S. contributed equally to this study.
Requests for data sharing may be submitted to Paolo Strati (pstrati@mdanderson.org).
The full-text version of this article contains a data supplement.