Key Points
Increased risk of infection-related hospital admission is independently associated with IDI >80% in older patients with DLBCL.
IPI score of 3 to 5, CIRS-G score ≥6, and low albumin are associated with infection-related death and are used for a predictive risk score.
Abstract
Infection-related morbidity and mortality are increased in older patients with diffuse large B-cell lymphoma (DLBCL) compared with population-matched controls. Key predictive factors for infection-related hospitalization during treatment with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and deaths as a result of infection in older patients during and after treatment with R-CHOP remain incompletely understood. For this study, 690 consecutively treated patients age 70 years or older who received full-dose or attenuated-dose R-CHOP treatment were analyzed for risk of infection-related hospitalization and infection-related death. Median age was 77 years, and 34.4% were 80 years old or older. Median follow-up was 2.8 years (range, 0.4-8.9 years). Patient and baseline disease characteristics were assessed in addition to intended dose intensity (IDI). Of all patients, 72% were not hospitalized with infection. In 331 patients receiving an IDI ≥80%, 33% were hospitalized with ≥1 infections compared with 23.3% of 355 patients receiving an IDI of <80% (odds ratio, 1.61; 95% confidence interval, 1.15-2.25; P = .006). An increased risk of infection-related admission was independently associated with IDI >80% across the whole cohort. Primary quinolone prophylaxis independently reduced infection-related admission. A total of 51 patients died as a result of infection. The 6-month, 12-month, 2-year, and 5-year cumulative incidences of infection-related death were 3.3%, 5.0%, 7.2%, and 11.1%, respectively. Key independent factors associated with infection-related death were an International Prognostic Index (IPI) score of 3 to 5, Cumulative Illness Rating Scale for Geriatrics (CIRS-G) score ≥6, and low albumin, which enabled us to generate a predictive risk score. We defined a smaller group (15%) of patients (IPI score of 0-2, albumin >36 g/L, CIRS-G score <6) in which no cases of infection-related deaths occurred at 5 years of follow-up. Whether patients at higher risk of infection-related death could be targeted with enhanced antimicrobial prophylaxis remains unknown and will require a randomized trial.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a curable malignancy in older patients when treated with full-dose or attenuated-dose anthracycline-based immunochemotherapy, typically rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).1-3 Despite this, patients of all ages receiving immunochemotherapy for DLBCL are at increased risk of death from infection.4 Risk of infection-related mortality remains elevated for up to 5 years after first-line treatment, and older patients are at higher risk compared with younger patients.4-7 A study of 190 US veterans age 80 years or older with DLBCL demonstrated a high treatment-related mortality (TRM) of 18%, with two-thirds of deaths associated with cycle 1 of therapy. An 11% TRM was seen during the first cycle of full-dose doxorubicin, whereas a 2% TRM was associated with a less-than-full dose of doxorubicin. Performance status (PS) was the most significant predictor of TRM.8 The German NHL-B2 trial showed the risk of infection-related death is highest after cycle 1 of CHOP in patients age 61 to 75 years old and is partially mitigated by prephase steroids with or without vincristine.9,10
Although use of R-CHOP in an older population has grown over the last decade,11,12 understanding who is at particular risk of TRM and specifically, infectious toxicity, remains relatively poorly understood; only small, heterogeneous studies that span broad age categories have been performed.6,7,13 There is still a lack of understanding regarding the key predictive factors for infection-related events and death as a result of infection. The relative importance of age, comorbidities, R-CHOP dose intensity, and disease-related factors remains incompletely understood.
We aimed to examine in detail the influence of baseline patient and disease characteristics, presentation, comorbidities, and intended dose intensity (IDI) in a large, consecutive group of older (age 70 years or older) DLBCL patients on the risk of both infection-related morbidity (ie, single or multiple admissions for infections) and infection-related mortality occurring during or after therapy.
Methods
The study received the National Health Service (NHS) service evaluation approval at each participating site, and it was conducted in accordance with the Declaration of Helsinki. Data on 690 consecutive patients were retrospectively collected across 8 centers in the United Kingdom from 2009 to 2018. Patients had untreated de novo DLBCL or untreated transformed indolent B-cell non-Hodgkin lymphoma (NHL). Patients who had leg-type DLBCL, posttransplantation lymphoproliferative disease, HIV, central nervous system involvement, or pretreated transformed indolent B-cell NHL were excluded. Patients received 1 to 8 cycles of R-CHOP21 (defined as a 21-day R-CHOP cycle) with curative intent. Patients must have received 1 or more cycles of therapy.
Detailed characteristics collected from medical records included sex, age, Eastern Cooperative Oncology Group PS (ECOG PS), B symptoms, International Prognostic Index (IPI) components, bulk (≥10 cm), hemoglobin, albumin, and Cumulative Illness Rating Scale for Geriatrics (CIRS-G) score. Whether R-CHOP cycle 1 (R-CHOP1) was administered on an inpatient basis was recorded. IDI was collected and was defined as the combined percentage dose of cyclophosphamide and doxorubicin at R-CHOP1. Because decisions regarding subsequent dosing and the consequent relative dose intensity (RDI) have the potential to be closely confounded by infection-related admissions, RDI was intentionally excluded from further analysis. Primary quinolone antibiotic prophylaxis was used only at a single institution from days 5 to 11 in R-CHOP21, and patients who received this prophylaxis represented 17% of the total patient cohort.
Statistical analysis
Progression-free survival (PFS), overall survival (OS), cumulative incidence of relapse, and influence of RDI and IDI on survival are reported elsewhere.3 Cumulative incidence of infection-related mortality (treating non–infection-related death without progression as a competing risk) was calculated from diagnosis to time of death. Patients who progressed were censored at progression whereas those who did not progress or die were censored when last seen alive and in remission. Follow-up was censored at 2 or 5 years for separate analyses. Competing risks survival regression was used to determine univariable and multivariable predictors of infection-related mortality.14 Multivariable analysis (MVA) performed using the Least Absolute Shrinkage and Selection Operator (LASSO) statistical formula penalized competing risk regression to account for relatively low numbers of events relative to number of predictors.
Infection-related admission was described as both a binary event and as the total number of events per patient. Care provided in a day unit did not equate to hospital admission(s). To make it easier to produce reliable, reproducible, consistent reporting, admissions were defined as being for neutropenic infection or non-neutropenic infection. All factors potentially directly related to infection-related mortality and infection-related admissions were analyzed by both standard and ordinal logistic regression.
In a prespecified analysis plan and to be consistent with published literature, IDI was analyzed as a categorical variable (full dose, ≥80%; attenuated dose, <80%) because most patients received either 100% or ∼50% dosage. Follow-up was censored in December 2018. Analyses were performed using Stata v15.1 (Stata Corp., College Station, TX) with the exception of LASSO competing risk regression (R package, crrp). The 95% confidence intervals (CIs) are presented for all estimates with P < .05 considered to be significant.
Results
Characteristics of 690 consecutive patients are provided in Table 1. Median age was 77.1 years (range, 70-96 years) and 34.4% were age 80 years or older. Median follow-up was 2.8 years (range, 0.4-8.9 years). Primary antibiotic quinolone prophylaxis was used in 17% (119 of 690) patients. Across 686 patients with complete data, the majority had no admissions with infection (72.1%). One hundred fourteen patients (16.6%) had 1 infection-related admission and 42 patients had 2 admissions (6.1%) (Table 2).
Baseline characteristics of the whole cohort
| Characteristic . | All patients (N = 690) . | Missing . | Unclassifiable . |
|---|---|---|---|
| Male sex | 350 (51) | ||
| Median age (range), y | 77.1 (70-96) | ||
| Tumor bulk >10 cm | 136 (20) | 3 | |
| B symptoms at diagnosis | 243 (35) | 4 | |
| IPI score of 3 to 5 | 451 (66) | 8 | |
| Hemoglobin ≤12.5 g/dL at diagnosis | 413 (58) | 1 | |
| Albumin ≤36 g/L at diagnosis | 362 (53) | 1 | |
| CIRS-G score <6 | 371 (54) | 8 | |
| Antibiotic prophylaxis | 119 (17) |
| Characteristic . | All patients (N = 690) . | Missing . | Unclassifiable . |
|---|---|---|---|
| Male sex | 350 (51) | ||
| Median age (range), y | 77.1 (70-96) | ||
| Tumor bulk >10 cm | 136 (20) | 3 | |
| B symptoms at diagnosis | 243 (35) | 4 | |
| IPI score of 3 to 5 | 451 (66) | 8 | |
| Hemoglobin ≤12.5 g/dL at diagnosis | 413 (58) | 1 | |
| Albumin ≤36 g/L at diagnosis | 362 (53) | 1 | |
| CIRS-G score <6 | 371 (54) | 8 | |
| Antibiotic prophylaxis | 119 (17) |
Data are presented as n (%) unless otherwise specified.
Infection-related admissions
| . | n (%) . |
|---|---|
| Total no. of patients | 686 |
| No. of infection-related admissions per patient | |
| 0 | 494 (72.1) |
| 1 | 114 (16.6) |
| 2 | 42 (6.1) |
| 3 | 20 (2.9) |
| 4 | 7 (1.0) |
| 5 | 7 (1.0) |
| 6 | 1 (0.2) |
| Unknown >0 | 1 (0.2) |
| Any infection admission | 192 (28.0) |
| . | n (%) . |
|---|---|
| Total no. of patients | 686 |
| No. of infection-related admissions per patient | |
| 0 | 494 (72.1) |
| 1 | 114 (16.6) |
| 2 | 42 (6.1) |
| 3 | 20 (2.9) |
| 4 | 7 (1.0) |
| 5 | 7 (1.0) |
| 6 | 1 (0.2) |
| Unknown >0 | 1 (0.2) |
| Any infection admission | 192 (28.0) |
Four patients had “not known” for infection-related admissions, so they are excluded from this analysis.
Infection-related admission
A total of 452 patients (66%) were age 70 to 80 years old and 238 patients (34%) were age 80 years or older (Table 3). Full-dose R-CHOP was more commonly given in younger patients. Of those age 70 to 80 years, 66% received an IDI >80% whereas in patients age 80 years old or older, 85% received an IDI <80%. In the 331 patients with available data across all ages who were receiving an IDI ≥80%, 109 (33%) were admitted with ≥1 infection-related events. In comparison, ≥1 infection-related admissions occurred in 83 (23.3%) of the 355 patients receiving IDI <80% who were eligible for analysis (odds ratio [OR], 1.61; 95% CI, 1.15-2.25; P = .006). In the subgroup of patients who were 80 years old or older, the risk of ≥1 infection-related admissions was 32.4% (11 of 34) in those with an IDI >80% vs 21.3% (43 of 202) in those with an IDI <80% (OR, 1.77; 95% CI, 0.80-3.91; P = .16). In this same group of patients age 80 years old or older, the mean rate of admission per cycle specifically with neutropenic fever was numerically higher at 7.4% for those with an IDI ≥80% vs 2.9% with an IDI <80% (rank-sum P = .06). Across all ages, the mean rate per R-CHOP cycle of an admission due to neutropenic infection was 7.9% in those with an IDI ≥80% compared with 3.3% in those with an IDI <80% (rank-sum P < .0001). This is in contrast to non-neutropenic infection or fever in which the risks per cycle across all patients were no different according to IDI (rank-sum P = .4). The rate of all-cause admissions was also not statistically different across any of the 4 groups divided by age and IDI.
Hospital admissions according to age and dose intensity
| Characteristic . | Age 70-79 y . | Age ≥80 y . | All patients (N = 690) . | ||
|---|---|---|---|---|---|
| IDI ≥80% (n = 299) . | IDI <80% (n = 153) . | IDI ≥80% (n = 35) . | IDI <80% (n = 203) . | ||
| Cycles of R-CHOP* | |||||
| Total | 1674 | 802 | 171 | 1030 | 3677 |
| Mean | 5.6 | 5.2 | 4.9 | 5.1 | 5.3 |
| Admissions per cycle | |||||
| Total (%)* | 335 (20.0) | 184 (22.9) | 48 (28.1) | 230 (22.3) | 797 (21.7) |
| Mean | 22.1 | 29.2 | 34.6 | 30.8 | 26.9 |
| Admissions* | |||||
| Median (range) | 1 (0-9) | 1 (0-6) | 1 (0-7) | 1 (0-9) | 1 (0-9) |
| Mean | 1.1 | 1.2 | 1.4 | 1.1 | 1.2 |
| Admissions during chemotherapy for infection | |||||
| Yes | 98 (33.0) | 40 (26.1) | 11 (32.4) | 43 (21.3) | 192 (28.0) |
| No | 199 (67.0) | 113 (73.9) | 23 (67.6) | 159 (78.7) | 494 (72.0) |
| Missing | 2 | 0 | 1 | 1 | 4 |
| Total cumulative inpatient days | |||||
| Median (range) | 2 (0-146) | 5 (0-120) | 5 (0-56) | 2 (0-92) | 2 (0-146) |
| Mean | 8.1 | 13.3 | 10.1 | 11.9 | 10.5 |
| All-cause admissions | |||||
| Yes | 164 (55.0) | 97 (63.4) | 20 (57.1) | 116 (57.1) | 397 (57.6) |
| No | 135 (45.3) | 56 (36.6) | 15 (42.9) | 87 (42.9) | 293 (42.5) |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Cycle 1 of R-CHOP received as inpatient | |||||
| Yes | 73 (24.5) | 67 (43.8) | 9 (26.5) | 72 (35.6) | 221 (32.2) |
| No | 225 (75.5) | 86 (56.2) | 25 (73.5) | 130 (64.4) | 466 (67.8) |
| Missing | 1 | 0 | 1 | 1 | 3 |
| Common causes of admission (mean rate % per cycle)* | |||||
| Neutropenic fever | 118 (7.9) | 29 (3.9) | 10 (7.4) | 23 (2.9) | 180 (5.5) |
| Non-neutropenic infection or fever | 59 (3.8) | 32 (5.8) | 9 (5.2) | 47 (6.0) | 147 (4.9) |
| Abdominal pain or distension | 12 (0.7) | 6 (1.2) | 2 (1.0) | 2 (0.2) | 22 (0.7) |
| Electrolyte or metabolic abnormality | 5 (0.3) | 8 (1.2) | 0 | 3 (0.2) | 16 (0.5) |
| Characteristic . | Age 70-79 y . | Age ≥80 y . | All patients (N = 690) . | ||
|---|---|---|---|---|---|
| IDI ≥80% (n = 299) . | IDI <80% (n = 153) . | IDI ≥80% (n = 35) . | IDI <80% (n = 203) . | ||
| Cycles of R-CHOP* | |||||
| Total | 1674 | 802 | 171 | 1030 | 3677 |
| Mean | 5.6 | 5.2 | 4.9 | 5.1 | 5.3 |
| Admissions per cycle | |||||
| Total (%)* | 335 (20.0) | 184 (22.9) | 48 (28.1) | 230 (22.3) | 797 (21.7) |
| Mean | 22.1 | 29.2 | 34.6 | 30.8 | 26.9 |
| Admissions* | |||||
| Median (range) | 1 (0-9) | 1 (0-6) | 1 (0-7) | 1 (0-9) | 1 (0-9) |
| Mean | 1.1 | 1.2 | 1.4 | 1.1 | 1.2 |
| Admissions during chemotherapy for infection | |||||
| Yes | 98 (33.0) | 40 (26.1) | 11 (32.4) | 43 (21.3) | 192 (28.0) |
| No | 199 (67.0) | 113 (73.9) | 23 (67.6) | 159 (78.7) | 494 (72.0) |
| Missing | 2 | 0 | 1 | 1 | 4 |
| Total cumulative inpatient days | |||||
| Median (range) | 2 (0-146) | 5 (0-120) | 5 (0-56) | 2 (0-92) | 2 (0-146) |
| Mean | 8.1 | 13.3 | 10.1 | 11.9 | 10.5 |
| All-cause admissions | |||||
| Yes | 164 (55.0) | 97 (63.4) | 20 (57.1) | 116 (57.1) | 397 (57.6) |
| No | 135 (45.3) | 56 (36.6) | 15 (42.9) | 87 (42.9) | 293 (42.5) |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Cycle 1 of R-CHOP received as inpatient | |||||
| Yes | 73 (24.5) | 67 (43.8) | 9 (26.5) | 72 (35.6) | 221 (32.2) |
| No | 225 (75.5) | 86 (56.2) | 25 (73.5) | 130 (64.4) | 466 (67.8) |
| Missing | 1 | 0 | 1 | 1 | 3 |
| Common causes of admission (mean rate % per cycle)* | |||||
| Neutropenic fever | 118 (7.9) | 29 (3.9) | 10 (7.4) | 23 (2.9) | 180 (5.5) |
| Non-neutropenic infection or fever | 59 (3.8) | 32 (5.8) | 9 (5.2) | 47 (6.0) | 147 (4.9) |
| Abdominal pain or distension | 12 (0.7) | 6 (1.2) | 2 (1.0) | 2 (0.2) | 22 (0.7) |
| Electrolyte or metabolic abnormality | 5 (0.3) | 8 (1.2) | 0 | 3 (0.2) | 16 (0.5) |
Data are presented as n (%) unless otherwise specified.
Excluding patients with unknown >0 infection-related admissions.
Infection-related admission and infection-related death: univariable and multivariable analyses
Patients were analyzed for 2 broad outcomes: risk of hospitalization due to infection and risk of death as a result of infection during or after R-CHOP treatment for up to 5 years. Results of univariable analysis and MVA for both methods are provided in supplemental Tables 1 and 2. According to standard logistic regression, patients younger than age 80 years, those with a baseline albumin ≤36 g/L, a baseline hemoglobin ≤12.5 g/dL, B symptoms, IPI score of 3 to 5, bulk, those receiving R-CHOP1 as an inpatient, those receiving an IDI ≥80% for cycle 1, and those receiving no antibiotic prophylaxis were all at increased risk of infection-related admissions. On MVA, only IDI ≥80% for cycle 1, low baseline albumin, and no primary antibiotic prophylaxis remained independently associated with increased risk of infection-related admission.
When all infection-related admissions were considered using ordinal logistic regression, similar results were obtained. However, patients with a CIRS-G score ≥6 had an increased risk of infection-related admission (OR, 1.42; 95% CI, 1.01-1.98; P = .04) and this was retained on MVA (OR, 1.44; 95% CI, 1.00-2.06; P = .05). This suggests that of patients who had an infection-related admission, patients with a CIRS-G score ≥6 tended to have more frequent, repeated infection-related admissions than those with a lower CIRS-G score. Within this model, patients with an IDI >80% had an increased infection-related risk on univariable (OR, 1.63; 95% CI, 1.17-2.29; P = .004) and multivariable (OR, 1.97; 95% CI, 1.31-2.96; P = .001) analysis. In addition, in this model, R-CHOP that was received on an inpatient basis remained a significant risk factor on multivariable analysis as did low baseline albumin and no primary antibiotic prophylaxis. There was no evidence of an interaction between age (younger than age 80 vs 80 years or older) and IDI (<80% vs ≥80%) either when they are the only variables in the model (P = .6) or when adjusting for all other variables (P = .4). The OR for the effect of IDI in patients younger than age 80 years was 1.39 (95% CI, 0.90-2.15) and the OR for effect of IDI in patients age 80 years or older was 1.77 (95% CI, 0.80-3.9). This suggests that there is no clear interaction between age, IDI, and infection-related admission, but that across the whole cohort, IDI ≥80% was a key independent risk factor for infection-related admission.
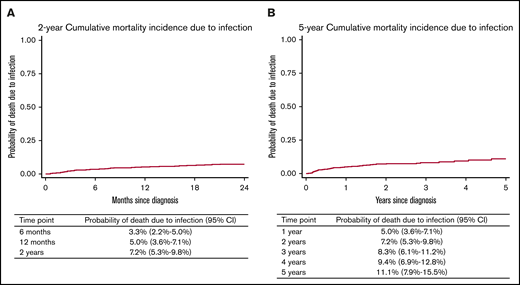
A total of 51 patients died as a result of infection. Median time from cycle 1 to death as a result of infection was 7 months (range, 0.5-60 months). Of those 51 patients, the median age was 79 years (range, 71-94 years), 86.3% had an IPI score of 3 to 5, 76.5% had a baseline albumin ≤36 g/L, and 72.5% had a baseline hemoglobin ≤12.5 g/dL; 46% started cycle 1 as an inpatient and 72% had a CIRS-G score of ≥6. Deaths as a result of infection were analyzed by using a competing risks model with censoring at relapse or progression or deaths as a result of other causes. The 6-month, 12-month, 2-year, and 5-year cumulative incidence of infection-related death was 3.3% (95% CI, 2.2%-5.0%), 5.0% (95% CI, 3.6%-7.1%), 7.2% (95% CI, 5.3%-9.8%), and 11.1% (95% CI, 7.9%-15.5%), respectively (Figure 1A-B).
Cumulative morality incidence due to infection. The 2-year (A) and the 5-year (B) cumulative mortality incidence due to infection.
Cumulative morality incidence due to infection. The 2-year (A) and the 5-year (B) cumulative mortality incidence due to infection.
Risk model for predicting infection-related mortality: cumulative incidence of death as a result of infection. N/A, not available.
Risk model for predicting infection-related mortality: cumulative incidence of death as a result of infection. N/A, not available.
LASSO regression showed that CIRS-G score of ≥6, albumin <36 g/L, IPI score of 3 to 5, and inpatient R-CHOP1 were independent risk factors for death as a result of infection at 2 years. At 5 years, CIRS-G score of ≥6, albumin <36 g/L, and IPI score of 3 to 5 remained independent factors for death as a result of infection; inpatient R-CHOP1 lost independent significance. IDI <80% had a borderline statistical protective effect at 2 years (hazard ratio [HR], 0.85; 95% CI, 0.70-1.02; P = .08) and 5 years (HR, 0.74; 95% CI, 0.50-1.03; P = .07), respectively, on MVA.
On the basis of the association of CIRS-G score of ≥6, albumin ≤36 g/L, and IPI score of 3 to 5 with infection-related death, we defined a weighted risk score based on MVA results. CIRS-G scores of ≥6 were assigned 2 points, IPI scores of 3 to 5 were assigned 1 point, and baseline albumin ≤36 g/L was assigned 1 point. Three distinct risk groups were defined: score 0 included 106 patients (15.5%), score 1 to 2 included 316 patients (46.4%), and score 3 to 4 included 259 patients (38.0%). Notably, patients with no risk factors had no infection-related deaths. There was a statistically significant and clinically meaningful difference between cohorts. The 2-year cumulative incidence of mortality as a result of infection for those with a score of 0 was 0%, score of 1 to 2 was 3.6% (95% CI, 1.8%-6.9%), and score of 3 to 4 was 15.2% (95% CI, 10.8%-21.1%). The 5-year cumulative incidence of mortality as a result of infection for those with a score of 0 was 0%, score of 1 to 2 was 8.3% (95% CI, 4.3%-15.8%), and score of 3 to 4 was 20.8% (95% CI, 14.0%-30.3%) (Figure 2).
Discussion
We describe infection-related morbidity and mortality outcomes in a large cohort of older patients with DLBCL who received either full- or attenuated-dose R-CHOP. To our knowledge, this is the largest series to study infection-related outcomes in patients age 70 years or older with such granularity. Several of our findings are noteworthy. First, the majority of older patients (72%) are not admitted with infection and receive outpatient-based, potentially curative R-CHOP without serious morbidity as a result of infection. Second, patients at higher risk of infection-related admission can be defined by a number of baseline factors such as high IPI score, low hemoglobin, and low albumin; however, independent risk factors were IDI ≥80% and low baseline albumin. Third, primary oral quinolone antimicrobial prophylaxis was also independently associated with a lower risk of infection-related admission but not infection-related death. Fourth, key independent factors associated with infection-related death were a high IPI score, high comorbidity burden (CIRS-G score of ≥6), and low albumin. Whether patients with a collection of these features could be targeted with enhanced antimicrobial prophylaxis is a point for debate, and primary oral quinolone antimicrobial prophylaxis did not influence risk for infection-related death. Fifth, we also defined a smaller group (low IPI score, normal albumin, low CIRS-G score) in which no cases of infection-related deaths occurred at 5 years of follow-up.
The importance of RDI and IDI to R-CHOP delivery has been investigated recently and remains somewhat contentious, particularly in patients age 80 years or older. In these patients, attenuated R-CHOP has been increasingly used and has formed the backbone of the first randomized trial (SENIOR) in patients exclusively age 80 years or older.15 We note that serious infection rates described in our series are numerically higher than those seen in prospective clinical trials in the older population. For example, an indirect comparison of infection-related admissions during R-CHOP in those age 80 years or older who are receiving IDI <80% in our series was 21.3% vs R-miniCHOP patients within the SENIOR trial who had an infection-related serious adverse event rate of 9.7%. This likely relates to patient selection, fitness, comorbidity burden, and possibly to the use of prephase therapy in the SENIOR trial. Morbidity as a result of infection for older patients with DLBCL is a more substantial problem than has been documented within trial populations and therefore requires independent study.
We demonstrate that across all patients age 70 years or older, those receiving an IDI ≥80% were at an independently higher risk of an infection-related admission during treatment with R-CHOP, although they were not at higher risk of dying as a result of infection. This increased risk is predominantly related to an increase in the rate of admissions resulting from neutropenic fever or infection per cycle rather than non-neutropenic fever or infection (rank-sum P < .0001) for episodes across the total number of R-CHOP cycles in patients receiving a higher IDI or all-cause admission. We note that across the whole cohort, attenuated-dose IDI <80% is used in patients with worse PS (52% vs 28%; ECOG PS, 2-4; OR, 2.8; P < .001) and increased comorbidity burden (55% vs 36%; CIRS-G score ≥6; OR, 2.2; P < .001). Lower IDI might protect patients from neutropenic fever, but we hypothesized that factors such as a high CIRS-G score and worse ECOG PS are likely to influence all other causes of admission.
Because attenuated R-CHOP is often given to patients with features associated with increased risk of infection and most commonly patients age 80 years or older, these data provide further reassurance regarding the tolerability profile of attenuated-dose R-CHOP in older patients with DLBCL. Our aim was not to specifically assess the role of IDI and survival. We used IDI as a marker of dose intensity within our analysis because of the enhanced risk of infection-related mortality after cycles 1 to 2 and because IDI correlated closely with RDI in our previous analysis.3 We have shown that IDI ≥80% is associated with an improved OS and PFS, with a marked difference in patients age 70 to 80 years but not in patients age 80 years or older. It is important therefore to recognize that although IDI >80% is associated with increased risk of infection, it is associated with improved OS in patients age 70 to 79 years old. It is recognized that a number of competing risks for clinical events are at play in the older population, including death as a result of unrelated causes. Choice of dose intensity must therefore take into account all of these competing factors: age, infection risk, and comorbidities.
Because it is common for the site of infection to be unknown when patients are admitted as febrile and unwell, we did not attempt to break down the specific site of infection. Although we recognize that this provides limited information in terms of infection sites, the categorization of neutropenic infection and non-neutropenic infection were intentionally used as simple, reliable, consistent, and clinically relevant end points. We recognize that the pattern of the type of infection at various follow-up time points might be different and is of interest; however, a detailed analysis of this was outside the scope of this work. Although we did not have a specific matched control cohort, previous data have described the increased infection-related events in this demographic up to 5 years.4 Moreover, recent published long-term follow-up data from 21 690 DLBCL patients16 has shown that when compared with survivors who had solid tumors, including breast cancer, prostate cancer, and melanoma, those patients who have survived DLBCL are more than 3 times more likely to develop viral or fungal pneumonia, meningitis, hypogammaglobulinemia, and immune-related cytopenias at up to 10 years of follow-up. It is therefore plausible that longer-term immune defects might influence the longer-term risk for these older patients. Our risk score provides some clarity regarding which patients are at risk, and it may enable enhanced follow-up planning. Examples of interventional approaches might include anti-encapsulated bacteria vaccinations, and monitoring of vaccine responses and immunoglobulin status with intravenous immunoglobulin replacement in high-risk groups.
Our data are limited, however, by their retrospective nature, with the inherent potential risks of interpreting medical records. It is possible that a small proportion of low-grade infections were not captured because of antimicrobials administered in primary care; however, this would not have influenced our primary analysis. We did not specifically collect a breakdown of the timing of infection according to specific cycle number, so we were not able to determine whether patients were at particular risk at a specific time point for admission with an infection. Despite this, it is well described that the peak risk for severe infection is early7,10 and this is probably unlikely to have an impact on our outcomes. We also did not specifically look at the role of prephase therapy or the utility of granulocyte colony-stimulating factor, primarily because of the challenge of collecting these data systematically from paper records, but we recognize the importance of this from data showing a reduction in TRM after the use of prephase therapy.2,10
Primary quinolone prophylaxis was used in 17% of our total cohort and was independently associated with a reduction in the risk of infection-related hospitalization by standard and ordinal logistic regression (HR, 0.28-0.29; P < .001) but not infection-related death. A randomized trial (TEAMM) of 977 myeloma patients17 has demonstrated that levofloxacin given for 12 weeks (vs placebo) during first-line chemotherapy reduced the risk of the composite end point of febrile episodes or death as a result of any cause within 12 weeks (27% vs 19%; HR, 0.66; 95% CI, 0.51-0.86; P = .0018) without increasing nosocomial pathogens. Our study demonstrated that patients with a higher IDI ≥80% in cycle 1 are at higher risk of being admitted for infection. This demonstrates the importance of judicious initial R-CHOP dosing in older patients or those with concerning comorbidities. Patients with low albumin, high IPI score, and a high comorbidity burden are at higher risk from death as a result of infection during or after treatment with R-CHOP. We recognize that the generalizability of the risk score would be more robust if it were validated in an independent cohort. Despite this, the risk score (IPI score of 3-5, CIRS-G score ≥6, albumin <36 g/L) described for infection-related death has shown that a substantial (38% of patients) high-risk population exists with a 2-year infection-related mortality of 15.2% and a 5-year infection-related mortality of 20.8%. No specific prospective trials have been performed for older patients treated with R-CHOP that test the role of antimicrobial prophylaxis in this setting. We demonstrate a cohort at higher risk in whom clinical trials of antibiotic prophylaxis could be performed to ask a similar question to the TEAMM trial.
For original data, please contact Toby A. Eyre via e-mail at toby.eyre@ouh.nhs.uk.
Acknowledgments
This work was supported by the Julian Starmer‐Smith Lymphoma Fund (T.A.E.) and by the National Institute for Health Research Oxford Biomedical Research Centre Programme (G.P.C. and T.A.E.).
The views expressed are those of the authors and not necessarily those of the funding bodies.
Authorship
Contribution: T.A.E. designed the study, coordinated the data collection, and wrote the manuscript; T.A.E., N.M.-C., C.H., H.P., S.B., J.G., J.W., A.M., C.P.F., M.B., R.O., P.F., and A.G. collected the majority of the data; A.A.K. and W.W. performed the statistical analysis; T.A.E., A.M., C.P.F., M.B., S.B., P.F., G.P.C., and C.S.R.H. and J.K. managed patients in the study; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toby A. Eyre, Department of Haematology, Cancer and Haematology Centre, Oxford University Hospitals NHS Trust, Churchill Hospital, Oxford OX3 7LE, United Kingdom; e-mail: toby.eyre@ouh.nhs.uk.
References
Author notes
The full-text version of this article contains a data supplement.