Key Points
Bleeding occurs most often in the first year of life, and a family history of VWD leads to earlier diagnosis among infants and toddlers.
Infants and toddlers with VWD experience higher rates of C-section, preterm birth, and low birth weight.
Abstract
Data on infants and toddlers (ITs) with von Willebrand disease (VWD) are lacking. We used data collected in the US Hemophilia Treatment Center Network (USHTCN) to describe birth characteristics, bleeding episodes, and complications experienced by 105 patients with VWD who were <2 years of age. In 68% of the patients, the reason for diagnostic testing was a family history of a bleeding disorder. The mean age at diagnosis was 7 months, with little variation by sex. Patients with type 2 VWD were diagnosed earlier than those with types 1 or 3 (P = .04), and those with a family history were diagnosed ∼4 months earlier than those with none (P < .001). Among the patients who experienced a bleeding event (70%), oral mucosa was the most common site of the initial bleeding episode (32%), followed by circumcision-related (12%) and intracranial/extracranial bleeding (10%). Forty-one percent of the initial bleeding events occurred before 6 months of age, and 68% of them occurred before the age of 1 year. Approximately 5% of the cohort experienced an intracranial hemorrhage; however, none was associated with delivery at birth. Bleeding patterns and rates were similar by sex (P = .40) and VWD type (P = .10). Forty-seven percent were treated with plasma-derived von Willebrand factor VIII concentrates. The results of this study indicate that a high percentage of ITs diagnosed with VWD and receiving care within the multidisciplinary structure of the USHTCN have a family history of VWD. In addition, bleeding events such as circumcision-related, oropharyngeal, and intracranial or extracranial episodes are common and are leading indicators for treatment.
Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder. It is caused by deficient or dysfunctional von Willebrand factor (VWF), a plasma protein essential to hemostasis. Diagnosis is typically established based on results of tests prompted by a positive family history, the presence of abnormal or unexplained bleeding, or abnormal laboratory results. The reported symptomatic and nonsymptomatic prevalence of VWD in the population, ranging from 0.01% to 1.3%, varies between studies, primarily because of differing case definitions.1-3
VWD is categorized as one of the main disease types (1, 2, and 3), based on the qualitative or quantitative defects in VWF. Type 1, characterized by partial deficiency of VWF in plasma, is the most common of the VWD types and typically results in milder bleeding manifestations.4,5 Type 2 includes numerous qualitative (rather than quantitative) abnormalities of VWF structure and functions and is subdivided into additional categories based on the presence and characteristics of VWF multimers and binding of VWF to factor VIII (FVIII).2,4 Type 3, the most severe and the rarest of the VWD types, is distinguished by the absence of VWF in plasma and platelets.4-7 Bleeding symptoms are variable and are influenced by type and severity of VWD, as well as other factors, such as age, sex, concomitant medications, physical activity, and comorbidities.
Although VWD can be diagnosed at any age, diagnosis at <2 years of age is difficult, because hemostatic challenges are rare, and bruising of extremities and head trauma are common, especially in toddlers as they learn to crawl and walk.8-10 Previous studies have described characteristics and bleeding complications in VWD among older children and adults; however, data on infants and toddlers (ITs) with VWD are lacking. Information regarding delivery and perinatal management of infants with VWD is also limited. Such information can be helpful to guide obstetric practices for women with VWD and neonatal practices for their potentially affected infants.
The purpose of this study was to describe diagnostic information, birth characteristics, bleeding episodes, and complications experienced by ITs with VWD who were enrolled in the Centers for Disease Control and Prevention (CDC) Universal Data Collection (UDC) surveillance system established in the US Hemophilia Treatment Center Network (USHTCN). The UDC represents one of the largest prospective surveillance systems of patients with VWD diagnosed before 2 years of age.
Methods
UDC was conducted from 1998 through 2011 to monitor the health of people with bleeding disorders who are receiving care at any of the (then) 135 federally funded Hemophilia Treatment Centers (HTCs) in the United States.11 In the UDC, data from patients between the ages of 0 and 23 months are available from 2003 through 2011. Data were collected by trained HTC staff at enrollment and then as often as 6-month intervals at routine clinic visits. A registration form was used to collect baseline and historical data on sex, race/ethnicity (self-reported), VWD diagnosis, age at diagnosis, date of visit, month and year of birth, member of household with a bleeding disorder, age at first HTC visit, reason for diagnostic testing (mother is the known carrier, other family history, bleeding symptom, and other or unknown), method of delivery, vitamin K administration at birth, weight and length at birth, gestational age at delivery, and bleeding history, including the age and site of the first bleeding episode. The presence of vitamin K administration at birth and HTC contact before birth were obtained from parent recall or extracted from the review of available medical records. A semiannual visit form (the first completed at the time of registration) was used to collect information on health insurance, bleeding episodes that occurred after enrollment, and HTC utilization during comprehensive clinic visits. The project protocol was approved by the institutional review boards of all participating institutions and parents/guardians gave informed consent before enrollment of their ITs. The study was conducted in accordance with the Declaration of Helsinki.
Month and year of birth data were converted to a birth date by assuming the 15th of the month, and the resulting date was subtracted from the registration date to calculate age at registration. ITs who were diagnosed prenatally via amniocentesis were included in a prenatal- to 6-month age category. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Hispanic, and other races (including Asian/Pacific Islander, and American Indian/Alaskan Native). Information about type of health insurance was obtained from the first semiannual visit form and was categorized as private (commercial insurance), public (Medicare, Medicaid, state plan, and Tricare), or unknown. Birth weight and length were classified as low (<2.5 kg weight; <45.7 cm length), normal (2.5-4.1 kg weight; 45.7-55.8 cm length), and high (>4.1 kg weight; 55.8 cm length). Birth before 37 weeks was considered preterm, and birth at 37 weeks and later was considered full term.
Laboratory definitions for VWD and diagnostic criteria7,12 for subtypes were at the discretion of the HTC physician. Subtypes of VWD type 2 were collapsed into a type 2 category. VWD types recorded from an “other, specify” option were reviewed and reclassified into type 1, 2, or 3 or unknown type. In 8 cases, "other, specify" included nonspecific text responses such as “von Willebrand’s,” “VWD other,” “VWD type, other,” or “VWD type, unknown”; these responses were classified into the “unknown” VWD type category. Classification of a family history of bleeding disorder was derived by combining the 2 variables, reason for diagnostic testing, and member of the household with a bleeding disorder. When a member of the household had a bleeding disorder or the diagnosis was made because of family history of VWD, the patient was assigned to the positive family history category. Although the frequencies of unknown response options are displayed in the tables, calculations of the percentage distributions for perinatal and other descriptive variables do not include responses of “unknown” in the denominator.
Data analysis
Data from registration and semiannual visit forms completed before 2 years of age were included in the analysis. Patient demographics and clinical characteristics were compared by sex, type of VWD, age at diagnosis, and age at first bleeding event, using the χ2, Fisher’s exact, and Wilcoxon rank sum tests, as appropriate. All analyses were performed in SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Among the 105 patients <2 years of age, 61 (58%) were male and 44 (42%) were female. Table 1 shows the sex distribution and other demographic and clinical characteristics of the ITs. Among the ITs where VWD type was known, type 1 (63%) was the most common, followed by types 2 (28%) and 3 (9%). The mean age at enrollment was 14 months; more than 75% of the ITs were enrolled in the study after their first birthday. Most of the patients were White non-Hispanic (82%) and had private health insurance (61%). Seventy-nine percent of the patients had a family history of bleeding disorders, and a family history of VWD prompted diagnostic testing in 68% of patients (Table 1).
Demographics at enrollment of ITs <2 years of age with VWD
| . | All (N = 105) . | |
|---|---|---|
| n . | % . | |
| Sex | ||
| Female | 44 | 41.9 |
| Male | 61 | 58.1 |
| Race/ethnicity | ||
| White (non-Hispanic) | 86 | 81.9 |
| Hispanic | 10 | 9.5 |
| Black or other race (non-Hispanic) | 9 | 8.6 |
| Insurance status* | ||
| Private | 61 | 61.0 |
| Public | 39 | 39.0 |
| Unknown | 5 | — |
| VWD type* | ||
| 1 | 61 | 62.9 |
| 2 | 27 | 27.8 |
| 3 | 9 | 9.3 |
| Unknown | 8 | — |
| Age at diagnosis† | ||
| Prenatal-6 mo | 52 | 49.5 |
| 7-23 mo | 52 | 49.5 |
| Reason for diagnostic testing* | ||
| Mother known carrier | 27 | 27.3 |
| Other family history | 40 | 40.4 |
| Bleeding symptom | 32 | 32.3 |
| Unknown | 6 | — |
| Family history of bleeding disorder‡ | ||
| Mother known carrier | 27 | 25.7 |
| Other family history | 56 | 53.4 |
| No family history | 22 | 20.9 |
| HTC contacted before delivery* | ||
| Yes | 30 | 30.3 |
| No | 69 | 69.7 |
| Unknown | 6 | — |
| Method of delivery* | ||
| Vaginal (including vacuum) | 61 | 62.9 |
| Elective C-section | 19 | 19.6 |
| Nonelective C-section | 17 | 17.5 |
| Unknown | 8 | — |
| Vitamin K administered at birth* | ||
| Yes | 64 | 92.8 |
| No | 5 | 7.2 |
| Unknown | 36 | — |
| Birth weight* | ||
| High birth weight (>4.1 kg) | § | ∼5% |
| Normal birth weight (2.5-4.1 kg) | 65 | 82.3 |
| Low birth weight (<2.5 kg) | 10 | 12.7 |
| Unknown birth weight | § | — |
| Preterm birth* | ||
| No (≥37 weeks at delivery) | 87 | 86.1 |
| Yes (31-36 weeks at delivery) | 14 | 13.9 |
| Unknown age at delivery | § | — |
| . | All (N = 105) . | |
|---|---|---|
| n . | % . | |
| Sex | ||
| Female | 44 | 41.9 |
| Male | 61 | 58.1 |
| Race/ethnicity | ||
| White (non-Hispanic) | 86 | 81.9 |
| Hispanic | 10 | 9.5 |
| Black or other race (non-Hispanic) | 9 | 8.6 |
| Insurance status* | ||
| Private | 61 | 61.0 |
| Public | 39 | 39.0 |
| Unknown | 5 | — |
| VWD type* | ||
| 1 | 61 | 62.9 |
| 2 | 27 | 27.8 |
| 3 | 9 | 9.3 |
| Unknown | 8 | — |
| Age at diagnosis† | ||
| Prenatal-6 mo | 52 | 49.5 |
| 7-23 mo | 52 | 49.5 |
| Reason for diagnostic testing* | ||
| Mother known carrier | 27 | 27.3 |
| Other family history | 40 | 40.4 |
| Bleeding symptom | 32 | 32.3 |
| Unknown | 6 | — |
| Family history of bleeding disorder‡ | ||
| Mother known carrier | 27 | 25.7 |
| Other family history | 56 | 53.4 |
| No family history | 22 | 20.9 |
| HTC contacted before delivery* | ||
| Yes | 30 | 30.3 |
| No | 69 | 69.7 |
| Unknown | 6 | — |
| Method of delivery* | ||
| Vaginal (including vacuum) | 61 | 62.9 |
| Elective C-section | 19 | 19.6 |
| Nonelective C-section | 17 | 17.5 |
| Unknown | 8 | — |
| Vitamin K administered at birth* | ||
| Yes | 64 | 92.8 |
| No | 5 | 7.2 |
| Unknown | 36 | — |
| Birth weight* | ||
| High birth weight (>4.1 kg) | § | ∼5% |
| Normal birth weight (2.5-4.1 kg) | 65 | 82.3 |
| Low birth weight (<2.5 kg) | 10 | 12.7 |
| Unknown birth weight | § | — |
| Preterm birth* | ||
| No (≥37 weeks at delivery) | 87 | 86.1 |
| Yes (31-36 weeks at delivery) | 14 | 13.9 |
| Unknown age at delivery | § | — |
Calculations of percentage of distributions do not include response of “unknown” in the denominator.
One person with unknown age at diagnosis excluded from the data.
‡Classification of a family history of bleeding disorder was derived by combining the 2 variables, reason for diagnostic testing, and a member of the household with a bleeding disorder. When a member of the household had a bleeding disorder or the diagnosis was the result of a mother being a carrier or another family member having VWD, the patient was assigned to the positive family history category.
Counts fewer than 5 have been suppressed to protect patient confidentiality. Additional cells may be suppressed to prevent derivation of these counts by subtraction.
Birth and delivery characteristics
Among those for whom birth and delivery data were reported, the majority of the ITs were full term (86%), had normal weight (82%) and length (89%), and were delivered vaginally (63%) (Table 1). Among the 14% who were preterm, 79% were born between 34 and 36 weeks. At birth, administration of vitamin K was documented for 93%; however, this estimate is based on only a subset of our total sample, given that vitamin K data were missing for 34% (Table 1). Clotting factor concentrate was administered to <5% of the patients within 24 hours of birth.
Family history
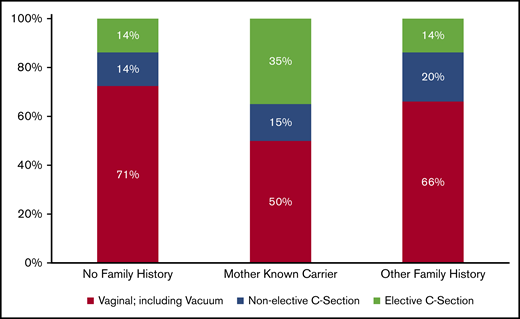
Seventy-nine percent of the patients had a family history of bleeding disorders, as mentioned earlier, family history of VWD prompted diagnostic testing in 68% of patients (Table 1). Distributed by VWD type, patients with type 2 disease were more likely to have a family history (93%) than were those with type 1 (77%) or 3 (44%; P = .02). For 70% of the infants, there was no recorded HTC contact before delivery. In the 30% of infants for whom HTC contact before delivery was recorded, all had a family history of bleeding disorders. In addition, family history significantly influenced method of delivery (P = .01). Although the proportion of nonelective Cesarian sections (C-sections) was similar among ITs with and without a maternal or other family history of bleeding disorders, elective C-sections were significantly more common among patients born to mothers who were known carriers of VWD (35%) than in those with no family history (14%; P = .02) and those with other family members with VWD (14%; P = .01; Figure 1).
Distribution of method of delivery by family history among ITs <2 years of age with a diagnosis of VWD. Eight patients were removed because their method of delivery was unknown. Percentages may not equal 100% because of rounding.
Distribution of method of delivery by family history among ITs <2 years of age with a diagnosis of VWD. Eight patients were removed because their method of delivery was unknown. Percentages may not equal 100% because of rounding.
Timing of VWD diagnosis
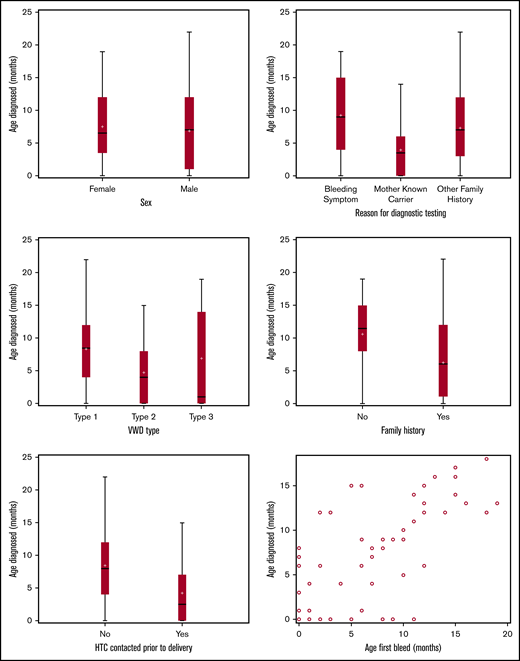
The age at diagnosis ranged from prenatal to 22 months, with half of the patients diagnosed within the first 6 months of life and 70% by 12 months. The overall mean age at diagnosis was 7 months, with little variation by sex (P = .54). The mean age at diagnosis of type 2 was 4.7 months compared with 8.0 and 6.8 months for types 1 and 3, respectively (P = .04). More specifically, 81% of ITs with type 2 VWD were diagnosed within the first year compared with 66% with type 1 and 55% with type 3 disease (Figure 2).
On average, ITs who had a family history of bleeding disorders were diagnosed 4 months earlier than those who did not (P < .001). Furthermore, patients born to mothers who were known carriers of VWD were diagnosed earlier than those with other family members who had a bleeding disorder and before patients with no family history. As expected, because of the relationship between family history and HTC contact, HTC contact before delivery was also associated with earlier diagnosis (P < .001; Figure 2).
Bleeding and diagnosis
Sixty-six percent of ITs experienced their first bleeding episode before UDC enrollment. Considering the age at diagnosis in relation to age at first bleeding event, 22% of ITs experienced a bleeding episode before VWD was diagnosed, 22% experienced a bleeding event at the same age as diagnosis, and just over 50% of patients were diagnosed before their first event. Among those patients with disease diagnosed before the bleeding episode, 92% had a family member with VWD.
Bleed sites and complications
Among the ITs who experienced a bleeding episode before or during the surveillance period (70%), oral mucosa was the most common site for initial bleeding (32%), followed by circumcision site (12%) and the head (intracranial or extracranial) (10%). Other sites of first bleeding episodes among ITs were cutaneous (7%), foot (after a heel stick; 7%), nasal (epistaxis; 4%), intramuscular (injection site; 4%), and gastrointestinal (3%). The site of bleeding was unclassified or unknown in 20% of the patients. Sites of first bleeding event were similar among male and female ITs. Forty-one percent of the initial bleeding events occurred before 6 months of age and 68% occurred before 1 year of age.
Considering the first episode in combination with subsequent bleeding episodes, there were 274 bleeding episodes among 73 ITs. Most of the bleeding episodes were oral/nasal episodes (38 patients experienced 166 episodes), or soft tissue hematomas (15 patients experienced 57 episodes; Table 2). Head injuries, including skull fractures, were also common (13 patients experienced 19 episodes; Table 2). Approximately 5% of the patients in this study experienced an intracranial hemorrhage (ICH). All ICHs were subdural: 80% were associated with trauma and 20% were spontaneous. Forty percent of ITs with ICH experienced long-term neurological effects, such as focal neurological deficits, seizure disorder, or hydrocephaly. ICH was primarily confirmed by computed tomographic scan; no ICH was associated with vaginal or C-section delivery.
Initial and subsequent bleeding events by location among ITs <2 years of age with VWD
| . | ITs with a bleeding episode, n (%) (N = 73)* . | Bleeding episodes, n (%) (N = 274) . |
|---|---|---|
| Bleeds from head injuries (including skull fractures) | 13 (17.8) | 19 (6.9) |
| Circumcision | 9 (12.3) | 9 (3.3) |
| Gastrointestinal (upper or lower) | † (†) | 3 (1.1) |
| Genitourinary renal | † (†) | 5 (1.8) |
| Intracranial hemorrhages | † (†) | 7 (2.5) |
| Joint | † (†) | 3 (1.1) |
| Oral/nasal | 38 (52.1) | 166 (60.6) |
| Soft tissue | 15 (20.5) | 57 (20.8) |
| Venipuncture/heel stick/surgical site | † (†) | 5 (1.8) |
| . | ITs with a bleeding episode, n (%) (N = 73)* . | Bleeding episodes, n (%) (N = 274) . |
|---|---|---|
| Bleeds from head injuries (including skull fractures) | 13 (17.8) | 19 (6.9) |
| Circumcision | 9 (12.3) | 9 (3.3) |
| Gastrointestinal (upper or lower) | † (†) | 3 (1.1) |
| Genitourinary renal | † (†) | 5 (1.8) |
| Intracranial hemorrhages | † (†) | 7 (2.5) |
| Joint | † (†) | 3 (1.1) |
| Oral/nasal | 38 (52.1) | 166 (60.6) |
| Soft tissue | 15 (20.5) | 57 (20.8) |
| Venipuncture/heel stick/surgical site | † (†) | 5 (1.8) |
Percentages do not total 100% because an infant could have multiple bleeding episodes.
Counts <5 have been suppressed to protect patient confidentiality. Additional cells may be suppressed to prevent derivation of these counts by subtraction.
Approximately 64% of ITs received treatment products to prevent perioperative bleeding or to treat a bleeding episode, including 47% that were treated with plasma-derived VWF-containing FVIII concentrates, 32% with aminocaproic acid, and 14% with IV or intranasal desmopressin. No patients in our study received topical hemostatic agents.
Discussion
This report describes characteristics and complications experienced by patients <2 years of age who had a VWD diagnosis and participated in the UDC surveillance project. According to the Community Counts HTC Population Profile data collected by the CDC,13 ∼1% of patients with VWD receiving care in US HTCs from 2012 through 2018 were <2 years of age. The 105 ITs in this study correspond to ∼1% of the total VWD population of 7269 patients enrolled in UDC between 2003 and 2011.
Compared with the distribution of race and ethnicity among children in the United States, Whites in our study (82% vs 50%) were overrepresented, whereas other races including Blacks, Asian/Pacific Islanders, American Indian/Alaskan Natives (9% vs 23%), and Hispanics (10% vs 25%) were underrepresented.14 Other data on the racial and ethnic distribution of infants with VWD outside of the HTC network are lacking. A previous study of children with VWD aged 2 to 12 years in the UDC project included a larger sample and larger proportion of racially and ethnically diverse participants.15 It is unclear why the racial and ethnic distribution in our subset of patients differed from that of the older children, but the lack of diversity in this study population limits the conclusions that can be made about bleeding and other outcomes among ITs of other races and ethnicities.
The distribution of VWD type in this study differed from that previously reported in most studies of patients with VWD. Approximately 63% of the ITs in our study had type 1 VWD, compared with a reported 75% in larger studies with wider age ranges (reviewed by Sadler16 ). This difference suggests that milder forms of VWD may be more difficult to diagnose and thus are less likely to be recognized early in life.
Method of delivery and perinatal outcome
Delivery when bleeding disorders are present poses considerable risk for both mother and infant. Experts generally agree that delivery should be achieved by the least traumatic method to minimize risk and that, when possible, instrumental deliveries and invasive intrapartum monitoring techniques should be avoided.17,18
We found that more than half of the ITs were delivered vaginally; however, a higher percentage of ITs with a maternal history of a bleeding disorder were delivered by C-section when compared with ITs without a maternal history; this difference was driven by an increase in elective C-sections among mothers with a bleeding disorder. The total C-section proportion was also higher than the US national average during the surveillance period (37% vs 31%).19 In general, research recommends that delivery be achieved by the least traumatic method.20 Our current data, in particular the lack of difference in perinatal ICH risk by delivery type, do not support the routine recommendation for C-section in this population. However, regardless of the method of delivery, infants born to mothers who are carriers of VWD require care coordination among the adult and pediatric hematologists, obstetrician, and neonatologist during pregnancy and after birth, to decrease the risk to both the mother and infant.17,21 Delivery plans should include an attempt to avoid unpredictable or prolonged labor and ensure that all the necessary health care providers are available at the time of delivery.20
Approximately 14% of the patients in this population were born preterm and 13% had a low birth weight. These proportions were higher than national preterm birth rates (∼12% and ∼8%, respectively), especially given that the study population was mostly non-Hispanic White women with private health insurance.22-24 From 2007 through 2014, the US preterm birth rate among non-Hispanic White women was ∼10.8%, and ∼7.1% of infants were born with low birth weight, whereas among the non-Hispanic Black women, the preterm birth rate and low birth weight were 17.4% and 13.5%, respectively.23,24 Further studies are needed to investigate whether the higher rates of preterm birth and low birth weight are related to VWD or other factors.
VWD diagnosis and bleeding
VWD was diagnosed in most patients by 7 months of age. ITs were diagnosed earlier when a family member, especially the mother, had an existing VWD diagnosis, when she had ever experienced a bleeding event, or when an HTC was contacted before delivery. Earlier diagnosis in ITs with family history may imply pediatric hematologist and/or obstetric engagement and increased monitoring for this population.
We identified that among ITs with VWD, bleeding episodes were common, even before they became mobile. These findings were consistent with those in other studies that have attempted to describe patterns of bleeding and bruising among children and have found that bleeding, especially in infants, may be indicative of either abuse/trauma or the presence of a severe bleeding disorder.10,25,26 Although the other studies did not specifically address the age range of our cohort, findings of earlier and increased bleeding events among patients with more severe forms of VWD were consistent.
The most common initial bleeding episodes in this sample of ITs were in the oral mucosa and circumcision sites. Older children and adults with VWD have been reported to present with spontaneous epistaxis or oral cavity bleeding, prolonged bleeding after skin laceration, spontaneous gastrointestinal bleeding, or, in women, menorrhagia.5,15,25,27,28 Our findings suggest that bleeding complications for ITs may be distinct from those in older children and adults with VWD. In the ITs in this study, sex was not associated with frequency of bleeding events or site, which contrasts with results of a previous study of older children that demonstrated that prepubescent boys experienced more significant bleeding than girls did.29
Treatment of bleeding
Among the two-thirds of the patients who had undergone intervention to prevent or treat bleeding, most received either plasma-derived VWF/FVIII concentrates or antifibrinolytics. The low rate of treatment with desmopressin (14%) is not surprising, given that, in very young patients, desmopressin is contraindicated because of the higher risk of hyponatremia and seizures in this age group.26,31 The UDC project ended in 2013, and we would expect to see even lower rates of use of desmopressin in the present-day management of ITs with VWD, given the safety concerns. Our data source does not enable us to examine whether desmopressin was given intranasally or IV, or administered while the patients were in the hospital with laboratory sodium monitoring.
Limitations
By design, the UDC surveillance system captured issues relating to ITs with bleeding disorders that are seen in the USHTCN. UDC did not include detailed data on bleeding episodes, exposure days, bleeding scores, treatment product usage for surgical interactions, VWF/antigen levels, or VWF ristocetin cofactor activity. Based on how the data collection was defined, we were not able to distinguish whether the mother was a carrier or had a diagnosis of VWD. In addition, to protect patient identity and to promote meaningful comparisons, the UDC data use guidelines restrict reporting cell counts <5; therefore, because we had a small cohort, some characteristics and combinations of characteristics were not evaluated. The study population was, with >80% being non-Hispanic White, so the impact of race/ethnicity and social determinants of health on VWD could not be adequately evaluated. Finally, for some factors assessed in this study, a substantial number of observations were missing data, notably length at birth and vitamin K administration.
Conclusions
This study contributes to the understanding of ITs with VWD. It highlights the role and importance of family history in the diagnosis of VWD and the higher rate of C-sections, as well as higher rates of preterm birth and low birth weight among ITs with early VWD diagnoses (eg, <2 years of age). The study also indicates that initial bleeding episodes were most commonly oropharyngeal, related to circumcision, or intracranial or extracranial and that most initial bleeding episodes occurred within the first year of life. Data on common sites of bleeding in ITs with VWD are particularly important, because this area has not been well studied. Other studies have recommended a multidisciplinary care approach to provide early diagnosis and optimal care for this population.17,21 Specialized HTCs are uniquely positioned to offer such multidisciplinary care, including genetic counselors throughout the prepartum period who work to increase expectant mothers’ understanding of the risks associated with having a child with VWD, and adult and pediatric hematologists, obstetrician-gynecologists, genetic counselors, nurses, and social workers throughout the pre- and postpartum period who seek to optimize outcomes and disease management.
For information on how to access Universal Data Collection data and how to submit a proposal for analysis, please see the “Organizational Structure and Guidelines for the CDC Cooperative Studies in the Prevention of Bleeding Disorder Complications through Hemophilia Treatment Centers” at https://www.cdc.gov/ncbddd/blooddisorders/udc/documents/DSA-organizational-structure-with-sep-2011-amendment-508comp.pdf.
Acknowledgments
The authors thank the staff of the USHTCN for recruiting patients to the UDC surveillance project and collecting the data and the regional coordinators, regional directors, patients, and their families for their participation.
The UDC project was funded by a cooperative agreement (“Prevention of Bleeding Disorder Complications through Regional Hemophilia Treatment Centers”) between the CDC and the USHTCN, which comprises >130 clinical centers located throughout the United States.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Authorship
Contribution: B.D., K.A., and J.M.S. conceived and designed the study; S.H.O. evaluated VWD subtype coding for categorization; B.D. performed the data analyses; B.D. drafted the manuscript with the remaining authors providing critical expert input with regard to clinical importance, surveillance methodology and epidemiology; and all authors made substantial contributions to the interpretation of the data and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brandi Dupervil, Centers for Disease Control and Prevention, 4770 Buford Hwy, Mail-Stop MS S 104-4, Atlanta, GA 30341-3717; e-mail: inm4@cdc.gov.