Key Points
Selection of unrelated donors using KIR allele typing was feasible for most transplant recipients.
Donor KIR3DL1-Weak Inhibition associates with decreased relapse incidence in patients with AML after HCT.
Abstract
Donor KIR and recipient HLA combinations that minimize inhibition and favor activation of the NK repertoire are associated with improved outcomes after allogeneic hematopoietic cell transplantation (HCT) in patients with myeloid neoplasia. We prospectively evaluated a weighted donor ranking algorithm designed to prioritize HLA-compatible unrelated donors (URDs) with weak inhibitory KIR3DL1/HLA-Bw4 interaction, followed by donors with nontolerized activating KIR2DS1, and finally those with KIR centromeric B haplotype. During donor evaluation, we performed KIR genotyping and ranked 2079 URDs for 527 subjects with myelodysplastic syndrome (MDS) or acute myelogenous leukemia (AML). Among all patients, 394 (75%) had at least 1 KIR-advantageous donor, and 263 (50%) underwent HCT. In patients with AML, KIR3DL1 weak inhibition provided protection from relapse. Compared with KIR3DL1-Weak Inhibiting donors, KIR3DL1-Noninteracting donors were associated with increased risk of relapse (HR, 2.97; 95% CI, 1.33-6.64; P = .008) and inferior event-free survival (EFS; HR, 2.14; 95% CI, 1.16-3.95; P = .015). KIR3DL1-Strong Inhibiting donors were associated with HR, 1.65 (95% CI, 0.66-4.08; P = .25) for AML relapse and HR, 1.6 (95% CI, 0.81-3.17; P = .1) for EFS when compared with the use of KIR3DL1-weak inhibiting donors. Donor KIR2DS1/HLA-C1 status and centromeric KIR haplotype-B content were not associated with decreased risk of AML relapse. There was no benefit to KIR-based donor selection in patients with MDS. This study demonstrates that donor KIR typing is feasible, and prioritization of donors with certain KIR3DL1 genotypes may confer a protection from relapse after HCT in patients with AML.
Introduction
Natural killer (NK) cells are innate immune cells with the ability to mediate potent cellular cytotoxicity against malignant cells without prior sensitization, thereby participating in the graft-versus-leukemia (GVL) phenomenon that occurs after allogeneic hematopoietic cell transplantation (HCT) in patients with myeloid neoplasia.1 Titration of NK cell effector response occurs in large part due to expression of killer immunoglobulinlike receptors (KIR), both inhibitory and activating, and their interaction with class I HLA molecules.2 Individuals may exhibit from 8 to 15 different KIR genes, leading to significant population diversity by KIR gene content alone, further amplified by substantial allelic polymorphism.2 The interaction between inhibitory KIR and their HLA class I ligands educates NK cells during development to mount a cytotoxic effect against target cells that lack self-HLA, while simultaneously promoting tolerance to cells that express self-HLA molecules. Although loss of HLA class I expression can occur on some tumor and virally infected cells, most cells, including leukemia, upregulate HLA class I expression in a minimally inflamed environment.3 Such expression of HLA class I proteins could subsequently dampen NK cell–mediated GVL via signaling through inhibitory KIR.4 Avoidance of donors with potential for strong NK inhibition and selection of donors with potential for weak inhibition may therefore promote NK cell reactivity and increase leukemia control.
Several approaches have been tested in large, retrospective registry-based studies designed to determine whether KIR-based donor groupings are an effective biomarker for prevention of relapse after URD HCT. These include studies that test the impact of interaction strength between inhibitory KIR and HLA class I ligands and examine the influence of donor activating KIR content, alone5,6 or in the presence of tolerizing ligand.3,7-9 The inhibitory KIR3DL1 is associated with AML relapse and survival after HCT, conferring favorable outcomes in the setting of lack of inhibition when its HLA-Bw4 ligand is missing or with decreased inhibition when the donor KIR3DL1 subtype is paired with a patient HLA-Bw4 subtype encoding proteins with weak interaction.3,7 We previously demonstrated in a retrospective analysis of 1328 HCT recipients with a diagnosis of AML that KIR3DL1-Weak Inhibiting donor-recipient interactions associated with decreased relapse and improved overall survival (OS).3 A subsequent, similarly large study conducted by Schetelig and colleagues using a different registry of AML patients did not replicate these results.10 At this time, it remains unclear whether KIR3DL1-based URD donor selection is helpful in preventing relapse and in particular whether the benefit seen in the earlier study is dependent on specific transplant conditions. KIR3DL1-based URD selection also has not been explored in patients with myelodysplasia syndrome (MDS).
Among the activating KIR, the telomeric KIR2DS1 interacts with HLA-CLys80 allotypes, collectively referred to as the HLA-C2 ligand group. Donor-recipient pairs homozygous for HLA-C2 alleles have been observed to have poor HCT outcomes, with higher rates of relapse and lower rates of survival.11-15 These outcomes may be related to tolerization of KIR2DS1+ NK cells, which exhibit an increasingly hyporesponsive phenotype, commensurate with the environmental dose of HLA-C2.14 In contrast, KIR2DS1+ donors with at least 1 HLA-C allele encoding a HLA-CAsn80 allotype (collectively referred to as HLA-C1 ligands) are associated with protection from AML relapse after URD HCT.12,13 Finally, it has been reported that URDs exhibiting a KIR “haplotype-B” and specifically characterized by activating KIR in the centromeric portion (the cenB partial haplotype), are also associated with improved relapse and OS in HCT recipients with AML, where donors homozygous for cenB (cenBB) appear to confer the greatest benefit.5,13,16 By comparison, donors exhibiting only KIR haplotype-A, which contains minimal if any activating KIR, are associated with the highest risk of relapse.5 The 3 mechanisms were compared in a large retrospective study, with KIR3DL1 inhibition emerging as the most influential in protecting patients with AML from relapse.3
In the current study, we sought to determine whether the same protective KIR/HLA associations identified in retrospective studies for patients with AML are feasible in real-time donor selection and are beneficial to transplant outcomes of patients with myeloid diseases. We find that KIR-based donor selection is feasible and, specifically, that selection based on KIR3DL1 inhibition can mitigate relapse in patients with AML but not in those with MDS who undergo allogeneic HCT.
Methods
Patient inclusion criteria and protection of human subjects
Eligible subjects were individuals with a diagnosis of MDS or AML with at least 1 HLA 7/8 or 8/8 URD (HLA-A, -B, -C, and -DRB1) requested to undergo confirmatory HLA typing and KIR genotyping from 2013 through 2019 at Memorial Sloan Kettering Cancer Center (MSKCC). Subjects who subsequently underwent HCT at MSKCC were included in the HCT outcomes analysis. All subjects provided informed consent for retrospective research. This analysis was approved by the Institutional Review Board and Privacy Board of MSKCC and was conducted in accordance with the Declaration of Helsinki.
KIR gene and allele typing and KIR/HLA-based donor ranking algorithm
KIR gene typing was performed by the American Red Cross (Philadelphia, PA) using the KIR Genotyping SSP Kit (One Lambda; Canoga Park, CA), according to the manufacturer’s instructions. KIR3DL1 allele-group typing was performed as previously described, assigning donors based on compound KIR3DL1 alleles into high-expression allele groups, low-expression allele groups, null groups without surface KIR3DL1 expression, or homozygosity for KIR3DS1.17 In combination with recipient HLA genotype, donors were assessed for “KIR advantage,” based on published models of NK reactivity associated with improved HCT outcomes, and prioritized, in descending order: strength of inhibition of donor KIR3DL1 by recipient HLA-B,3 presence of donor HLA-C1/KIR2DS1,12 and centromeric KIR haplotype content.5 Donors were placed in 3 groups based on potential for KIR3DL1 inhibition using previously described allele groups (supplemental Tables 1 and 2): donor KIR3DL1/recipient HLA-Bw4 combinations with strong inhibition potential (KIR3DL1-Strong Inhibiting), noninteracting donor KIR3DL1/recipient HLA-B combinations (KIR3DL1-Noninteracting), and donor KIR3DL1/recipient HLA-Bw4 combinations with weak inhibition potential (KIR3DL1-Weak Inhibiting). KIR3DL1 inhibition status was weighted the heaviest, with highest priority given to KIR3DL1-Weak Inhibiting donors. KIR3DL1-Weak Inhibiting and KIR3DL1-Noninteracting donors underwent further prioritization based on KIR2DS1/HLA-C1 status. The lowest tier prioritized cenBB donors over the remaining donors. HLA-C2 homozygous and/or KIR3DL1-Strong Inhibiting donors were considered unfavorable, even if other favorable genotypes were present.3,12,13,18 URD KIR allele typing was obtained within 72 hours of receipt of DNA in the laboratory, and donor rankings were provided to treating physicians in real time before donor selection. Treating physicians made the final choice with respect to the number of donors typed and the donor chosen for HCT and could elect to increase the number of donors evaluated based on the KIR status of previously evaluated donors. KIR genotyping was used to prioritize donors between similarly HLA-matched donors. Selection of donors based on KIR genotype was recommended, but not required.
Clinical end points and statistical methodology
Disease stage and assessment of relapse were determined according to standard criteria.19,20 All end points were assessed from the time of transplantation. The χ2 test for trend was used to test for trends in donor availability. The Wilcoxon rank-sum test was used to evaluate continuous measurements. OS and EFS were estimated by Kaplan-Meier methodology. The incidence of relapse and nonrelapse mortality (NRM) were estimated using cumulative incidence functions. Deaths were attributed to relapse or NRM causes. Cox proportional hazards regression evaluated univariate and multivariate associations with OS, and cause-specific proportional hazards regression was used to evaluate associations with relapse risk and NRM. The proportional hazards assumption was assessed according to the methods proposed by Grambsch and Therneau.21 Statistical analyses were performed using R: A language and environment for statistical computing, version 3.5.
Results
Patient characteristics and donor results in all subjects evaluated for HCT
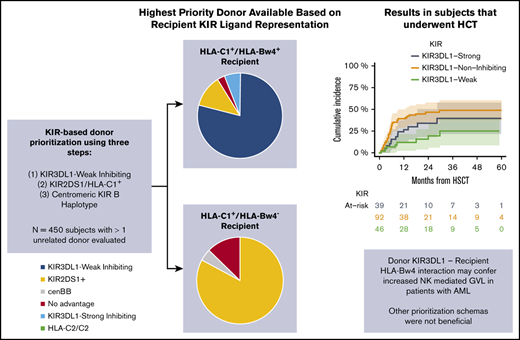
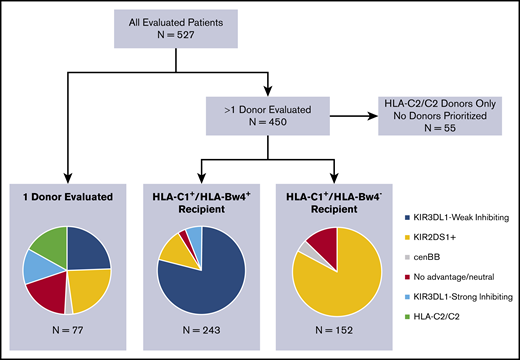
A total of 2079 donors underwent confirmatory HLA and KIR genotyping of 527 subjects with a diagnosis of MDS (n = 200) or AML (n = 327) and had been evaluated for HCT. A median of 4 donors were evaluated per patient (range, 1-12). KIR ligand and KIR gene/allele typing frequencies are presented in Table 1 and supplemental Tables 3 and 4. Frequencies of prioritized donor groups according to the algorithm are shown in Figure 1. Among the 450 patients with >1 donor evaluated, 55 had only donor options that exhibited homozygosity for HLA-C2 and were therefore deemed disadvantageous. Of the 395 recipients with HLA-C1+ donors, 243 recipients (61.5%) were also HLA-Bw4+ and underwent KIR3DL1-based donor prioritization, the most heavily weighted among the criteria for KIR advantage. Among all donors evaluated for these subjects (n = 1102), 407 (37%) were considered KIR3DL1-Weak Inhibiting and were therefore prioritized in rank. In contrast, 337 donors (31%) were considered KIR3DL1-Strong Inhibiting and were deprioritized. The remaining 358 donors (32.5%) were KIR3DL1-Noninteracting, most being HLA-Bw6 homozygous. Considering all donors evaluated for each patient, 192 of the 243 HLA-Bw4+ subjects (79%) had at least 1 KIR3DL1-Weak Inhibiting donor available. Importantly, 124 subjects (51%) had a mixture of both KIR3DL1-Weak Inhibiting and KIR3DL1-Strong Inhibiting donors, presenting a choice of donors based on KIR3DL1/HLA-Bw4 interaction.
Recipient KIR ligand and donor KIR genotypes
| . | All subjects . | Transplant recipients . |
|---|---|---|
| Recipient KIRl ligands | ||
| Total | 527 | 263 |
| HLA-Bw4-I80 composite | 201 (38.1) | 74 (28.1) |
| HLA-Bw4-T80 composite | 139 (26.4) | 92 (35.0) |
| HLA-Bw6/Bw6 | 187 (35.5) | 97 (36.9) |
| HLA-C1/x | 459 (87.0) | 234 (89.0) |
| HLA-C2/C2 | 68 (12.9) | 29 (11.0) |
| Donor KIR genotypes/compound allotypes | ||
| Total | 2079 | 263 |
| CenAA | 902 (43.4) | 126 (47.9) |
| CenAB | 986 (47.4) | 116 (44.1) |
| CenBB | 191 (9.2) | 21 (8.0) |
| KIR3DL1-Weak Inhibiting | 489 (23.5) | 64 (24.3) |
| KIR3DL1-Noninteracting | 1096 (52.7) | 139 (52.8) |
| KIR3DL1-Strong Inhibiting | 498 (23.9) | 60 (22.8) |
| HLA-C1+/KIR2DS1+ | 706 (33.9) | 111 (42.2) |
| HLA-C1+/KIR2DS1− | 1137 (54.7) | 124 (47.1) |
| HLA-C2/C2 | 236 (11.4) | 28 (10.6) |
| . | All subjects . | Transplant recipients . |
|---|---|---|
| Recipient KIRl ligands | ||
| Total | 527 | 263 |
| HLA-Bw4-I80 composite | 201 (38.1) | 74 (28.1) |
| HLA-Bw4-T80 composite | 139 (26.4) | 92 (35.0) |
| HLA-Bw6/Bw6 | 187 (35.5) | 97 (36.9) |
| HLA-C1/x | 459 (87.0) | 234 (89.0) |
| HLA-C2/C2 | 68 (12.9) | 29 (11.0) |
| Donor KIR genotypes/compound allotypes | ||
| Total | 2079 | 263 |
| CenAA | 902 (43.4) | 126 (47.9) |
| CenAB | 986 (47.4) | 116 (44.1) |
| CenBB | 191 (9.2) | 21 (8.0) |
| KIR3DL1-Weak Inhibiting | 489 (23.5) | 64 (24.3) |
| KIR3DL1-Noninteracting | 1096 (52.7) | 139 (52.8) |
| KIR3DL1-Strong Inhibiting | 498 (23.9) | 60 (22.8) |
| HLA-C1+/KIR2DS1+ | 706 (33.9) | 111 (42.2) |
| HLA-C1+/KIR2DS1− | 1137 (54.7) | 124 (47.1) |
| HLA-C2/C2 | 236 (11.4) | 28 (10.6) |
Data are the number of subjects or transplant recipients (percentage of total group).
Identification of the best available donor based on KIR genotypes, using the weighted tiered algorithm in all evaluated patients. Recipients with >1 donor are divided based on KIR ligand: HLA-C1+/HLA-Bw4+ recipients had donors prioritized for KIR3DL1 inhibition, followed by KIR2DS1, and then cenBB. HLA-C1+/HLA-Bw4− recipients had donors prioritized for KIR2DS1, followed by cenBB.
Identification of the best available donor based on KIR genotypes, using the weighted tiered algorithm in all evaluated patients. Recipients with >1 donor are divided based on KIR ligand: HLA-C1+/HLA-Bw4+ recipients had donors prioritized for KIR3DL1 inhibition, followed by KIR2DS1, and then cenBB. HLA-C1+/HLA-Bw4− recipients had donors prioritized for KIR2DS1, followed by cenBB.
KIR2DS1-based donor prioritization, the second tier of the donor selection algorithm, was relevant for 188 subjects, due either to lack of HLA-Bw4 (HLA-Bw6/Bw6 recipient, n = 152) or lack of KIR3DL1-Weak Inhibiting donors available for an HLA-Bw4+ recipient (n = 36). Among the 863 donors evaluated for these subjects, 339 donors (39%) exhibited the KIR2DS1 genotype, providing 155 of 188 subjects (82%) with at least 1 KIR2DS1+ donor. Eight of the 33 HLA-C1+ subjects with neither a KIR2DS1+ donor nor a KIR3DL1-Weak Inhibiting donor had an available donor with a cenBB KIR genotype, the last tier within the donor selection algorithm.
In total, 70 of 450 subjects (15.6%) had only disadvantageous donors available, due to either donor HLA-C2 homozygosity (n = 55) or the availability of only KIR3DL1-Strong Inhibiting donors (n = 15). An additional 25 of the 450 subjects (6%) had only donors with no known KIR advantage. The remaining 355 of 450 subjects (79%) had at least 1 KIR-advantageous donor available to them from a group comprising KIR3DL1-Weak Inhibiting donors, KIR2DS1+/HLA-C1+ donors, and cenBB donors.
Evaluation of greater numbers of donors was associated with an increased probability of identifying an advantageous donor based on inhibitory KIR3DL1 interactions (χ2 test for trend, P < .0001), presence of KIR2DS1/C1+ (P < .0001), or centromeric haplotype B content (Figure 2; P < .0001). Among HLA-Bw4+ recipients, the probability of having a KIR3DL1-Weak Inhibiting donor was 43% if only 1 donor underwent typing but increased to 79.6% if 3 donors were typed. In HLA-C1+ recipients the probabilities of having a KIR2DS1+ or a cenBB donor were 45% and 11%, respectively, if 1 donor was typed and increased to 69% and 22% if 3 donors were typed, respectively.
Probability of identifying a KIR-advantageous donor based on the number of donors who undergo confirmatory HLA typing for an individual patient. (A) Probability of identifying a KIR3DL1-Weak Inhibiting donor for HLA-C1+/HLA-Bw4+ recipients. (B) Probability of identifying a KIR2DS1+ (blue) or cenBB (red) donor for all HLA-C1+ recipients.
Probability of identifying a KIR-advantageous donor based on the number of donors who undergo confirmatory HLA typing for an individual patient. (A) Probability of identifying a KIR3DL1-Weak Inhibiting donor for HLA-C1+/HLA-Bw4+ recipients. (B) Probability of identifying a KIR2DS1+ (blue) or cenBB (red) donor for all HLA-C1+ recipients.
Outcomes in subjects who underwent allogeneic HCT
Among the subjects evaluated for HCT, 263 subjects (50%) underwent the procedure with an URD well matched for HLA. Characteristics of transplant recipients are outlined in Table 2. The median donor age was 28 years (range, 18-60), and, among all donors, 121 (46%) were cytomegalovirus (CMV) seropositive. Fifteen subjects underwent HLA-mismatched donor HCT, of which 14 were KIR ligand matched. One HLA-C2/C2 patient received an allograft from an HLA-C1/C2 donor. In the entire cohort, the 24-month OS was 60% (95% CI, 54-67) and EFS was 48% (95% CI, 42-55). The 24-month cumulative incidence of relapse was 35% (95% CI, 29-41) and NRM was 17% (95% CI, 13-22).
Demographics of allogeneic HCT recipients
| Demographic . | Data . | |
|---|---|---|
| Total | 263 | |
| Median follow-up (IQR), mo | 16.0 (6.9-30.1) | |
| Mean age (range), y | 60.0 (21.7-78.4) | |
| HCT-CI | ||
| 0-1 | 80 (30) | |
| 2 | 43 (16) | |
| 3+ | 140 (53) | |
| Conditioning | ||
| Ablative | 173 (66) | |
| Reduced or nonmyeloablative | 90 (34) | |
| Diagnosis | ||
| AML | 167 (63) | |
| MDS | 96 (37) | |
| HLA match | ||
| 8/8 | 248 (94) | |
| 7/8 | 15 (6) | |
| GVHD prophylaxis | ||
| CD34+ selection | 121 (46.0) | |
| Calcineurin inhibitor based | 142 (54.0) | |
| Refined disease risk index | ||
| Low/intermediate | 142 (54.0) | |
| High | 91 (34.6) | |
| Very high | 30 (11.4) | |
| Demographic . | Data . | |
|---|---|---|
| Total | 263 | |
| Median follow-up (IQR), mo | 16.0 (6.9-30.1) | |
| Mean age (range), y | 60.0 (21.7-78.4) | |
| HCT-CI | ||
| 0-1 | 80 (30) | |
| 2 | 43 (16) | |
| 3+ | 140 (53) | |
| Conditioning | ||
| Ablative | 173 (66) | |
| Reduced or nonmyeloablative | 90 (34) | |
| Diagnosis | ||
| AML | 167 (63) | |
| MDS | 96 (37) | |
| HLA match | ||
| 8/8 | 248 (94) | |
| 7/8 | 15 (6) | |
| GVHD prophylaxis | ||
| CD34+ selection | 121 (46.0) | |
| Calcineurin inhibitor based | 142 (54.0) | |
| Refined disease risk index | ||
| Low/intermediate | 142 (54.0) | |
| High | 91 (34.6) | |
| Very high | 30 (11.4) | |
Data are number of patients (percentage of total transplant recipients), unless otherwise stated.
Analysis outcomes based on independent KIR-HLA donor ranking schemas
Among the selected KIR-advantageous donors, there was a diversity of advantage type, even though KIR3DL1-Weak Inhibiting donors were recommended above all others. We could therefore examine each KIR donor stratification tool separately in univariate analyses in patients with MDS and in those with AML.
Given that there are few data to support a role of KIR-based selection in patients with MDS, we first sought to determine whether the patients benefited from any of the individual donor ranking schemas. We did not observe a benefit of KIR3DL1-based, donor KIR2DS1/HLA-C1–based, or centromeric haplotype–based donor selection in this population. Comprehensive results of the hazards for relapse, OS, EFS, and NRM are provided in supplemental Table 6.
We then examined the role of the individual donor-ranking schemas in patients with AML. Neither donor centromeric B haplotype content nor donor KIR2DS1/HLA-C1 content was associated with improved outcomes in this cohort (Table 3). In contrast, there was a protective relapse benefit in patients with AML who received allografts from KIR3DL1-Weak Inhibiting donors. Compared with KIR3DL1-Weak Inhibiting donors, use of KIR3DL1-Noninteracting donors resulted in an increased risk of relapse (HR, 3.03; 95% CI, 1.41-6.5; P = .004), and use of KIR3DL1 Strong-Inhibiting donors trended toward an increased risk of relapse incidence (HR, 1.98; 95% CI, 0.82-4.79; P = .13; Table 3; Figure 3). Consequently, recipients of KIR3DL1-Weak Inhibiting donors had reduced incidence of relapse and improved EFS compared with recipients of KIR3DL1-Noninteracting or -Strong Inhibiting donors (Table 3). NRM was similar in recipients of KIR3DL1-Weak Inhibiting, Noninteracting, and Strong Inhibiting donors (Figure 3; Table 3).
Univariate hazards for transplantation outcomes in patients with AML, according to different KIR-based donor grouping tools
| . | . | n . | Survival . | P . | EFS . | P . | Relapse . | P . | Treatment-related mortality . | P . | Acute GVHD (100 d) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KIR3DL1 Inhibition | ||||||||||||
| KIR3DL1-Weak Inhibiting | 46 | Reference | Reference | Reference | Reference | Reference | ||||||
| KIR3DL1-Noninteracting | 92 | 1.84 (0.98-3.43) | .058 | 2.16 (1.21-3.84) | .009 | 3.03 (1.41-6.5) | .004 | 1.13 (0.44-2.88) | .795 | 1.17 (0.65-2.11) | .604 | |
| KIR3DL1-Strong Inhibiting | 39 | 1.55 (0.76-3.16) | .231 | 1.81 (0.94-3.49) | .077 | 1.98 (0.82-4.79) | .128 | 1.63 (0.61-4.38) | .334 | 1.58 (0.81-3.07) | .18 | |
| KIR3DL1, 2 groups | ||||||||||||
| KIR3DL1-Weak Inhibiting | 46 | Reference | Reference | Reference | Reference | Reference | ||||||
| KIR3DL1-Noninteracting or Strong Inhibiting | 131 | 1.74 (0.95-3.17) | .073 | 2.04 (1.17-3.56) | .012 | 2.68 (1.27-5.64) | .01 | 1.3 (0.55-3.07) | .546 | 1.29 (0.74-2.25) | .376 | |
| KIR2DS1 | ||||||||||||
| KIR2DS1-/HLA-C1+ | 126 | Reference | Reference | Reference | Reference | Reference | ||||||
| KIR2DS1+/HLA-C1+ | 108 | 0.85 (0.53-1.36) | .495 | 1.07 (0.7-1.62) | .758 | 1.19 (0.72-1.97) | .506 | 0.85 (0.4-1.8) | .667 | 1.1 (0.69-1.75) | .702 | |
| Centromeric haplotype B content | ||||||||||||
| CenAA | 88 | Reference | Reference | Reference | Reference | Reference | ||||||
| CenAB | 75 | 0.67 (0.41-1.1) | .115 | 0.67 (0.43-1.05) | .078 | 0.66 (0.39-1.14) | .137 | 0.69 (0.32-1.5) | .347 | 0.79 (0.48-1.3) | .354 | |
| CenBB | 14 | 1.07 (0.45-2.53) | .879 | 1.01 (0.46-2.24) | .976 | 1.04 (0.41-2.67) | .931 | 0.94 (0.22-4.14) | .938 | 0.76 (0.3-1.93) | .562 | |
| Combined ranking schemas | ||||||||||||
| KIR advantageous | 87 | Reference | Reference | Reference | Reference | Reference | ||||||
| No ranking | 34 | 1.5 (0.8-2.79) | .206 | 1.23 (0.7-2.17) | .475 | 0.96 (0.47-1.97) | .913 | 2.04 (0.78-5.38) | .148 | 0.61 (0.29-1.28) | .191 | |
| KIR disadvantageous | 56 | 1.33 (0.79-2.24) | .29 | 1.14 (0.71-1.83) | .578 | 0.99 (0.56-1.74) | .965 | 1.61 (0.68-3.79) | .278 | 1.18 (0.71-1.96) | .527 | |
| . | . | n . | Survival . | P . | EFS . | P . | Relapse . | P . | Treatment-related mortality . | P . | Acute GVHD (100 d) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KIR3DL1 Inhibition | ||||||||||||
| KIR3DL1-Weak Inhibiting | 46 | Reference | Reference | Reference | Reference | Reference | ||||||
| KIR3DL1-Noninteracting | 92 | 1.84 (0.98-3.43) | .058 | 2.16 (1.21-3.84) | .009 | 3.03 (1.41-6.5) | .004 | 1.13 (0.44-2.88) | .795 | 1.17 (0.65-2.11) | .604 | |
| KIR3DL1-Strong Inhibiting | 39 | 1.55 (0.76-3.16) | .231 | 1.81 (0.94-3.49) | .077 | 1.98 (0.82-4.79) | .128 | 1.63 (0.61-4.38) | .334 | 1.58 (0.81-3.07) | .18 | |
| KIR3DL1, 2 groups | ||||||||||||
| KIR3DL1-Weak Inhibiting | 46 | Reference | Reference | Reference | Reference | Reference | ||||||
| KIR3DL1-Noninteracting or Strong Inhibiting | 131 | 1.74 (0.95-3.17) | .073 | 2.04 (1.17-3.56) | .012 | 2.68 (1.27-5.64) | .01 | 1.3 (0.55-3.07) | .546 | 1.29 (0.74-2.25) | .376 | |
| KIR2DS1 | ||||||||||||
| KIR2DS1-/HLA-C1+ | 126 | Reference | Reference | Reference | Reference | Reference | ||||||
| KIR2DS1+/HLA-C1+ | 108 | 0.85 (0.53-1.36) | .495 | 1.07 (0.7-1.62) | .758 | 1.19 (0.72-1.97) | .506 | 0.85 (0.4-1.8) | .667 | 1.1 (0.69-1.75) | .702 | |
| Centromeric haplotype B content | ||||||||||||
| CenAA | 88 | Reference | Reference | Reference | Reference | Reference | ||||||
| CenAB | 75 | 0.67 (0.41-1.1) | .115 | 0.67 (0.43-1.05) | .078 | 0.66 (0.39-1.14) | .137 | 0.69 (0.32-1.5) | .347 | 0.79 (0.48-1.3) | .354 | |
| CenBB | 14 | 1.07 (0.45-2.53) | .879 | 1.01 (0.46-2.24) | .976 | 1.04 (0.41-2.67) | .931 | 0.94 (0.22-4.14) | .938 | 0.76 (0.3-1.93) | .562 | |
| Combined ranking schemas | ||||||||||||
| KIR advantageous | 87 | Reference | Reference | Reference | Reference | Reference | ||||||
| No ranking | 34 | 1.5 (0.8-2.79) | .206 | 1.23 (0.7-2.17) | .475 | 0.96 (0.47-1.97) | .913 | 2.04 (0.78-5.38) | .148 | 0.61 (0.29-1.28) | .191 | |
| KIR disadvantageous | 56 | 1.33 (0.79-2.24) | .29 | 1.14 (0.71-1.83) | .578 | 0.99 (0.56-1.74) | .965 | 1.61 (0.68-3.79) | .278 | 1.18 (0.71-1.96) | .527 | |
Outcomes in recipients of KIR3DL1-Weak Inhibiting compared with KIR3DL1-Strong Inhibiting or KIR3DL1- Noninteracting donor recipients in patients with AML. OS (A), cumulative incidence of relapse (B), cumulative incidence of NRM (C), and cumulative incidence of acute GVHD (D).
Outcomes in recipients of KIR3DL1-Weak Inhibiting compared with KIR3DL1-Strong Inhibiting or KIR3DL1- Noninteracting donor recipients in patients with AML. OS (A), cumulative incidence of relapse (B), cumulative incidence of NRM (C), and cumulative incidence of acute GVHD (D).
To adjust for significant clinical covariates, we performed a multivariate analysis of outcomes in recipients of allografts from KIR3DL1-Weak Inhibiting donors compared with recipients of allografts from KIR3DL1-Strong Inhibiting or KIR3DL1-Noninteracting donors, with adjustment for patient age, conditioning intensity, patient hematopoietic cell transplant comorbidity index, disease histology, donor CMV serostatus, refined disease risk index, use of T-cell depletion, and donor/recipient HLA-matching status (Table 4). Compared with recipients of KIR3DL1-Weak Inhibiting donors, increased relapse incidence was observed in recipients of KIR3DL1-Noninteracting donor allografts (HR, 2.97; 95% CI, 1.33-6.64; P = .008), whereas statistically similar relapse incidence was observed in recipients of KIR3DL1-Strong Inhibiting donor allografts (HR, 1.65; 95% CI, 0.66-4.08; P = .28). This outcome results in worse EFS in recipients of KIR3DL1-Noninteracting donor allografts (HR, 2.14; 95% CI, 1.16-3.95; P = .02) and similar EFS in recipients of KIR3DL1-Strong Inhibiting donor allografts (HR, 1.6; 95% CI, 0.81-3.17; P = .17; Table 4).
Multivariate hazards for transplantation outcomes in patients with AML, based on donor KIR3DL1 inhibition potential
| . | OS . | . | EFS . | . | Relapse . | . |
|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| KIR3DL1-Weak Inhibiting | Reference | Reference | Reference | |||
| KIR3DL1-Noninteracting | 1.83 (0.94-3.56) | .077 | 2.14 (1.16-3.95) | .015 | 2.97 (1.33-6.64) | .008 |
| KIR3DL1-Strong Inhibiting | 1.4 (0.67-2.94) | .367 | 1.6 (0.81-3.17) | .174 | 1.65 (0.66-4.08) | .281 |
| . | OS . | . | EFS . | . | Relapse . | . |
|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| KIR3DL1-Weak Inhibiting | Reference | Reference | Reference | |||
| KIR3DL1-Noninteracting | 1.83 (0.94-3.56) | .077 | 2.14 (1.16-3.95) | .015 | 2.97 (1.33-6.64) | .008 |
| KIR3DL1-Strong Inhibiting | 1.4 (0.67-2.94) | .367 | 1.6 (0.81-3.17) | .174 | 1.65 (0.66-4.08) | .281 |
We then sought to determine whether the protection in relapse from KIR3DL1-Weak Inhibiting donors could be related to an increase in acute graft-versus-host disease (GVHD). The risk for day +100 grade 2 to 4 acute GVHD in recipients of KIR3DL1-Weak Inhibiting donor allografts after HCT was similar to that of recipients of KIR3DL1-Noninteracting donors or recipients of KIR3DL1-Strong Inhibiting donors (Table 3). These data indicate that protection from relapse in this cohort was not associated with an increased incidence of grade 2 to 4 acute GVHD.
Analysis of outcomes based on the combined KIR-HLA donor ranking system
Donors were ranked in real time with selection according to a combined algorithm that considered KIR3DL1-Weak Inhibiting, KIR2DS1/HLA-C1, and cenBB donors collectively as “KIR-advantageous.” Using this combined ranking system, the first or second ranked donor was selected for transplant in 181 of 263 subjects (68.8%). We found that 126 subjects received an allograft from a KIR-advantageous donor, 83 subjects received an allograft from a KIR-disadvantageous donor (54 recipients had KIR3DL1-Strong Inhibiting donors, and 29 recipients had HLA-C2 homozygous donors), and 54 subjects underwent HCT with donors with no known KIR advantage. When all groups of presumed “KIR advantageous” donors were combined, there was no association with improvement in OS compared with that of nonadvantageous donors in patients with AML (Table 3) or MDS (supplemental Table 6).
Feasibility of selection of URDs based on KIR genotyping
KIR3DL1-based selection did not appear to alter other significant parameters relevant to donor selection. The median days from formal search to transplantation was similar between recipients with KIR3DL1-Weak Inhibiting donors compared with recipients with KIR3DL1-Noninteracting or -Strong Inhibiting donors (86.5 days; interquartile range [IQR], 63-122 vs 88 days; IQR, 63-154; P = .7, respectively). The median donor age for KIR3DL1-Weak Inhibiting, Noninteracting, and Strong Inhibiting donors was 29, 28, and 30 years, respectively. The frequency of CMV seropositivity for KIR3DL1-Weak Inhibiting, -Noninteracting, and -Strong Inhibiting donors was 40%, 48%, and 47%, respectively.
We evaluated whether the ranking process led to the desired enrichment of KIR3DL1-Weak Inhibiting donors in the target population of HLA-C1+/HLA-Bw4+ recipients for whom >1 donor was evaluated. For all potential donors evaluated for HLA-C1+/HLA-Bw4+ patients who underwent HCT, the frequency of KIR3DL1-Weak Inhibiting and KIR3DL1-Strong Inhibiting donors was 35% and 31%, respectively. In comparison, donors ultimately chosen for HLA-C1+/HLA-Bw4+ recipients were KIR3DL1-Weak Inhibiting and KIR3DL1-Strong Inhibiting in 41% and 37%, respectively (P = .2), suggesting that the ranking process did not enrich for KIR3DL1-Weak Inhibiting donors.
Discussion
We demonstrate that use of patient and donor HLA and KIR genotyping to prioritize donors is feasible in the context of prospective donor selection. Donors with greater KIR3DL1-mediated NK alloreactivity related to weak KIR3DL1 inhibition confers protection from AML relapse after HCT using a well-HLA allele–matched URD.
Selection of the URD to use in HCT is one of the most important, modifiable factors in the overall transplant design. In an era of advances in transplant supportive care, relapse remains the most pressing cause of post-HCT mortality, and methods that result in reduced relapse without increasing GVHD are critical to improving survival in transplant recipients. These data demonstrate that an immunogenetics tool based on NK biology may be used to address a patient-centered problem without inciting toxicity. We further showed that use of KIR/HLA genotyping to prioritize donors is feasible in a real-world, prospective donor selection framework and that the selection of a KIR-advantageous donor is not associated with a significant increase in the time to HCT. Increasing incorporation of KIR genotyping into donor registries will make selection of donors based on this parameter more accessible to transplant clinicians in the future.
In the current study, we combined models, using a tiered approach to KIR-based URD selection, first prioritizing donors based on KIR3DL1 inhibition, followed by selection of donors with HLA-C1+/KIR2DS1+, and finally cenBB. The tiers of the schema were organized based on published associations with decreased relapse noted in previous large retrospective studies, the majority of which were based on in vitro mechanistic studies of NK function.3,22,23 Although most subjects underwent transplant with the first- or second-ranked donor, according to the tiered ranking system, we found that only KIR3DL1 inhibition by HLA-Bw4 had a significant impact on relapse and survival in this cohort, where donor KIR3DL1 allotypes with weak inhibitory interaction with patient HLA-B allotypes were associated with protection from relapse. Use of donors with KIR2DS1/HLA-C1 or cenBB did not extend the posttransplant benefit. The sample size contained in this single center trial is most likely too small to definitively rule out a benefit in donor cenB content or KIR2DS1, but rather suggests that the effect size from KIR3DL1-based effects may be larger.
Importantly, our finding that weak inhibition KIR3DL1/HLA-B compound genotypes is associated with protection against AML relapse and enhanced EFS confirms our original observation made in a large, retrospective, registry-based study and supports the application of KIR allele typing in donor selection.3 One important difference, however, is the lack of relapse benefit that was seen in the registry-based study in patients with KIR3DL1-Noninteracting donors, who are largely HLA-Bw6 homozygous. In the retrospective study, use of KIR3DL1-Noninteracting donors yielded a probability of relapse and OS similar to KIR3DL1-Weak Inhibiting donors, when compared with KIR3DL1-Strong Inhibiting donors.3 In the current analysis, however, KIR3DL1-Noninteracting donor recipients had similarly poor OS to KIR3DL1-Strong Inhibiting donor recipients, whereas KIR3DL1-Weak Inhibiting donors remained protective. A possible explanation for this finding is that weak inhibition KIR3DL1/HLA-Bw4 combinations are also combinations that confer increased NK cell responsiveness via NK cell education, whereas most KIR3DL1-Noninteracting donors are HLA-Bw6 homozygous, leading to an uneducated, hyporesponsive KIR3DL1+ NK cell population due to the absence of the educating HLA-Bw4 epitope. This implies that despite the prospect of in vivo inhibition for donor NK cells with weakly inhibiting KIR3DL1/HLA-Bw4 potential, the heightened responsiveness still provides disease control. In highly inflammatory conditions, even uneducated NK cells develop higher responsiveness.24 Such an environment may have occurred in older transplants with total body irradiation and/or complicated by infection, leading to improved outcomes for the KIR3DL1-Noninteracting group in older studies.3,9
Before the initiation of this study, there has not been an extensive analysis of KIR-based donor selection in patients with MDS.9,25,26 Because most patients at our center undergo HCT for MDS with excess blasts, we hypothesized that KIR-based donor selection would still confer some benefit and extended our donor ranking process to patients with this diagnosis. Our results support registry-based conclusions that KIR-based donor selection does not confer a benefit to patients with MDS. Whether a subpopulation of MDS patients could benefit from the intervention is unclear, as small sample numbers precluded subcohort analysis. Similarly, the number of recipients undergoing allografts from an HLA 7/8-matched donor in this study were small. These patients were included in previous studies of KIR-based donor grouping tools.3,9,12,13 We elected to include these patients in this study, but there are too few to support a broad conclusion as to the benefit of KIR-based donor grouping in this specific population.
It should be noted that a number of large retrospective studies have not confirmed a relationship between protection from myeloid disease relapse and donor KIR genotype.10,25,27 Given that retrospective studies have yielded inconsistent results, multicenter studies are critical in the determination of the effectiveness of this tool.10 At least 2 prospective studies have been conducted to address this scientific question in HLA well-matched URD HCT.28 The first of these studies prioritized centromeric haplotype B content and has completed accrual.29 In that study (registered at www.clinicaltrials.gov as #NCT01288222) 2080 donors were evaluated for 535 subjects, of whom 247 subsequently underwent HCT. In the transplant recipients, 9.3% underwent HCT with a cenBB donor, and an additional 19% underwent HCT with a cenAB donor. Encouragingly, the authors noted no prolongation in the donor acquisition time between subjects who underwent HCT from donors who were KIR genotyped vs recipients from donors who were not KIR genotyped. The researchers recently reported a benefit of KIR B-haplotype donors in an enlarged cohort that included study subjects as well as patients previously treated at their center.16 The second multicenter prospective study (registered at www.clinicaltrials.gov as #NCT02450708) uses the same weighted, tiered system as the one presented here in patients receiving an allogeneic HCT from an HLA well-matched URD for the treatment of AML. Confirmation in a multicenter study that a KIR/HLA-based intervention in donor selection results in improved transplant outcomes will solidify the practice of incorporating KIR typing in URD selection for patients undergoing HCT for AML. In the interim, measures designed to eliminate barriers to selection of a KIR3DL1-Weak Inhibiting donor should be further explored in subsequent studies.
The authors welcome collaborations with respect to these data. For inquiries, please e-mail the corresponding author (hsuk@mskcc.org).
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant K23 HL140134-01A1 and National Center for Advancing Translational Sciences grant UL1-TR-002384 (B.C.S.); National Cancer Institute grant P01 CA23766, and National Institute of Allergy and Infectious Diseases grant U01 AI25651 (J.-B.L., S.P., and K.C.H.); and National Cancer Institute Cancer Center Support Grant P30 CA008748. The development of the KIR3DL1 multiplex PCR assay used for subtyping donors and the process for KIR- and HLA-based selection of hematopoietic stem cells was supported by the National Cancer Institute (CA2907068A1) (K.C.H.).
Authorship
Contribution: B.C.S., J.-B.L.L., K.C.H., A.A., C.C., E.D., M.N., B.S., D.W., R.T., E.P., A.A.J., S.G., and S.P. acquired the data; B.C.S., J.-B.L.L., S.D., and K.C.H. analyzed the data and prepared the manuscript with input from all authors; and K.C.H. conceived and designed the study.
Conflict-of-interest disclosure: K.C.H. has a patent application on the processes used for subtyping donors and the KIR- and HLA-based selection of hematopoietic stem cells used in this study. The remaining authors declare no competing financial interests.
Correspondence: Katharine C. Hsu, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY, 10065; e-mail: hsuk@mskcc.org.
References
Author notes
The full-text version of this article contains a data supplement.