Key Points
The MGUS transformation risk varied depending on the diagnosed RDs.
The risk of progression is doubled for MGUS patients with non-Ab–mediated RDs compared with those without concomitant RDs.
Abstract
Monoclonal gammopathy of undetermined significance (MGUS), a premalignant condition, is associated with various chronic inflammatory rheumatic diseases (RDs) and is frequently observed as an incidental finding during routine work-up. The association of MGUS and chronic RDs is well established, but the impact of RDs on the risk of transformation into overt multiple myeloma (MM) has not been evaluated so far. MGUS patients diagnosed between January 2000 and August 2016 were identified and screened for concomitant RDs. RDs were grouped into antibody (Ab)-mediated RDs and non-Ab–mediated RDs (polymyalgia rheumatica, large-vessel giant cell arteritis, spondyloarthritis, and gout). Progression to MM was defined as a categorical (yes/no) or continuous time-dependent (time to progression) variable. Of 2935 MGUS patients, 255 (9%) had a concomitant RD. MGUS patients diagnosed with non-Ab–mediated RDs had a doubled risk of progression compared with those without a concomitant RD (hazard ratio, 2.1; 95% CI, 1.1-3.9; P = .02). These data translate into a 5-year risk of progression of 4% in MGUS patients without rheumatologic comorbidity, 10% in those with concomitant non-Ab–mediated RDS, and 2% in those with Ab-mediated RDs. By using the complex risk stratification model that includes myeloma protein (M-protein) concentration, immunoglobulin type, and level of free light chain ratio as variables, patients with non-Ab–mediated RDs (n = 57) had the highest risk for progression (hazard ratio, 6.8; 95% CI, 1.5-30.7; P = .01) compared with patients with Ab-mediated RDs (n = 77). Chronic inflammatory diseases have an impact on the risk of MGUS progressing into overt MM, with a doubled risk of transformation observed in patients with non-Ab–mediated RDs. Future research can elucidate whether comorbidities such as RDs should be included in currently applied prognostic MGUS scores.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic condition characterized by an increase of the immunoglobulin (Ig) fraction with an inherent risk of progression to multiple myeloma (MM) or other related hematologic malignancies. According to epidemiological studies, MGUS affects up to 3.2% of people older than age 50 years,1 whereas the overall risk of progressing to MM or any other related diseases is 1% per year.2 Diagnosis of MGUS relies on serum levels of myeloma protein (M-protein) < 30 g/L and clonal cell fraction in bone marrow <10% as well as the exclusion of evident MM, other B‐cell proliferative disorders, or amyloidosis.3 However, at time of diagnosis, the prognosis and final outcome of this asymptomatic condition remain unclear. Main risk factors for progression in MGUS of non-IgG isotype are an M-protein concentration of at least 15 g/L and an abnormal serum free light chain ratio.4,5 It is assumed that most MM patients pass through an MGUS phase, which provides compelling evidence for MGUS as a precondition of MM.6
It is commonly accepted that many chronic inflammatory diseases increase the risk of cancer. For example, patients with ulcerative colitis carry a 2.4-fold risk of developing colorectal cancer compared with the general population, but the risk is significantly reduced when sustained disease is controlled by anti-inflammatory agents.7 The recent CANTOS trial, which was originally designed to confirm the beneficial effects of anti-interleukin-1β (anti-IL-1β) therapy in atherosclerosis, revealed a reduction of risk for lung cancer in patients treated with anti-IL-1β.8 Data are limited on how chronic inflammation contributes to the development of hematologic diseases, including the role of concomitant chronic inflammatory rheumatic diseases (RDs) on the process of MGUS transforming to MM. Notably, MGUS is more prevalent in patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) than in healthy individuals9-11 and is associated with the severity of the disease as well as an increased rate of other hematologic cancers.9,12-15 Moreover, persistent B-cell activation together with increased free light chain levels may lead to lymphoma and MM in primary Sjögren syndrome.16,17 Single studies or case series further emphasize a possible association of MGUS with gout, polymyalgia rheumatica (PMR), and ankylosing spondylitis (AS).11,18-22
Thus, it was the aim of this study to define the prevalence of concomitant chronic inflammatory RDs in a cohort of MGUS patients, determine the clinical outcome of MGUS with and without rheumatic comorbidity, and define the impact of RDs as a risk factor for progression to MM, AL amyloidosis, or Waldenström macroglobulinemia (WM).
Methods
Patients
In this retrospective study, all patients with a diagnosis of MGUS between January 2000 and August 2016 were screened for concomitant chronic inflammatory RDs by reviewing their medical records. MGUS was diagnosed according to the International Myeloma Working Group (IMWG) criteria3 with missing bone marrow evaluation being a limitation in most cases. RDs were diagnosed and treated according to currently available clinical practice by 3 experienced rheumatologists and stratified into the following 2 groups: (1) antibody (Ab)-mediated RDs (RA, connective tissue diseases including systemic lupus erythematosus, Sjögren syndrome, mixed connective tissue disease, systemic sclerosis, and antineutrophil cytoplasmic antibody [ANCA]–associated vasculitis) and (2) non-Ab–mediated RDs (PMR, large-vessel giant cell arteritis [LV-GCA], spondyloarthritis [SpA], and gout).
Overall survival (OS), progression-free survival (PFS), and risk factors for disease progression were compared between patients with MGUS and patients with MGUS with concomitant RDs (MGUS-RD). The study was approved by the local institutional ethics committee (vote number: 1144/2017) and was conducted in accordance with the principles of the Declaration of Helsinki.
Statistical analysis
Descriptive statistics were used to summarize the samples, laboratory measures, and disease outcomes stratified by patients with MGUS or MGUS-RD. All event summaries refer to the first hematologic malignancy (PFS) and death (OS). To evaluate differences between patients with MGUS or MGUS-RD, we used the χ2 test, the Mann-Whitney U test, and survival analysis (Kaplan-Meier curves, log-rank test). We calculated crude incidence rates as the number of events divided by the total number of person-years at risk after a diagnosis of MGUS with 95% confidence intervals (CIs) according to a Poisson distribution. We estimated unadjusted cumulative 1-year risks for mortality and progression defined as the probability of the event within the first year after an MGUS diagnosis. By using Cox proportional hazards models, we examined the hazard ratio (HR) associations between progression for patients with MGUS or MGUS-RD and potential risk factors. The time scale for calculation of the Cox proportional hazards models was months from MGUS diagnosis. For visualization and presentation within the tables, the time scale was changed to years. The proportional hazards assumption was tested by inspecting Kaplan-Meier curves and using Schoenfeld residuals. All tests for statistical significance were two-sided. P values < .05 were considered statistically significant, and for point estimators, we provide 95% CIs. Statistical evaluation was performed using SPSS version 24.0 statistical software (SPSS, Chicago, IL) and Stata version 14 (StataCorp, College Station, TX).
Results
Patients
Of 2935 patients with MGUS, 255 (9%) were identified as having a concomitant RD: 86 patients (34%) had a connective tissue disease or ANCA-associated vasculitis, 68 (27%) had RA, 47 (18%) had PMR or LV-GCA, 32 (13%) had gout, and 22 (9%) had SpA. The vast majority of MGUS-RD patients (62%) had an IgG MGUS, whereas other MGUS entities such as IgM in 19%, IgA in 11%, IgD in 0.4%, and light-chain only MGUS in 3% were less frequently observed. Table 1 provides a detailed overview of the patients’ characteristics.
Demographic data and laboratory features at diagnosis and comparison of the MGUS and MGUS-RD cohorts
| Parameter . | MGUS (n = 2680) . | MGUS-RD (n = 255) . | ||
|---|---|---|---|---|
| Median age (range), y | 68 (18-97) | 65 (25-92) | ||
| Sex* | ||||
| Female | 1117 | 42 | 46 | 57 |
| Male | 1563 | 58 | 109 | 43 |
| Ig heavy chain* | ||||
| IgG | 1808 | 68 | 158 | 62 |
| IgM | 491 | 18 | 48 | 19 |
| IgA | 209 | 8 | 28 | 11 |
| IgD | 0 | 0 | 1 | 0.4 |
| Biclonal gammopathy | ||||
| IgG + IgM | 92 | 3 | 9 | 4 |
| IgG + IgA | 35 | 1 | 4 | 1 |
| IgA + IgM | 5 | 0.2 | 0 | 0 |
| IgA + IgG + IgM | 2 | 0.1 | 0 | 0 |
| Light chain only | 38 | 1 | 7 | 3 |
| Ig light chain | ||||
| Kappa | 1531 | 57 | 136 | 53 |
| Lambda | 983 | 37 | 103 | 40 |
| Both | 153 | 6 | 13 | 5 |
| Not measurable | 13 | 0.5 | 3 | 1 |
| Lactate dehydrogenase >ULN | 679 | 27 | 56 | 24 |
| Hemoglobin ≤12 g/dL | 968 | 37 | 101 | 40 |
| Median M-protein (range), g/dL | 0.34 (0.01-2.89) | 0.35 (0.07-2.54) | ||
| Plasma cells in bone marrow | 619 | 44 | ||
| Median percentage (IQR) | 1.5 (0.5-3.0) | 2.0 (1.0-3.0) | ||
| C-reactive protein >ULN | 1484 | 57 | 159 | 63 |
| Positive rheumatoid factor* | 67 | 14 | 67 | 32 |
| Positive ACPA* | 5 | 2 | 38 | 25 |
| Positive ANCA* | 4 | 1 | 7 | 6 |
| Positive ANA* | 104 | 12 | 61 | 28 |
| MGUS with RD | ||||
| RA | 68 | 27 | ||
| SpA | 22 | 9 | ||
| Connective tissue diseases or ANCA-associated vasculitis | 86 | 34 | ||
| PMR/large vessel-giant cell arteritis | 47 | 18 | ||
| Gouty arthritis | 32 | 13 | ||
| Treatment received | ||||
| Yes | 225 | 88 | ||
| No | 30 | 12 | ||
| DMARDs | 15 | 6 | ||
| Biologic agents | 2 | 1 | ||
| GC | 64 | 25 | ||
| Biological agent + glucocorticoids | 4 | 2 | ||
| DMARDs + biologic agents | 2 | 1 | ||
| DMARDs + glucocorticoids | 74 | 29 | ||
| DMARDs + biologic agents + glucocorticoids | 29 | 11 | ||
| Others | 35 | 14 | ||
| Parameter . | MGUS (n = 2680) . | MGUS-RD (n = 255) . | ||
|---|---|---|---|---|
| Median age (range), y | 68 (18-97) | 65 (25-92) | ||
| Sex* | ||||
| Female | 1117 | 42 | 46 | 57 |
| Male | 1563 | 58 | 109 | 43 |
| Ig heavy chain* | ||||
| IgG | 1808 | 68 | 158 | 62 |
| IgM | 491 | 18 | 48 | 19 |
| IgA | 209 | 8 | 28 | 11 |
| IgD | 0 | 0 | 1 | 0.4 |
| Biclonal gammopathy | ||||
| IgG + IgM | 92 | 3 | 9 | 4 |
| IgG + IgA | 35 | 1 | 4 | 1 |
| IgA + IgM | 5 | 0.2 | 0 | 0 |
| IgA + IgG + IgM | 2 | 0.1 | 0 | 0 |
| Light chain only | 38 | 1 | 7 | 3 |
| Ig light chain | ||||
| Kappa | 1531 | 57 | 136 | 53 |
| Lambda | 983 | 37 | 103 | 40 |
| Both | 153 | 6 | 13 | 5 |
| Not measurable | 13 | 0.5 | 3 | 1 |
| Lactate dehydrogenase >ULN | 679 | 27 | 56 | 24 |
| Hemoglobin ≤12 g/dL | 968 | 37 | 101 | 40 |
| Median M-protein (range), g/dL | 0.34 (0.01-2.89) | 0.35 (0.07-2.54) | ||
| Plasma cells in bone marrow | 619 | 44 | ||
| Median percentage (IQR) | 1.5 (0.5-3.0) | 2.0 (1.0-3.0) | ||
| C-reactive protein >ULN | 1484 | 57 | 159 | 63 |
| Positive rheumatoid factor* | 67 | 14 | 67 | 32 |
| Positive ACPA* | 5 | 2 | 38 | 25 |
| Positive ANCA* | 4 | 1 | 7 | 6 |
| Positive ANA* | 104 | 12 | 61 | 28 |
| MGUS with RD | ||||
| RA | 68 | 27 | ||
| SpA | 22 | 9 | ||
| Connective tissue diseases or ANCA-associated vasculitis | 86 | 34 | ||
| PMR/large vessel-giant cell arteritis | 47 | 18 | ||
| Gouty arthritis | 32 | 13 | ||
| Treatment received | ||||
| Yes | 225 | 88 | ||
| No | 30 | 12 | ||
| DMARDs | 15 | 6 | ||
| Biologic agents | 2 | 1 | ||
| GC | 64 | 25 | ||
| Biological agent + glucocorticoids | 4 | 2 | ||
| DMARDs + biologic agents | 2 | 1 | ||
| DMARDs + glucocorticoids | 74 | 29 | ||
| DMARDs + biologic agents + glucocorticoids | 29 | 11 | ||
| Others | 35 | 14 | ||
All data are n (%), unless otherwise indicated.
ACPA, anti-citrullinated protein/peptide antibody; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; DMARDs, disease-modifying antirheumatic drugs; GC, glucocorticoids; ULN, upper limit of normal.
Indicates significant results of exact χ2 or Mann-Whitney U tests. A P value < .05 was considered statistically significant. No correction for multiple testing was applied.
Clinical outcome and survival analysis in MGUS patients with and without RDs
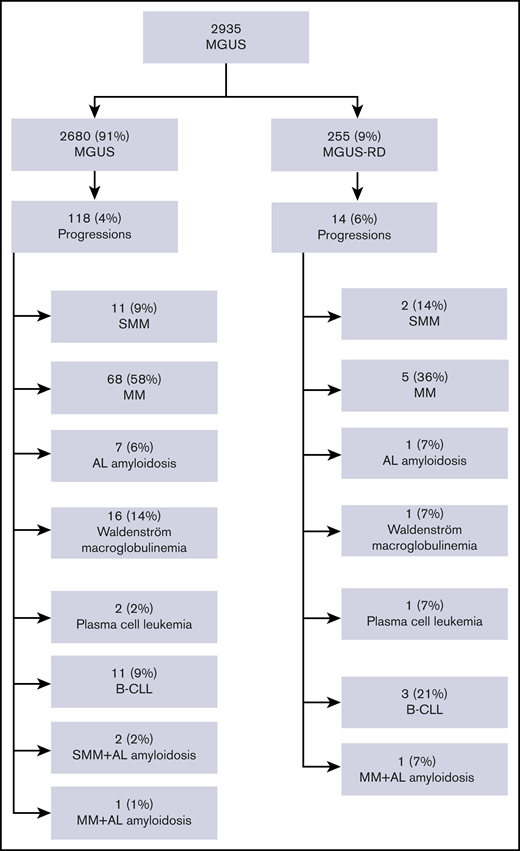
The overall follow-up time for the 2935 patients was 13.694 person-years with a median follow-up of 3.2 years (interquartile range [IQR], 0.7-7.2 years] and a maximum observation time of 33.7 years. Median time at risk was 4.6 years (IQR, 0.6-9.0 years) for MGUS-RD patients and 3.1 years (IQR, 0.6-7.0 years) for MGUS patients. Forty-eight MGUS-RD patients (19%) and 608 MGUS patients (23%) died during follow-up. Of the whole study cohort, 132 patients (4.5%) progressed. MM or other lymphoproliferative disorders were observed in 14 MGUS-RD patients (6%) and in 118 MGUS patients without rheumatic comorbidity (4%) (P = .3). In both MGUS cohorts, patients most frequently developed MM. A detailed overview of clinical outcome is depicted in Figure 1.
Study population overview with respect to transformation events. B-CLL, B-cell chronic lymphocytic leukemia; SMM, smoldering multiple myeloma.
Study population overview with respect to transformation events. B-CLL, B-cell chronic lymphocytic leukemia; SMM, smoldering multiple myeloma.
The median survival time in the overall cohort was 22 years. MGUS-RD patients revealed a significant superior OS when compared with MGUS patients (HR, 1.5; 95% CI, 1.1-2.0; P < .01). In all, 75% of the MGUS-RD patients survived 10.1 years, and 75% of the MGUS patients were alive after 5.6 years. The estimated risk for death within the first year after diagnosis was 7% (95% CI, 4%-12%) in the MGUS-RD group and 13% (95% CI, 11%-14%) in the MGUS group. Accordingly, the estimated 5-year mortality rate was significantly lower with 36% (95% CI, 25%-51%) in MGUS-RD patients compared with 64% (95% CI, 58%-70%) per 1000 person-years in MGUS patients.
Risk of progression in MGUS patients with and without RDs
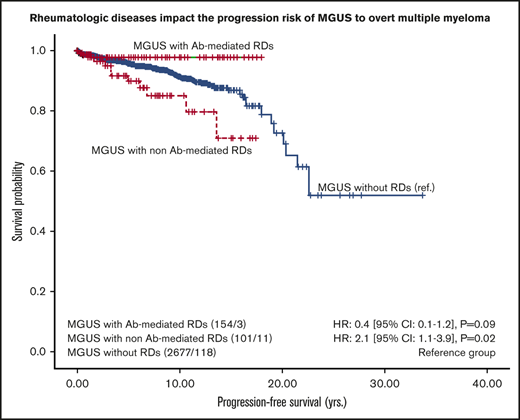
The progression rate during the whole follow-up period was 10 events per 1000 person-years (95% CI, 8.4-11.9 person-years), which translates as 132 progressions in 13 189 person-years at risk. Another possible interpretation: if 1000 people were observed for 1 year, there would be 10 progressions on average. By analyzing the Kaplan-Meier curves for different types of rheumatic patients, we identified 2 strata of MGUS patients who had different progression risk profiles: 101 MGUS patients with non-Ab–mediated RDs were at higher risk for progression compared with 154 MGUS patients with Ab-mediated RDs (11% vs 2%; P = .02). By using the univariable Cox regression model, patients with non-Ab–mediated RDs had a twofold higher risk of progression (HR, 2.1; 95% CI, 1.1-3.9; P = .02), whereas patients with Ab-mediated RDs had a 60% reduced risk of progression (HR, 0.4; 95% CI, 0.1-1.2), which did not reach statistical significance (P = .09; Figure 2). The 5-year progression rates were estimated to be 4% for MGUS patients without rheumatologic comorbidities, 10% for those with concomitant non-Ab–mediated RDs, and 2% for those with Ab-mediated RDs.
PFS of MGUS patients with and without RDs. PFS is given in years calculated from the time of MGUS diagnosis and stratified by MGUS vs MGUS-RD. RDs were grouped as follows: (1) Ab-mediated RDs (RA, connective tissue diseases such as systematic lupus erythematosus, Sjögren syndrome, mixed connective tissue disease, systemic sclerosis/ANCA-associated vasculitis) and (2) non-Ab–mediated RDs (including PMR, LV-GCA, SpA, and gout). The number of patients at risk, the number of events (eg, 154/3), and HRs [95% CIs] are shown.
PFS of MGUS patients with and without RDs. PFS is given in years calculated from the time of MGUS diagnosis and stratified by MGUS vs MGUS-RD. RDs were grouped as follows: (1) Ab-mediated RDs (RA, connective tissue diseases such as systematic lupus erythematosus, Sjögren syndrome, mixed connective tissue disease, systemic sclerosis/ANCA-associated vasculitis) and (2) non-Ab–mediated RDs (including PMR, LV-GCA, SpA, and gout). The number of patients at risk, the number of events (eg, 154/3), and HRs [95% CIs] are shown.
Risk factor analysis for estimating disease progression
MGUS patients with and without RDs were additionally evaluated for traditional clinical risk factors that impact the likelihood of disease progression. First, we evaluated the impact of the type of MGUS paraprotein. Compared with the IgG isotype group for the whole MGUS cohort as a reference (based on its high incidence), patients with light chain only disease faced a sixfold risk of progression (HR, 6.5; 95% CI, 2.9-14.4; P < .001) and those patients with IgA subtype had an HR of 1.9 (95% CI, 1.1-3.2; P = .03). The latter observation for IgA was similar in the MGUS-RD subgroup (HR, 2.9; 95% CI, 0.8-9.9; P = .09).
A more complex risk-stratification model that includes M-protein concentration, Ig type, and level of serum free light chain ratio as variables allowed patients to be segregated into 3 risk groups: the high- and intermediate-risk groups, which had at least 2 risk factors (n = 1470), had a twofold risk (HR, 2.0; 95% CI, 1.4-3.0; P < .001) for progressive disease compared with the low-risk group (all factors normal; n = 1445).
Progression was more likely among intermediate- and high-risk patients with MGUS-RD (n = 134) than among low-risk patients with MGUS-RD (n = 116) (HR, 9.6; 95% CI, 1.2-73.3; P = .03). Within the intermediate- and high-risk MGUS-RD cohorts, again the patient group with non-Ab–mediated RD (n = 57) had the highest risk for progression (HR, 6.8; 95% CI, 1.5-30.7; P = .01) compared with patients with Ab-mediated RD (n = 77). No significant difference between these groups was detected in the low-risk cohort. However, low-risk patients with MGUS-RDs had a 70% reduced risk for progressive disease compared with low-risk MGUS patients without RDs (HR, 0.3; 95% CI, 0.04-2.0; P = .21) (Figure 3).
PFS of low-risk vs intermediate-/high-risk MGUS patients irrespective of concomitant RD. PFS of patients with or without rheumatic impairment in years from MGUS diagnosis stratified by the risk group profile. The risk group profile is based on currently applied transformation risk scores that include the amount of M-protein, Ig type, and free light chain ratio. The number of patients at risk, the number of events (eg, 1329/33), and HRs [95% CIs] are shown.
PFS of low-risk vs intermediate-/high-risk MGUS patients irrespective of concomitant RD. PFS of patients with or without rheumatic impairment in years from MGUS diagnosis stratified by the risk group profile. The risk group profile is based on currently applied transformation risk scores that include the amount of M-protein, Ig type, and free light chain ratio. The number of patients at risk, the number of events (eg, 1329/33), and HRs [95% CIs] are shown.
M-protein concentration in MGUS patients without RDs was significantly associated with progressive disease (Plog rank < .001), whereas no association was found in the MGUS-RD group. Hematologic progression was independent from antibody status (rheumatoid factor, anti-citrullinated protein/peptide antibodies, antinuclear antibodies, ANCA) or other laboratory parameters (lactate dehydrogenase, C-reactive protein, hemoglobin).
Treatments within the MGUS patients with RDs
Of the 255 MGUS-RD patients who received a specific antirheumatic or anti-inflammatory treatment, 225 (88%) were treated with disease modifying antirheumatic drugs, biologic agents, glucocorticoids, and other drugs (eg, nonsteroidal anti-inflammatory drugs [NSAIDs]) in different combinations (Table 1). All patients with RA, 86% of patients with SpA, 80% of patients with connective tissue disease or ANCA-associated vasculitis, 87% of patients with PMR or LV-GCA, and 88% of patients with gout received specific anti-inflammatory medications. More treatment-naïve MGUS-RD patients progressed to hematologic malignancy when compared with patients receiving any therapy (HR, 2.0; 95% CI, 0.6-7.1; P = .3) or applied for the intermediate- and high-risk cohort (HR, 2.1; 95% CI, 0.6-7.8; P = .3). However, none of the comparisons reached statistical significance (supplemental Table 1).
To investigate whether different therapies may have an impact on the outcome of Ab-mediated RD patients vs non-Ab–mediated RD patients, we split these groups into additional subgroups (ie, treated and nontreated patients). This factor was not associated with time to progression in Ab-mediated RD (P = .5), but we did find a significant difference in the non-Ab–mediated RD group. The small subgroup of 13 patients without any therapy had a worse PFS when compared with the 88 patients who received treatment (P < .02). Furthermore, we found that 112 (73%) of 154 Ab-mediated RD patients compared with 60 (60%) of 101 non-Ab–mediated RD patients received glucocorticoids, which did not have an impact on progression time to overt myeloma (P = .8).
Discussion
Here we provide compelling evidence that chronic inflammatory RDs have an impact on the disease biology of MGUS by modulating the risk of transformation, which resulted in a twofold increased probability for the development of MM or related lymphoproliferative malignancies in non-Ab–mediated RDs when compared with the MGUS cohort without RDs. Our results fit well with recent advancements that emphasize chronic inflammation as a cancer risk factor23,24 and as an additional cancer hallmark.25 Furthermore, the relevance of MGUS for estimating disease activity and outcome in rheumatic inflammatory diseases is best reflected by its inclusion in the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) and lymphoma risk stratification models in primary Sjögren syndrome.26
MGUS is a well-described premalignant condition that has a 1% annual risk for transformation to overt MM.2 The 5-year risk for progression in the MGUS group without RD presented here was 4%, which is in line with previous reports.2 In contrast, MGUS patients who have concomitant non-Ab–mediated RDs revealed a 5-year risk of progression of 10%, indicating a twofold risk of progression to MM or related disorders. Although the association of MGUS and chronic inflammatory diseases is well established, the impact of concomitant RDs on the outcome of MGUS remains elusive so far.
MM patients who had previously experienced MGUS showed an increased rate of comorbidities compared with those who were first diagnosed with MM, reflecting the fact that MGUS is most often diagnosed during work-up for an unrelated disease.27 This holds true for patients with chronic inflammatory RDs in whom MGUS is most frequently diagnosed as an incidental finding during follow-up.11 A recently published large population-based study from Sweden revealed a 1.2- to 1.4-fold increased risk of death in MM and MGUS patients with a previous history of autoimmune disease.28 Focusing on the different autoimmune diseases included, the excess mortality was confirmed only for a previous history of ulcerative colitis, whereas in other autoimmune conditions, a mortality rate equal to or even lower than that found in controls was observed. In addition, the results in our study were inconsistent, because MGUS patients with concomitant Ab-mediated RDs had a protective effect with a 60% reduced 5-year risk of progression; consequently, we observed an equal OS rate for the 3 different MGUS groups.
The sensitivity analyses that focused on risk stratification models used for MGUS revealed the highest risk for progression in non-Ab–mediated RD patients within the intermediate- and high-risk MGUS cohorts, confirming the robustness of our data. We decided to stratify our MGUS patients with concomitant RDs into those with Ab-mediated and non-Ab–mediated RDs based on a pathogenetic point of view, although different RD entities were summarized. At this time, we can only speculate on possible reasons for these diverging results: one explanation might be that in Ab-associated RDs the paraproteinemia is essentially assembled by the excess amount of circulating disease-related antibodies, whereas in non-Ab–associated RDs, the paraproteinemia results from a true clonal outgrowth of pathological plasma cells. It is well established that autoantibodies are contained within the gamma peak of the serum electrophoresis, but the proportional extent has not yet been addressed systematically in Ab-mediated RDs. In a historical case series on the association of RA with plasma cell and lymphocytic neoplasms, there was a question of whether the M-protein may possess antibody activity.29 In addition, the increased risk of progression in non-Ab–mediated RDs may be related to shared underlying common genetic factors or chronically immune-driven mechanisms that result in more severe cases of MM and MGUS.
Indeed, IL-1β, one of the main drivers in inflammation,30 plays a critical role in the pathogenesis of PMR,31,32 gouty arthritis, and SMM.33,34 The central role of IL-1β is underpinned by the drastic therapeutic efficacy of IL-1β blocking therapies in gouty arthritis and also in smoldering multiple myeloma,35-37 creating a possible link between these diseases and our results. A Swedish population-based registry study also demonstrated increased risks of 2.6 and 7.7 for the development of MM in patients with a history of PMR and LV-GCA, respectively,11 but subanalyses from another register study demonstrated no excess mortality in MGUS and MM patients with concomitant PMR or LV-GCA.28 Although different outcome parameters were addressed (MGUS progression to MM vs MM-associated excess mortality), the discrepancy might rely on the fact that data from the latter study retrieved patients with autoimmune diseases by searching the Swedish Inpatients Register, which might reflect only the subgroup of severely ill patients but not the whole spectrum of diseased patients. In line with our results, ankylosing spondylitis was associated with an overall higher risk of 1.8 for MM in a meta-analysis,38 whereas data are lacking on gouty arthritis and its linkage to MGUS and MM.
In our study, MGUS-RD patients revealed a significant superior OS when compared with MGUS patients. Close follow-up of MGUS patients because of concomitant diseases such as RDs positively influenced the disease course regarding the outcome of MM27 because of early diagnosis and medical interference, which might also explain our findings.
The influence of immunomodulatory therapies on the prevalence and outcome of MGUS and MM is still a matter of debate.39 Although case series raised concerns regarding an increased risk for the development of MGUS or MM under treatment with tumor necrosis factor (TNF) inhibitors,40 a cross-sectional cohort study that included psoriasis and psoriatic arthritis patients demonstrated no difference between patients treated with tumor necrosis factor blocker and those treated with conventional disease modifying antirheumatic drugs.41 In various clinical settings (eg, Schnitzler syndrome, RA, and concomitant MGUS or MM), different biologicals (anakinra, abatacept, and tocilizumab) seemed to be safe, and they had a stabilizing effect on paraproteinemia or plasma cell dyscrasia.42-44 In addition, in our MGUS-RD cohort, intermediate- and high-risk patients who received any rheumatic therapy seem to be stable and have less frequently observed progression. However, our evaluation of the influence of treatments on the risk of progression was limited by small sample size, and the results did not reach statistical significance.
A standardized treatment strategy for MGUS-RD patients has not yet been established. The primary goal for these patients should be suppression of both the RD activity and the monoclonal Ig concentration. Whether refining the currently applied MGUS risk stratification model4,5 by incorporating rheumatologic comorbidities is warranted must be addressed by future research.
We observed a prevalence of 9% of patients with concomitant RD within an MGUS cohort from a tertiary care university hospital, which corresponds to the reported MGUS prevalence of 7% in RD patients from a Swedish Registry study.11 But contrasts with the described 21% prevalence of autoimmune diseases among MGUS patients in another registry study that focused on chronic inflammatory RDs and also included other autoimmune disorders such as inflammatory bowel disease and pernicious anemia did not allow a direct comparison.28
Our study is limited by the retrospective design and incomplete characterization of MGUS-RD patients. Data on medical history and disease-related outcome parameters were lacking. Thus, it was not possible to evaluate the potential influence of disease duration and activity on the outcome or risk of progression of MGUS.
In conclusion, we found a high prevalence of concomitant chronic inflammatory RDs in MGUS patients. The risk of progression varied depending on which RDs were diagnosed, and patients with non-Ab–mediated RDs had doubled risk of transformation and a 5-year risk of progression. Future studies are necessary to further elucidate the impact of proinflammatory processes and immunosuppressive therapies on how MGUS evolves and its risk of progression.
Presented as a poster at the 2019 Annual Meeting of the German Society for Hematology and Medical Oncology, Berlin, Germany, 11-15 October 2019.
Request data via e-mail to Normann Steiner at normann.steiner@i-med.ac.at.
Authorship
Contribution: N.S., A.-L.P., and W.P. identified the patients from the local database; N.S., C.D., D.M., and G.G. analyzed and interpreted the data; N.S., C.D., D.M., A.-L.P., G.G., A.M.W., D.W., and E.G. wrote the paper; and G.G. performed the statistical evaluation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Normann Steiner, Department of Internal Medicine V, Haematology and Medical Oncology, Medical University of Innsbruck, Anichstr 35, A-6020 Innsbruck, Austria; e-mail: normann.steiner@i-med.ac.at.
References
Author notes
N.S., G.G., C.D., and E.G. contributed equally to this work.
The full-text version of this article contains a data supplement.

![PFS of MGUS patients with and without RDs. PFS is given in years calculated from the time of MGUS diagnosis and stratified by MGUS vs MGUS-RD. RDs were grouped as follows: (1) Ab-mediated RDs (RA, connective tissue diseases such as systematic lupus erythematosus, Sjögren syndrome, mixed connective tissue disease, systemic sclerosis/ANCA-associated vasculitis) and (2) non-Ab–mediated RDs (including PMR, LV-GCA, SpA, and gout). The number of patients at risk, the number of events (eg, 154/3), and HRs [95% CIs] are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/6/10.1182_bloodadvances.2020003193/2/m_advancesadv2020003193f2.png?Expires=1770895068&Signature=zeqKRHXTG21X8ey05e9UlorW8gPO88Bim2V0s2ZyoFCsNBdBSoByxRIGKhMDNvVtbIRHYhdROeLf6S1sG8w5Atq9KIpCO3FJd1lr-KxqXJYUtaq0fn~hk4-8MWv4Bo3N~ycQ80~5t5dICpIPaweFS~yc3G9h~xBaDL7N2siKBjKcxihJ~1v71zE1h7qH8p4NwoAfy1wP1oqzBV6wA0vXcVmt37r2jdn~ODNZ-XmhZV9f~Mzn4pdfRabCE-G2mkiFl6NfbcPhzXcEYHINxrpioqRgTOZosRDT0WHpdc2aC3FJqNHGlxiZgAmBXGzH2pYNZHuwNk~92RlE3gfCjpn-yw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![PFS of low-risk vs intermediate-/high-risk MGUS patients irrespective of concomitant RD. PFS of patients with or without rheumatic impairment in years from MGUS diagnosis stratified by the risk group profile. The risk group profile is based on currently applied transformation risk scores that include the amount of M-protein, Ig type, and free light chain ratio. The number of patients at risk, the number of events (eg, 1329/33), and HRs [95% CIs] are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/6/10.1182_bloodadvances.2020003193/2/m_advancesadv2020003193f3.png?Expires=1770895068&Signature=L~jHusFYSQz4ZwUywafkaQmhgvY-YTraJaLkiWk7FtNsZoXE97OPZKvMs~yhuOX6XrxwOwyrsHTJ5DXtH7KlkR23G6mh0ul1yIg7KtQsLdU0XN6UeaDTHJpfOmF3~kEBMv17beR~yBVnvVKJSfbIXgm32ABlkSMkGnKgdds9hUiFYcKV9mLJTdGsxExCyK2WAndv8S-pzvh~41ggDiYcxc6z6chCUA3eyiM1wHck5QtTaotvPOdW3ZRwetK75K64Li69v~2EgbNHYMeGpLVuIpnBvppTyKfJjk9vRwKOqwEG5dhF2zFutVM9J3OeTqZ84AxfGCR8ganIjxd42Rzcvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)