Key Points
Older patients with FL have similar early disease outcomes to younger patients.
Age alone should not disqualify older FL patients from standard treatments or RCTs.
Abstract
Limited data exist to describe the clinical features and outcomes for elderly patients with follicular lymphoma (FL). The Follicular Lymphoma Analysis of Surrogacy Hypothesis (FLASH) group performed a prospectively planned pooled analysis of individual patient data from first-line randomized controlled trials (RCTs) and examined associations between age (≤70 vs >70 years), clinical characteristics, and FL outcomes. We identified 18 multicenter clinical RCTs in the FLASH database that enrolled elderly patients (>70 years). Primary end points were early disease outcomes, CR24 and CR30, and progression-free survival (PFS) at 24 months (PFS24). Secondary end points were PFS and overall survival (OS). We identified 5922 previously untreated FL patients from 18 RCTs. Patients age >70 years (vs ≤70 years) more commonly had elevated lactate dehydrogenase, hemoglobin <12 g/dL, ECOG PS ≥2, and elevated β2-microglobulin. Median follow-up was 5.6 years. Patients >70 years did not differ from patients ≤70 years in rates of CR24, CR30, or PFS24. With a median OS of 14.6 years for all patients, median OS was 7.4 and 15.7 years for patients >70 and ≤70 years of age, respectively (hazard ratio = 2.35; 95% confidence interval = 2.03-2.73; P < .001). Age >70 years was a significant predictor of OS and PFS due to higher rates of death without progression, but not PFS24, CR24, or CR30. FL patients >70 years treated on trials have similar early disease outcomes to younger patients. There is no disease-specific outcome difference between age groups. Age alone should not disqualify patients from standard treatments or RCTs.
Introduction
Follicular lymphoma (FL) is among the most common forms of indolent non-Hodgkin lymphoma (NHL), with an estimated 14 000 new cases diagnosed in the United States in 2016. FL increases with age, with a median age at diagnosis in the seventh decade.1 Older adults comprise the majority of FL diagnoses, and are more likely to have competing comorbid conditions influencing treatment selection, drug metabolism, tolerance to therapy and treatment related complications. Age also is an adverse prognostic marker in NHL, affecting therapeutic outcome and subsequent survival.2,3 Because most chemotherapy trials in FL have included primarily younger patients, the impact of age on disease progression or treatment success is not fully understood. However, some data suggest that older age is not associated with higher-risk disease or inferior efficacy of therapy.4
Use of the anti-CD20 antibody rituximab either alone or in combination with chemotherapy has led to dramatically improved survival in FL.5-7 Concurrently, advances in the molecular, genetic, and clinical characterization of FL have improved the understanding of prognosis in various subgroups, such as those with early relapse, transformation, and refractory disease.8,9 Early disease recurrence is a robust marker of poor survival in FL, and is of particular importance to describe in older patients, who have fewer aggressive treatment options available at the time of early relapse.10,11 Given the long natural history of FL, and the current unprecedented growth of the population aged 65 years and older in the United States and Europe, a deeper understanding of disease-specific outcomes for older patients with FL is required to identify unmet needs in treatment efficacy and tolerability to optimize outcomes and quality of life (per US Census Bureau population projections).
To this end, the Follicular Lymphoma Analysis of Surrogacy Hypothesis (FLASH) group performed a prospectively planned pooled analysis of individual patient data (IPD) from first-line randomized controlled trials (RCTs) to examine the associations between age (>70 vs ≤70 years), clinical characteristics, and FL outcomes to assess disease-related morbidity, treatment response, and survival in older patients with FL.
Methods
Details of the FLASH analysis are previously published.11 Patients were included if they had untreated FL enrolled in 1 of the 21 randomized, multicenter clinical trials included in the FLASH database. We excluded studies that did not enroll any older patients (>70 years). We identified 18 randomized multicenter studies in FLASH that enrolled older patients (>70 years). From these 18 studies, 5922 previously untreated FL patients were included for this analysis. Age was determined at trial enrollment. We also analyzed a subgroup of 3450 patients who received treatments containing rituximab.
Primary end points were early disease outcomes, complete response (CR) at 24 (CR24) and 30 (CR30) months, and progression-free survival (PFS) at 24 months (PFS24). CR30 has been previously validated as a surrogate end point for PFS in FL in the pivotal FLASH analysis,11 whereas CR24 demonstrated strong patient-level correlation but fell short of predefined surrogacy criteria for trial-level correlation (demonstrated in a post hoc sensitivity analysis). Secondary end points were PFS and overall survival (OS). CR24 and CR30 were defined as whether the patient had a disease response of CR at 24 months and 30 months after enrollment. PFS24 was defined as the proportion of patients progression-free and alive 24 months after enrollment. OS was defined as time from enrollment to the date of death due to any cause. PFS was defined as time from enrollment to the date of progression or death, due to any cause, whichever came first.
Patient characteristics were summarized by age group, and the χ2 test was used to test for differences between the 2 groups. For binary outcomes (CR24 and CR30), generalized estimation equations (GEEs) with logit link and compound symmetry working correlation were used to take into account the correlation between patients within the same trial while adjusting for potential confounders. For time-to-event outcomes, the Kaplan-Meier method and log-rank test were used for univariate estimation and comparison; Cox proportional hazard modeling stratified by trial was used for multivariable analyses. Cumulative incidence function (CIF) was used to model time to progression while treating death without prior disease progression as a competing risk, and to model time to death after disease progression while treating death without prior disease progression as a competing risk using the Fine and Gray model.12 The Gray k-sample test13 was used to evaluate differences between treatment groups. For PFS24, direct adjusted survival probabilities and standard errors for both age groups were calculated at 24 months based on stratified Cox regression models. These probabilities were then compared using a 2-proportion z-test with pooled standard error. The variables adjusted in these models included the Follicular Lymphoma International Prognostic Index (FLIPI) score without the age component, Eastern Cooperative Oncology Group Performance Status (ECOG PS; ≥2 vs <2), and rituximab use.
Results
We identified 5922 patients with previously untreated FL from 18 RCTs (Table 1). Trial characteristics are noted in Figure 1. Patient characteristics are noted in Table 2. A majority of patients (63.0%; n = 3728) were ≤60 years of age, 27.9% were 61 to 70 years (n = 1652), 8.8% were 71 to 80 years (n = 523), and 0.3% were >80 years (n = 19). Patients age >70 years (vs ≤70 years) more commonly had elevated LDH (42% vs 36%; P = .0159), hemoglobin <12 g/dL (27% vs 19%; P < .001), ECOG PS ≥2 (8.8% vs 5.0%; P < .001), and elevated β2-microglobulin (68% vs 49%; P < .001). Less often, they had ≥5 lymph nodes involved (54% vs 65%; P < .001), and had similar FLIPI scores without the age component (P = .172). There were no major differences between groups in Ann Arbor stage (94% vs 95% stage III/IV; P = .604) or rituximab use (62% vs 58%; P = .090).
Studies included
| Reference, year . | Study name . | Regimen used . | Total, N . | Elderly, % . |
|---|---|---|---|---|
| Peterson et al,16 2003 | CALGB7951 | CTX vs CHOP-B | 189 | 7.4 |
| Hochster et al,17 2009 | E1496 | MR vs Obs after CVP | 285 | 15.1 |
| Hagenbeek et al,18 2006 | EORTC20921 | Fludarabine vs CVP | 231 | 6.9 |
| Salles et al,19 2008 | FL2000 | CHVP+I vs R-CHVP+I | 358 | 11.2 |
| Solal-Celigny et al,20 1993; Solal-Celigny et al,21 1998; Bachy et al,22 2010 | GELF862 | CHVP vs CHVP+I | 242 | 0.8 |
| Ladetto et al,23 2008 | GITMO | R-HDS vs R-CHOP | 134 | 0.7 |
| Nickenig et al,24 2006 | GLSG1996 | CHOP vs MCP | 536 | 8.0 |
| Hiddemann et al,25 2005 | GLSG2000 | CHOP vs R-CHOP | 1040 | 8.6 |
| Salles et al,26 2008 | GOELAMS052 | CHVP vs FM | 85 | 17.6 |
| Marcus et al,27 2008 | M39021 | CVP vs CVP+R | 322 | 7.5 |
| Herold et al,28 2007 | OSHO39 | MCP vs R-MCP | 207 | 5.3 |
| Kimby et al,29 2015 | ML16865 | R vs R+IFN | 228 | 11.8 |
| Vitolo et al,30 2013 | ML17638 | R vs Obs after R-FND | 234 | 17.5 |
| Morschhauser et al,31 2008 | FIT | 90Y-ibritumomab tiuxetan vs Obs for consolidation therapy | 414 | 5.1 |
| Herold et al,32 2006 | OSHO19 | BOP vs CVP | 75 | 12.0 |
| Salles et al,33 2011 | PRIMA | R vs Obs maintenance | 1018 | 9.8 |
| Ghielmini et al,34 2004; Martinelli et al,35 2010 | SAKK35/98 | Therapy vs Obs after R | 45 | 8.9 |
| Rummel et al,36 2013 | NHL12003 | B-R vs R-CHOP | 279 | 15.1 |
| Reference, year . | Study name . | Regimen used . | Total, N . | Elderly, % . |
|---|---|---|---|---|
| Peterson et al,16 2003 | CALGB7951 | CTX vs CHOP-B | 189 | 7.4 |
| Hochster et al,17 2009 | E1496 | MR vs Obs after CVP | 285 | 15.1 |
| Hagenbeek et al,18 2006 | EORTC20921 | Fludarabine vs CVP | 231 | 6.9 |
| Salles et al,19 2008 | FL2000 | CHVP+I vs R-CHVP+I | 358 | 11.2 |
| Solal-Celigny et al,20 1993; Solal-Celigny et al,21 1998; Bachy et al,22 2010 | GELF862 | CHVP vs CHVP+I | 242 | 0.8 |
| Ladetto et al,23 2008 | GITMO | R-HDS vs R-CHOP | 134 | 0.7 |
| Nickenig et al,24 2006 | GLSG1996 | CHOP vs MCP | 536 | 8.0 |
| Hiddemann et al,25 2005 | GLSG2000 | CHOP vs R-CHOP | 1040 | 8.6 |
| Salles et al,26 2008 | GOELAMS052 | CHVP vs FM | 85 | 17.6 |
| Marcus et al,27 2008 | M39021 | CVP vs CVP+R | 322 | 7.5 |
| Herold et al,28 2007 | OSHO39 | MCP vs R-MCP | 207 | 5.3 |
| Kimby et al,29 2015 | ML16865 | R vs R+IFN | 228 | 11.8 |
| Vitolo et al,30 2013 | ML17638 | R vs Obs after R-FND | 234 | 17.5 |
| Morschhauser et al,31 2008 | FIT | 90Y-ibritumomab tiuxetan vs Obs for consolidation therapy | 414 | 5.1 |
| Herold et al,32 2006 | OSHO19 | BOP vs CVP | 75 | 12.0 |
| Salles et al,33 2011 | PRIMA | R vs Obs maintenance | 1018 | 9.8 |
| Ghielmini et al,34 2004; Martinelli et al,35 2010 | SAKK35/98 | Therapy vs Obs after R | 45 | 8.9 |
| Rummel et al,36 2013 | NHL12003 | B-R vs R-CHOP | 279 | 15.1 |
BOP, bleomycin, vincristine, and cisplatin; B-R, bendamustine and rituximab; CALGB, Cancer and Leukemia Group B; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CHOP-B, cyclophosphamide, doxorubicin, vincristine, prednisone, and bleomycin; CHVP, cyclophosphamide, Adriamycin (doxorubicin), etoposide, and prednisolone; CHVP+I, cyclophosphamide, Adriamycin, etoposide, and prednisolone plus interferon; CTX, cyclophosphamide; CVP, cyclophosphamide, vincristine, and prednisone; CVP+R, cyclophosphamide, vincristine, and prednisone plus rituximab; EORTC, European Organisation for Research and Treatment of Cancer; FIT, First-Line Indolent Trial; FL, Follicular Lymphoma; FM, fludarabine and mitoxantrone; GELF, Groupe d’Etude Lymphomes Folliculaire; GITMO, Gruppo Italiano Trapianti di Midollo Osseo; GLSG, German Low Grade Lymphoma Study Group; GOELAMS, Groupe Ouest-Est des Leucémies Aiguës et Maladies du Sang; MCP, mitoxantrone, chlorambucil, and prednisolone; MR, maintenance rituximab; NHL, Non-Hodgkin Lymphoma; Obs, observation; OSHO, Ostdeutsche Studiengruppe Haematologie/Oncologie; PRIMA, Primary Rituximab and Maintenance; R, rituximab; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHVP + I, rituximab, cyclophosphamide, Adriamycin, etoposide, and prednisolone plus interferon; R-FND, rituximab, fludarabine, mitoxantrone, and dexamethasone; R-HDS, high-dose sequential chemotherapy with rituximab; R+IFN, rituximab plus interferon; R-MCP, rituximab, mitoxantrone, chlorambucil, and prednisolone; SAKK, Swiss Group for Clinical Cancer Research.
Patient characteristics
| . | Age ≤70 y, N = 5380 . | Age >70 y, N = 542 . | Total, N = 5922 . | P . |
|---|---|---|---|---|
| Age, y | <.0001* | |||
| Mean (SD) | 53.7 (10.10) | 74.0 (3.00) | 55.6 (11.29) | |
| Median (range) | 55.0 (17.8, 70.0) | 73.2 (70.0, 90.1) | 56.3 (17.8, 90.1) | |
| Sex, n (%) | .0042† | |||
| Female | 2661 (49.5) | 303 (55.9) | 2964 (50.1) | |
| Male | 2719 (50.5) | 239 (44.1) | 2958 (49.9) | |
| ECOG PS, n (%) | .0004† | |||
| 0-1 | 4299 (95.0) | 444 (91.2) | 4743 (94.6) | |
| ≥2 | 227 (5.0) | 43 (8.8) | 270 (5.4) | |
| Missing | 854 | 55 | 909 | |
| FLIPI, n (%) | <.0001† | |||
| Low | 989 (21.8) | 12 (2.6) | 1001 (20.0) | |
| Intermediate | 1753 (38.7) | 124 (26.7) | 1877 (37.5) | |
| High | 1792 (39.5) | 329 (70.8) | 2121 (42.4) | |
| Missing | 846 | 77 | 923 | |
| FLIPI score without age, n (%) | .17† | |||
| 0 | 145 (3.4) | 12 (2.8) | 157 (3.4) | |
| 1 | 1148 (27.1) | 115 (26.9) | 1263 (27.1) | |
| 2 | 1786 (42.1) | 163 (38.2) | 1949 (41.8) | |
| 3 | 922 (21.7) | 103 (24.1) | 1025 (22.0) | |
| 4 | 240 (5.7) | 34 (8.0) | 274 (5.9) | |
| Missing | 1139 | 115 | 1254 | |
| Ann Arbor stage, n (%) | .60† | |||
| I/II | 273 (5.2) | 31 (5.7) | 304 (5.3) | |
| III/IV | 4960 (94.8) | 509 (94.3) | 5469 (94.7) | |
| Missing | 147 | 2 | 149 | |
| Nodal sites, n (%) | <.0001† | |||
| <5 | 1248 (35.0) | 171 (45.6) | 1419 (36.0) | |
| ≥5 | 2314 (65.0) | 204 (54.4) | 2518 (64.0) | |
| Missing | 1818 | 167 | 1985 | |
| LDH at baseline, n (%) | .0159† | |||
| >ULN | 1394 (35.7) | 164 (41.8) | 1558 (36.3) | |
| ≤ULN | 2511 (64.3) | 228 (58.2) | 2739 (63.7) | |
| Missing | 1475 | 150 | 1625 | |
| HGB at baseline, n (%) | <.0001† | |||
| ≥12 g/dL | 3309 (81.4) | 300 (72.8) | 3609 (80.6) | |
| <12 g/dL | 756 (18.6) | 112 (27.2) | 868 (19.4) | |
| Missing | 1315 | 130 | 1445 | |
| β-2 at baseline, n (%) | <.0001† | |||
| >ULN | 928 (48.5) | 120 (67.8) | 1048 (50.2) | |
| ≤ULN | 984 (51.5) | 57 (32.2) | 1041 (49.8) | |
| Missing | 3468 | 365 | 3833 | |
| Rituximab, n (%) | .0898† | |||
| No rituximab | 2236 (41.8) | 204 (38.0) | 2440 (41.4) | |
| Rituximab | 3117 (58.2) | 333 (62.0) | 3450 (58.6) | |
| Missing | 27 | 5 | 32 |
| . | Age ≤70 y, N = 5380 . | Age >70 y, N = 542 . | Total, N = 5922 . | P . |
|---|---|---|---|---|
| Age, y | <.0001* | |||
| Mean (SD) | 53.7 (10.10) | 74.0 (3.00) | 55.6 (11.29) | |
| Median (range) | 55.0 (17.8, 70.0) | 73.2 (70.0, 90.1) | 56.3 (17.8, 90.1) | |
| Sex, n (%) | .0042† | |||
| Female | 2661 (49.5) | 303 (55.9) | 2964 (50.1) | |
| Male | 2719 (50.5) | 239 (44.1) | 2958 (49.9) | |
| ECOG PS, n (%) | .0004† | |||
| 0-1 | 4299 (95.0) | 444 (91.2) | 4743 (94.6) | |
| ≥2 | 227 (5.0) | 43 (8.8) | 270 (5.4) | |
| Missing | 854 | 55 | 909 | |
| FLIPI, n (%) | <.0001† | |||
| Low | 989 (21.8) | 12 (2.6) | 1001 (20.0) | |
| Intermediate | 1753 (38.7) | 124 (26.7) | 1877 (37.5) | |
| High | 1792 (39.5) | 329 (70.8) | 2121 (42.4) | |
| Missing | 846 | 77 | 923 | |
| FLIPI score without age, n (%) | .17† | |||
| 0 | 145 (3.4) | 12 (2.8) | 157 (3.4) | |
| 1 | 1148 (27.1) | 115 (26.9) | 1263 (27.1) | |
| 2 | 1786 (42.1) | 163 (38.2) | 1949 (41.8) | |
| 3 | 922 (21.7) | 103 (24.1) | 1025 (22.0) | |
| 4 | 240 (5.7) | 34 (8.0) | 274 (5.9) | |
| Missing | 1139 | 115 | 1254 | |
| Ann Arbor stage, n (%) | .60† | |||
| I/II | 273 (5.2) | 31 (5.7) | 304 (5.3) | |
| III/IV | 4960 (94.8) | 509 (94.3) | 5469 (94.7) | |
| Missing | 147 | 2 | 149 | |
| Nodal sites, n (%) | <.0001† | |||
| <5 | 1248 (35.0) | 171 (45.6) | 1419 (36.0) | |
| ≥5 | 2314 (65.0) | 204 (54.4) | 2518 (64.0) | |
| Missing | 1818 | 167 | 1985 | |
| LDH at baseline, n (%) | .0159† | |||
| >ULN | 1394 (35.7) | 164 (41.8) | 1558 (36.3) | |
| ≤ULN | 2511 (64.3) | 228 (58.2) | 2739 (63.7) | |
| Missing | 1475 | 150 | 1625 | |
| HGB at baseline, n (%) | <.0001† | |||
| ≥12 g/dL | 3309 (81.4) | 300 (72.8) | 3609 (80.6) | |
| <12 g/dL | 756 (18.6) | 112 (27.2) | 868 (19.4) | |
| Missing | 1315 | 130 | 1445 | |
| β-2 at baseline, n (%) | <.0001† | |||
| >ULN | 928 (48.5) | 120 (67.8) | 1048 (50.2) | |
| ≤ULN | 984 (51.5) | 57 (32.2) | 1041 (49.8) | |
| Missing | 3468 | 365 | 3833 | |
| Rituximab, n (%) | .0898† | |||
| No rituximab | 2236 (41.8) | 204 (38.0) | 2440 (41.4) | |
| Rituximab | 3117 (58.2) | 333 (62.0) | 3450 (58.6) | |
| Missing | 27 | 5 | 32 |
ECOG PS, Eastern Cooperative Oncology Group; HGB, hemoglobin; LDH, lactate dehydrogenase; SD, standard deviation; ULN, upper limit of normal.
Kruskal-Wallis P value.
χ2P value.
Rates of CR24 (29.6% vs 32.3%) and CR30 (31.8% vs 34.3%) differed only slightly between patients >70 years and patients ≤70 years. GEE models adjusted for FLIPI (without age), PS, and rituximab use did not show significant differences in odds of achieving CR24 (odds ratio [OR] = 0.80; 95% confidence interval [CI] = 0.61-1.06; P = .119) or CR30 (OR = 0.80; 95% CI = 0.61-1.05; P = .109) between patients >70 years and patients ≤70 years. Rates for CR24 (38.5% vs 39.9%) and CR30 (41.6% vs 43.2%) were comparably similar between patients >70 years and patients ≤70 years for the subset of patients who were treated with regimens containing rituximab. Adjusted GEE models for CR24 (OR = 0.91; 95% CI = 0.72-1.16; P = .439) and CR30 (OR = 0.90; 95% CI = 0.73-1.10; P = .311) also remained consistent when looking only at patients who received rituximab.
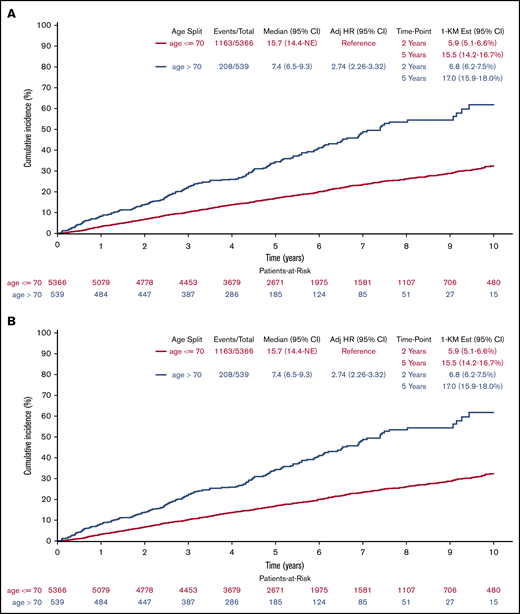
PFS (Figure 2A) was shorter (log-rank P < .001), but clinically similar in patients >70 years of age when compared with patients ≤70 years with medians of 3.1 years (95% CI = 2.7-3.5) and 3.8 years (3.6-4.0), respectively. Results remained consistent after multivariable adjustment (HR = 1.32; 95% CI = 1.15-1.53; P < .001). Using CIF methods (Figure 2B) with disease progression as primary event of interest and death without prior disease progression as a competing risk, cumulative incidence of progression between age groups no longer differed in both univariate (Gray k-sample P = .965) and multivariable (HR = 1.01; 95% CI = 0.85-1.19; P = .942) analyses, with medians of 4.2 years (95% CI = 3.5-5.1) and 4.2 years (95% CI = 4.0-4.6), respectively, for patients >70 years and patients ≤70 years; the cumulative incidence of death without prior disease progression differed in both univariate (Gray k-sample P < .001) and multivariable analyses (HR = 4.45; 95% CI = 3.05-6.48; P < .001) with medians not reached for either age group. Results for the rituximab subgroup remained consistent, with the total analysis population when treating progression and deaths without prior progression as events (log-rank P < .001; median PFS, 4.1 years vs 5.9 years; adjusted HR = 1.26; 95% CI = 1.04-1.52; P = .020). The rituximab subgroup results differed from the overall population when looking at the cumulative incidence of progression (Gray k-sample P = .090; median time to progression, 4.5 years vs 7.2 years; adjusted HR = 1.11; 95% CI = 0.91-1.35; P = .305) while treating death without prior disease progression as a competing risk (Gray k-sample P < .001; medians not reached; adjusted HR = 4.77; 95% CI = 2.66-8.54; P < .001).
Cumulative incidence of progression. (A) Or death by age (derived from Kaplan-Meier [KM] estimates). (B) By age (treating death without previous progression as competing risk). Adj, adjusted; Est, estimated.
Cumulative incidence of progression. (A) Or death by age (derived from Kaplan-Meier [KM] estimates). (B) By age (treating death without previous progression as competing risk). Adj, adjusted; Est, estimated.
No clinically relevant difference in PFS24 rates (P = .057), estimated from stratified Cox regression models, was observed when comparing patients >70 years and patients ≤70 years with rates of 0.663 (95% CI = 0.615-0.711) and 0.712 (95% CI = 0.698-0.727), respectively. Results were similar when looking at the patients in the rituximab subgroup (P = .349) with consistent rates of PFS24 across age groups of 0.748 (95% CI = 0.693-0.803) and 0.775 (95% CI = 0.758-0.793), respectively, for patients >70 years and patients ≤70 years.
Median follow-up time was 5.1 years for patients >70 years and 5.6 years for patients ≤70 years. Unsurprisingly, OS (Figure 3A) was notably shorter (log-rank P < .001) in patients >70 years when compared with patients ≤70 years with medians of 7.4 years (95% CI = 6.5-9.3) and 15.7 years (14.4 to not reached), respectively. This result remained the same after multivariable adjustment (HR = 2.74; 95% CI = 2.26-3.32; P < .001). Using CIF methods (Figure 3B) with death after disease progression as primary event of interest and death without prior disease progression as a competing risk, time to death with prior disease progression remained shorter in both univariate (Gray k-sample P < .001) and multivariable (HR = 1.87; 95% CI = 1.45-2.40; P < .001) analyses in patients >70 years with a median of 10.8 years (95% CI = 9.1 to not reached) when compared with patients ≤70 years who did not reach the median (95% CI = 17.9 to not reached). The time to death without prior disease progression was also shorter in patients >70 years for both the univariate (Gray k-sample P < .001) and multivariable (HR = 4.24; 95% CI = 2.86-6.28; P < .001) analyses with medians not reached for either age group. Results for the rituximab subgroup remained consistent with the total analysis population when treating all deaths as events (log-rank P < .001; median 9.3 years vs not reached; adjusted HR = 2.47; 95% CI = 1.86-3.27; P < .001) and when treating only death with prior disease progression as events (Gray k-sample P < .001; median 11.4 years vs not reached; adjusted HR = 2.00; 95% CI = 1.41-2.85; P < .001) and death without prior disease progression as a competing risk (Gray k-sample P < .001; medians not reached; adjusted HR = 4.45; 95% CI = 2.38-8.29; P < .001).
Cumulative incidence of death. (A) All causes, by age (derived from Kaplan-Meier estimates). (B) Following lymphoma progression by age (treating death without previous lymphoma progression as competing risk). NE, not estimable.
Cumulative incidence of death. (A) All causes, by age (derived from Kaplan-Meier estimates). (B) Following lymphoma progression by age (treating death without previous lymphoma progression as competing risk). NE, not estimable.
Discussion
Our analysis of the FLASH data including 5922 patients demonstrates that patients with FL over the age of 70 years treated on frontline prospective randomized trials have similar disease-related outcomes to younger patients. In this population of patients enrolled on clinical trials, patients >70 years were more likely to have statistically significantly increased LDH, anemia, and poor performance status compared with younger patients. The survival differences observed in older adults were due to higher mortality following first progression in the elderly, compared with younger patients (5-year OS, 66% vs 83%; HR = 2.35 [95% CI, 2.03-2.73]).
There are limited data on the outcomes of older patients with FL outside of descriptive retrospective and Medicare/SEER analyses reporting a worse PS in elderly patients, and confirming the benefit of rituximab in frontline treatment.14,15 A study by Alig et al explored age-specific survival differences in older patients with FL, finding shorter failure-free survival in those >70 years due to death without progression.4 Our pooled analysis assembled IPD from 18 global randomized trials in FL enrolling patients spanning nearly 2 decades. To our knowledge, this analysis is the first to be based on integrated IPD from RCTs in lymphoma with a specific focus on older patients, and among the largest focusing on this patient population.
Of key importance, our data demonstrate that early end points and first-line PFS for FL patients >70 years are no different. This suggests that we should consider these patients similarly for first-line clinical trials and selection of first-line therapy of moderate intensity. However, because this population has additional risk of nonlymphoma death, it is paramount to consider trials with agents that take this risk into consideration for older patients with FL.
These data highlight important considerations in the approach to older patients with FL. One critical finding is that age alone should not disqualify patients with FL from standard treatments or RCTs. Increased emphasis is being placed on broader patient enrollment on clinical trials to improve access to novel therapies. Best practices are to have trials be more representative of all ages, especially those patients who are most vulnerable. An American Society of Clinical Oncology Advocacy Summit convened with several members of the US Congress to act on advancing policy priorities to improve patient access to cancer care especially on clinical trials. These initiatives should translate to the practicing clinician when faced with an older FL patient, who may be a study candidate, to optimize opportunities for trial participation. The second consideration from our analysis is that, in our patient population, older patients with FL did not show increased rates of risk factors commonly associated with poor outcomes. Although early relapse, refractory disease, or early transformation are reproducible predictors of negative outcomes, our data support that age >70 years does not predict early progression or lack of CR at 24 or 30 months.
Our data are limited by the inclusion of older studies using less contemporary chemotherapies, and as such, should be investigated in a population of patients treated with more novel therapies. Moreover, patients >70 years who are healthy enough to meet clinical trial eligibility criteria may be less likely to suffer from comorbidities as the average elderly patient. This could result in a healthier than normal elderly population, which may bias results. Additionally, although our study is among the largest to date evaluating older patients with FL, from nearly 6000 patients only 542 were over 70 years of age. As such, meaningful subgroup analysis to control for interventions and different exclusion/inclusion criteria were not possible.
Despite this, we demonstrate compelling data on survival patterns of older patients with FL that should be used in context of treating the average patient with FL, who is likely to be older, with medical comorbidities. The challenging landscape of aging and cancer continues to evolve as awareness increases on more effective and less-toxic treatments, and understanding geriatric syndromes predicting morbidity and mortality in older patients that may be considered in daily practice. Ultimately, the question of whether age affects presentation and outcomes in FL would best be addressed by prospectively evaluating all presentations of FL over the age of 70 years.
Requests for data may be e-mailed to the corresponding author, Carla Casulo (carla_casulo@urmc.rochester.edu).
Acknowledgments
The authors thank Roche/Genentech and Bristol Myers Squibb for sponsorship of the study, and Dan Sargeant for contributions.
Authorship
Contribution: C.C. and C.R.F. were core investigators, performed data analysis, and wrote the paper; J.G.D. and F.-S.O. performed data analysis and wrote the paper; E.H. and Q.S. were trial contributors, performed data analysis, and wrote the paper; B.A.P., H.S.H., P.B., M.H., M.R., and A.H. were trial contributors; M.L., W.H., R.M., E.K., F.M., U.V., and G.A.S. were trial contributors and wrote the paper; and T.N. was the sponsor.
Conflict-of-interest disclosure: C.C. received research funding from Verastem, Genentech, Bristol Myers Squibb (BMS), and Gilead Core. E.H. received institutional research funding and travel support from Roche. H.S.H. received honoraria from Genentech. M.L. received invitations to scientific meetings and institutional research support from, and has contracts with, AbbVie, Acerta, Amgen, Archigen, ADC Therapeutics, BeiGene, Celgene, Gilead, Johnson & Johnson (J&J), Jazz, Roche, Sandoz, and Takeda; was principal investigator (PI) or strategic investigator in studies supported by Celgene, J&J, and BeiGene; is a board member of Fondazione Italiana Linfomi; is a member of the European Hematology Association (EHA) Guidelines Committee; was a member of the Euro-MRD Board of the European Society for Medical Oncology (ESMO) Guidelines Committee (until to December 2018); and was vice president of Associazione Italiana Leucemie (Alessandria section). W.H. received honoraria and research support from Roche, Celgene, Bayer, and Janssen. R.M. received consulting fees and lecture fees from Takeda Pharmaceuticals, and travel support, consulting fees, and lecture fees from Roche. E.K. received grants and other support from Roche/Genentech, during the conduct of the study; received personal fees from Bayer, Genmab, Janssen, Gilead, Roche, and AbbVie; and received nonfinancial support from MEI Pharma, outside of the submitted work. M.H. received research funding from F. Hoffmann-La Roche and is a member on an entity’s board of directors or advisory committee for F. Hoffmann-La Roche, Gilead Sciences, Celgene, and Janssen. T.N. was employed by Roche/Genentech and has stocks in Roche/Genentech. F.M. was an advisor (advisory board) for Celgene, Roche, Gilead, Epizyme, and Verasteem, and received honoraria for scientific lectures from Celgene, Roche, Janssen, and AbbVie. M.R. received honoraria from Sandoz, Janssen, Celgene, Roche, and Roche AG, and received research funding from Roche AG. A.H. reports personal fees for advisory work from Takeda Oncology. U.V. served on advisory boards for Janssen, Celgene, and Gilead Sciences, and has received lecture fees from Roche, Celgene, Janssen, AbbVie, and Gilead Sciences. G.A.S. served on an advisory board for, provided consultancy services to, or participated in educational events for AbbVie, Amgen, Autolus, BMS/Celgene, Debiopharm, Genmab, Kite/Gilead, Epizyme, Janssen, Karyopharm, MorphoSys, Novartis, Roche, and Takeda. C.R.F. held consulting roles with AbbVie, Spectrum, Celgene, Denovo Biopharma, OptumRx, Karyopharm, Pharmacyclics LLC, an AbbVie Company, Janssen, Gilead, and Bayer; has received research funding from AbbVie, Acerta, Celgene, Gilead, Genentech/Roche, Janssen, Millennium/Takeda, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; and has received travel expenses from Genentech/Roche. The remaining authors declare no competing financial interests.
A complete list of the members of the FLASH group appears in “Appendix.”
Correspondence: Carla Casulo, Wilmot Cancer Institute, University of Rochester, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: carla_casulo@urmc.rochester.edu.
Appendix
Members of the FLASH group are: Christopher R. Flowers (University of Texas MD Anderson Cancer Center, Houston, TX), Nathan H. Fowler (University of Texas MD Anderson Cancer Center, Houston, TX), Anton Hagenbeek (Academic Medical Center, Amsterdam, The Netherlands), Michael Herold (HELIOS Kliniken, Erfurt, Germany), Wolfgang Hiddemann (Ludwig-Maximilians University Hospital, Munich, Germany), Eva Kimby (Karolinska Institute, Stockholm, Sweden), Marco Ladetto (Azienda Ospedaliera SS Antonio e Biagio e Cesare Arrigo, Alessandria, Italy), Robert Marcus (Addenbrooke's Hospital, Cambridge, United Kingdom), Franck Morschhauser (Service Université de Lille 2, Lille, France), Gilles Salles (Memorial Sloan Kettering Cancer Center, New York, NY), Umberto Vitolo (Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Turin, Italy), Emanuele Zucca (Swiss Group for Clinical Cancer Research, Bern, Switzerland), and Daniel J. Sargent (deceased) (Mayo Clinic, Rochester, MN).

![Cumulative incidence of progression. (A) Or death by age (derived from Kaplan-Meier [KM] estimates). (B) By age (treating death without previous progression as competing risk). Adj, adjusted; Est, estimated.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/6/10.1182_bloodadvances.2020002724/2/m_advancesadv2020002724f2.png?Expires=1769882085&Signature=HhKG2UCJVg1IkDh5PhMCL~JAwasdPyX3yEbWb3wGA61li5Dh~OMA2m3trMtHdU10BHXbZnDKczv4ygGaXSniLXaM4wXtIjUzhR2YzkOauZkwnQyqdLxUncCfK4oFWtTqVVsNhGwDmzPBY1EfDhM9TLfdlvUg998PnQvS59UGSiAxVjTN8EJyeyHlLO5wFkcNriHkXvZmP8KExP4h90gQnHk3uvmAj~xdReuoDIMMuEjUXnCtgMhJROb3UaC3XKt99Yuxiglha~nTdNBoqaf1JuYMFpAM8GBpbXf7B1Y3rAISuGaWunROAnPQHA-vkK80ZjsTMr5KiJElApyfT8cI5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)