Key Points
For untreated DLBCL, CHOP combined with dose-dense rituximab is not superior to standard R-CHOP.
Abstract
Rituximab plus cyclophosphamide-doxorubicin-vincristine-prednisone (R-CHOP) is the standard of care for untreated diffuse large B-cell lymphoma (DLBCL). However, the schedule for rituximab administration has not been optimized. To compare standard R-CHOP with CHOP plus dose-dense weekly rituximab (RW-CHOP) in patients with untreated DLBCL, we conducted a phase 2/3 study (JCOG0601, jRCTs031180139). Patients were randomly assigned to R-CHOP (CHOP-21 with 8 doses of rituximab once every 3 weeks [375 mg/m2]) or RW-CHOP (CHOP-21 with 8 doses of weekly rituximab [375 mg/m2]) groups. The primary end point of the phase 2 component was percent complete response (%CR) of the RW-CHOP arm, whereas that of the phase 3 component was progression-free survival (PFS). Between December 2007 and December 2014, 421 untreated patients were randomly assigned to R-CHOP (213 patients) or RW-CHOP (208 patients). The %CR in the RW-CHOP arm was 85.3% and therefore met the prespecified decision criteria for the phase 2 component. With a median follow-up of 63.4 months, the 3-year PFS and overall survival were 79.2% and 88.7% in the R-CHOP arm and 80.3% and 90.4% in the RW-CHOP arm, respectively. There was no significant difference in PFS (hazard ratio, 0.95; 90.6% confidence interval, 0.68-1.31). Although the safety profile and efficacy of RW-CHOP was comparable with R-CHOP and its tolerability was acceptable, weekly rituximab in combination with CHOP during the early treatment period did not improve PFS in untreated patients with DLBCL. This trial was registered at jrct.niph.go.jp as #jRCTs031180139.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma subtype worldwide. Current immunochemotherapy strategies can lead to long-term remission for many patients with DLBCL. However, approximately one-third of patients relapse or have refractory disease, which remains a major cause of mortality.1-7 Given the unfavorable outcomes of patients with recurrent DLBCL, a more effective first-line treatment is required.
Rituximab in combination with cyclophosphamide (CPA), doxorubicin (DOX), vincristine, and prednisolone (R-CHOP) has been a standard treatment of previously untreated DLBCL since the early 2000s.8,9 In general, R-CHOP comprises a CHOP regimen that is repeated every 3 weeks with 1-dose rituximab during each cycle, the schedule for which was derived from the first reported randomized trial.8 Although the combination of rituximab with CHOP is not only effective but also convenient, there is insufficient scientific rationale to support it and the schedule for rituximab administration has not been optimized.
In the initial development of single-agent rituximab for relapsed or refractory B-cell lymphomas, patients were treated with rituximab at a dose of 375 mg/m2 over 4 or 8 consecutive weekly infusions.10,11 The pharmacokinetics of rituximab differ substantially between individuals, and its serum half-life is up to more than 500 hours. Peak concentration therefore increases cumulatively with each weekly infusion.12 However, there are no data available about the effects of administering the drug every 3 weeks. In clinical studies of rituximab for relapsed low-grade B-cell lymphoma and mantle cell lymphoma, it was reported that patients with a high blood concentration of rituximab had a higher response rate and a longer progression-free survival (PFS).13,14 High concentration rituximab combined with DOX had a synergistic antitumor effect for drug-resistant lymphoma cell lines.15 This is important, because rapid tumor control is critical to improving the treatment outcomes of DLBCL patients.16,17 These findings suggest that maintaining a higher rituximab concentration and combining it with chemotherapy during the early treatment period improves treatment efficacy.
We developed the RW-CHOP regimen, which consists of a dose-dense, weekly administration of rituximab combined with standard CHOP during the early treatment period to increase the serum level of rituximab. A multicenter, randomized phase 2/3 study was then performed to compare RW-CHOP with standard R-CHOP in patients with previously untreated DLBCL.
Patients and methods
Eligibility criteria
Our inclusion criteria were as follows: previously untreated CD20-positive DLBCL according to the World Health Organization classification, third edition,18 excluding histologic transformation from other B-cell lymphomas or immunodeficiency-associated lymphoproliferative disorders; clinical stage I to IV disease diagnosed with a computed tomography scan (Ann Arbor classification); lymphoma cells in the peripheral blood numbering less than 10 × 103/μL; age 20 to 79 years; Eastern Cooperative Oncology Group performance status 0 to 2; no central nervous system involvement; at least 1 measurable lesion; white blood cell count ≥ 3.0 × 103/μL; absolute neutrophil count ≥ 1.0 × 103/μL; platelet count ≥ 100 × 103/μL; aspartate aminotransferase ≤ 150 U/L; alanine aminotransferase levels, male ≤ 210 U/L, female ≤ 115 U/L; total bilirubin level ≤ 2.0 mg/dL; serum creatinine level ≤ 2.0 mg/dL; PaO2 ≥ 65 mm Hg; and normal electrocardiogram and ejection fraction ≥ 50%. Exclusion criteria included glaucoma, any other malignancy, prior chemotherapy or radiotherapy, HIV infection, a positive test for hepatitis B virus (HBV) surface antigen and/or hepatitis C virus antibody, pregnancy or breast feeding, severe concomitant disease, uncontrolled diabetes mellitus, and uncontrolled hypertension.
Enrollment began in December 2007. At the beginning of the study, only patients with advanced stage disease and in a low or low-intermediate risk group as classified with the International Prognostic Index (IPI)19 were eligible. However, because of slow recruitment, these criteria were amended in September 2010 to permit the enrollment of the patients with any IPI risk and any clinical stage whose standard treatment was R-CHOP.
Written informed consent was obtained from all patients before enrollment, and the study protocol was approved by the Protocol Review Committee of Japan Clinical Oncology Group (JCOG) and the institutional review board of each participating center.
Study treatment
Fifty institutions participated in this randomized multicenter phase 2/3 study. Patients were randomized at the JCOG Data Center to receive either R-CHOP or RW-CHOP as per the minimization method of balancing the groups according to the institution, age ≤60 or ≥61 years and with or without a bulky mass (more than 5-cm diameter).
The R-CHOP regimen that was administered every 3 weeks consisted of rituximab 375 mg/m2 on day 1, CPA 750 mg/m2, DXR 50 mg/m2, and vincristine 1.4 mg/m2 (maximum 2 mg) administered on day 2, and prednisolone 100 mg for patient ≤64 years or 60 mg/m2 for patient ≥65 years administered on days 1 to 5. RW-CHOP consisted of the same dosages and schedule as CHOP, but with the addition of rituximab 375 mg/m2 on days 1, 8, 15, 22, 29, 36, 43, and 50. Six cycles of CHOP were given to stage I nonbulky patients, 8 cycles were given to stage I bulky and II to IV patients, and rituximab was given 8 times regardless of the number of CHOP cycles.
We recommended prophylaxis with trimethoprim-sulfamethoxazole against Pneumocystis pneumonia for patients in both treatment arms but not acyclovir against herpes virus because of medical insurance policy issues. Filgrastim, lenograstim, or nartograstim was initiated as primary prophylaxis for patients older than 65 years, patients who experienced febrile neutropenia during their previous cycle, or at the discretion of the treating physician. We recommended HBV DNA monitoring in patients who were HBs antibody or HBc antibody positive and preemptive antiviral therapy if HBV DNA was detectable. Consolidative radiotherapy and central nervous system prophylaxis were not permitted.
Study end points and assessment
The primary end point of the phase 2 component was investigator-assessed percent complete response (CR) in the RW-CHOP arm.
The primary end point of the phase 3 component was PFS, defined as the date from random assignment to disease progression, relapse after CR, or any cause of death in the intent-to-treat population. PFS was censored on the latest date when no progression was confirmed. Secondary end points included overall survival (OS), defined as the date from random assignment to any cause of death, and adverse events (AEs).
Tumor response was assessed by the investigators using computed tomography and positron emission tomography per the Revised Response Criteria for Malignant Lymphoma responses20 6 to 8 weeks after treatment completion. Positron emission tomography was recommended but not mandated at baseline and was required at the end of treatment. As posttreatment surveillance, a computed tomography scan from the neck through the pelvis every 6 months was recommended during the follow-up period. AEs were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Statistical methods
This study consisted of phase 2 and phase 3 components. The main objective of the phase 2 component was to evaluate whether RW-CHOP had comparable efficacy and safety to R-CHOP and to determine whether this study was appropriate to continue as a phase 3 study. Two interim analyses were planned. The first interim analysis was conducted during phase 2 to test whether the %CR of the RW-CHOP arm was superior to the predefined threshold (55%) with a 1-sided α = 0.10 and β = 0.10 to detect a 15% increase. The threshold data were based on the results of the standard CHOP regimen without rituximab. The phase 2 component required 68 patients. The Data and Safety Monitoring Committee reviewed %CR without comparison between arms and judged whether the study should be continued.
The main objective of the phase 3 component was to evaluate whether RW-CHOP improved the PFS of R-CHOP. At the beginning of the study, we assumed that the 3-year PFS following R-CHOP for patients with advanced stage disease and in a low or low-intermediate risk group with an IPI of 65% and an expected improvement of 10% with RW-CHOP required the enrollment of a total of 360 patients with a 1-sided α level of 5% to achieve 80% power. We then reevaluated statistical calculations at the time of protocol amendment because we expanded our eligibility criteria because of slow recruitment. Calculating the weighted average of the patient population for each IPI risk and the estimated PFS, we assumed that the 3-year PFS following R-CHOP for all patients was 66%, with an 9.5% expected improvement with RW-CHOP. This estimate was similar to our initial assumption. The power would be 80% when 154 events were observed in this setting, but regular study monitoring showed that the estimated 3-year PFS for all patients in this study was 80.5%, which was better than expected in advance. We reevaluated our statistical calculations to maintain our power and required 422 patients in total with a 1-sided α level of 5% to achieve 80% power, 7 years of accrual, and 3 years of follow-up. Conclusively, this study expected to detect a 7% improvement in 3-year PFS in the RW-CHOP arm compared with the R-CHOP arm, which was anticipated to have a 3-year PFS of 77%. A second interim analysis was performed to assess the necessity of further follow-up when all of patients had completed treatment. The Lan and DeMets method and O'Brien and Fleming type α-spending function were used to control the type I error for the primary end point. Throughout the study period, the researchers were blinded to the primary end point of the results of the interim analysis. Superiority of RW-CHOP as a primary end point was assessed by the 1-sided stratified log-rank test according to age (≤60/≥61 years old) and bulky mass (presence/absence). To summarize the difference between the arms, hazard ratios (HRs) with confidence intervals (CIs) were calculated using the Cox proportional hazard model. PFS and OS curves were estimated using the Kaplan-Meier method. All patient information forms were collected and managed at the JCOG Data Center where in-house interim monitoring was performed, and reports were semiannually reviewed by their Data and Safety Monitoring Committee. All statistical analyses were performed using SAS software, release 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
Between December 2007 and December 2014, a total of 422 patients were enrolled and randomized into treatment groups, but primary analysis was performed on 421 patients: 213 in the R-CHOP arm and 208 in the RW-CHOP arm. One patient was excluded because of consent withdrawal. The baseline characteristics of 421 patients were as follows (R-CHOP arm vs RW-CHOP arm): median age, 61 vs 62 years; male sex, 54.5% vs 55.8%; Ann Arbor stage I/II/III/IV, 14.6/32.9/26.8/25.8% vs 16.3/42.8/20.2/20.7%; and IPI score ≤ 2, 77.0% vs 87.5%. The R-CHOP arm contained more patients with unfavorable factors, stage IV disease (25.8% vs 20.7%), and IPI HI/H risk (23% vs 12.5%; Table 1). Of the 421 patients, 12 were ineligible (6, incorrect histopathologic diagnosis after registration; 4, complicated because of another malignancy; 1, no measurable lesion; 1, deviation of eligibility criteria; Figure 1). A central pathology review was performed in 95.2% (401 of 421) of cases, and 196 patients in the R-CHOP arm and 191 patients in the RW-CHOP arm were histologically confirmed to be eligible. The median time from diagnosis to treatment was 18 days in the R-CHOP arm and 20 days in the RW-CHOP arm.
Patient characteristics
| . | R-CHOP (N = 213), n (%) . | RW-CHOP (N = 208), n (%) . |
|---|---|---|
| Age, median (range), y | 61 (25-78) | 62 (20-79) |
| ≤60 | 98 (46.0) | 96 (46.2) |
| ≥61 | 115 (54.0) | 112 (53.8) |
| Sex | ||
| Male | 116 (54.5) | 116 (55.8) |
| Female | 97 (45.5) | 92 (44.2) |
| ECOG PS | ||
| 0-1 | 207 (97.2) | 202 (97.1) |
| 2 | 6 (2.8) | 6 (2.9) |
| Ann Arbor | ||
| I, nonbulky | 16 (7.5) | 20 (9.6) |
| I, bulky | 15 (7.0) | 14 (6.7) |
| II | 70 (32.9) | 89 (42.8) |
| III | 57 (26.8) | 42 (20.2) |
| IV | 55 (25.8) | 43 (20.7) |
| B symptom present | 25 (11.7) | 27 (13.0) |
| Bulky mass (≥5 cm) present | 101 (47.4) | 93 (44.7) |
| LDH > UNL | 110 (51.6) | 84 (40.4) |
| Extranodal sites ≥ 2 | 29 (13.6) | 16 (7.7) |
| IPI | ||
| Low | 97 (45.5) | 113 (54.3) |
| Low-intermediate | 67 (31.5) | 69 (33.2) |
| High-intermediate | 34 (16.0) | 18 (8.7) |
| High | 15 (7.0) | 8 (3.8) |
| . | R-CHOP (N = 213), n (%) . | RW-CHOP (N = 208), n (%) . |
|---|---|---|
| Age, median (range), y | 61 (25-78) | 62 (20-79) |
| ≤60 | 98 (46.0) | 96 (46.2) |
| ≥61 | 115 (54.0) | 112 (53.8) |
| Sex | ||
| Male | 116 (54.5) | 116 (55.8) |
| Female | 97 (45.5) | 92 (44.2) |
| ECOG PS | ||
| 0-1 | 207 (97.2) | 202 (97.1) |
| 2 | 6 (2.8) | 6 (2.9) |
| Ann Arbor | ||
| I, nonbulky | 16 (7.5) | 20 (9.6) |
| I, bulky | 15 (7.0) | 14 (6.7) |
| II | 70 (32.9) | 89 (42.8) |
| III | 57 (26.8) | 42 (20.2) |
| IV | 55 (25.8) | 43 (20.7) |
| B symptom present | 25 (11.7) | 27 (13.0) |
| Bulky mass (≥5 cm) present | 101 (47.4) | 93 (44.7) |
| LDH > UNL | 110 (51.6) | 84 (40.4) |
| Extranodal sites ≥ 2 | 29 (13.6) | 16 (7.7) |
| IPI | ||
| Low | 97 (45.5) | 113 (54.3) |
| Low-intermediate | 67 (31.5) | 69 (33.2) |
| High-intermediate | 34 (16.0) | 18 (8.7) |
| High | 15 (7.0) | 8 (3.8) |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, lactate dehydrogenase; UNL, upper normal limit.
Efficacy
At the first interim analysis, the %CR of 68 patients enrolled in the phase 2 component of the RW-CHOP arm was 85.3% (80% CI, 78.3-90.7), meeting the primary end point.
With a median follow-up of 63.4 months (range, 3.2-119.2) among all patients, 106 patients had a PFS event and 69 died. Figure 2 shows PFS and OS curves for all randomized patients. There was no significant difference in PFS between the arms (HR, 0.95; 90.6% CI, 0.68-1.31; 1-sided log-rank, P = .39). The 3-year PFS and OS were 79.2% (95% CI, 73.1%-84.1%) and 88.7% (95% CI, 83.3%-92.3%) in the R-CHOP arm and 80.3% (95% CI, 74.2%-85.1%) and 90.4% (95% CI, 85.5%-93.7%) in the RW-CHOP arm. Estimated 9-year PFS and OS were 68.0% (95% CI, 57.8%-76.3%) and 81.8% (95% CI, 74.7%-87.0%) in the R-CHOP arm and 70.9% (95% CI, 62.8%-77.5%) and 78.1% (95% CI, 70.0%-84.3%) in the RW-CHOP arm.
Kaplan-Meir curves by treatment arm. (A) PFS. (B) OS. There was no significant difference in PFS between the arms (HR, 0.95; 90.6% CI, 0.68-1.31; 1-sided log-rank, P = .39).
Kaplan-Meir curves by treatment arm. (A) PFS. (B) OS. There was no significant difference in PFS between the arms (HR, 0.95; 90.6% CI, 0.68-1.31; 1-sided log-rank, P = .39).
Among all randomized patients except for 1 patient who withdrew consent, the %CR and overall response rate were 77.0% (95% CI, 70.8%-82.5%) and 93.0% (95% CI, 88.7%-96.0%) in the R-CHOP arm and 82.2% (95% CI, 76.3%-87.2%) and 91.8% (95% CI, 87.2%-95.2%) in the RW-CHOP arm.
Similar results were observed in all eligible patients, and no significant difference was observed between the arms. These results were similar in patients histologically confirmed to have DLBCL by central pathology.
Subgroup analysis
Figure 3 compares PFS by arm and preplanned clinical subgroups, which include age, bulky mass, sex, B symptoms, and IPI risk group. No significant differences between the treatment arms were observed. Subgroup analyses were also performed for groups classified according to IPI. The 3-year PFS and OS was 84.4% (95% CI, 75.5%-90.3%) and 92.6% (95% CI, 85.1%-96.4%) for low risk; 75.9% (95% CI, 63.7%-84.5%) and 88.1% (95% CI, 77.5%-93.8%) for low-intermediate risk; 79.4% (95% CI, 61.6%-89.6%) and 85.3% (95% CI, 68.2%-93.6%) for high-intermediate risk; and 60.0% (95% CI, 31.8%-79.7%) and 73.3% (95% CI, 43.6%-89.1%) for high risk in the R-CHOP arm and 86.7% (95% CI, 78.9%-91.8%) and 93.8% (95% CI, 87.4%-97.0%) for low risk; 75.4% (95% CI, 63.4%-83.9%) and 89.9% (95% CI, 79.9%-95.0%) for low-intermediate risk; 77.8% (95% CI, 51.1%-91.0%) and 83.3% (95% CI, 56.8%-94.3%) for high-intermediate risk; and 37.5% (95% CI, 8.7%-67.4%) and 62.5% (95% CI, 22.9%-86.1%) for high risk in the RW-CHOP arm (supplemental Figure).
PFS by subset analysis. Post hoc comparison of PFS by arm in subgroups of clinical features, including age, bulky mass, sex, B symptoms, and IPI risk group. No significant differences between arms were observed.
PFS by subset analysis. Post hoc comparison of PFS by arm in subgroups of clinical features, including age, bulky mass, sex, B symptoms, and IPI risk group. No significant differences between arms were observed.
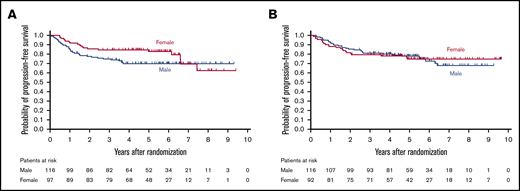
In a subgroup analysis, there was a trend toward male patients having a greater benefit from RW-CHOP than female patients (HR, 0.65; 95% CI, 0.38-1.3; Figure 4). Patient characteristics by sex were equivalent between arms (supplemental Table). There were no deviations between arms regarding factors that could affect outcome.
Kaplan-Meier curves for PFS by sex. (A) R-CHOP arm (n = 213). (B) RW-CHOP arm (n = 208).
Kaplan-Meier curves for PFS by sex. (A) R-CHOP arm (n = 213). (B) RW-CHOP arm (n = 208).
Treatment
All planed treatment courses were completed by 89.7% of the R-CHOP arm and 89.9% of the RW-CHOP arm. Reasons for early discontinuation included disease progression (R-CHOP, 3.8%; RW-CHOP, 2.9%), adverse events (R-CHOP, 2.3%; RW-CHOP, 4.3%), patient refusal due to AEs (R-CHOP, 1.9%; RW-CHOP, 1.9%) and other reasons (R-CHOP, 2.3%; RW-CHOP, 1.0%).
Relative dose intensity (RDI) was calculated as the dose intensity that was actually administered divided by the planned dose intensity for all 8 courses. Actual dose intensity was calculated as the actual total dose of each drug per body surface area divided by treatment duration. In the R-CHOP arm, the median RDI of rituximab, CPA, and DOX were 97.1% (range, 77.8%-101.2%), 95.5% (range, 13.3%-102.9%), and 95.5% (range, 54.1%-101.8%), respectively. In the RW-CHOP arm, the median RDI of rituximab, CPA, and DXR were 100.0% (range, 66.7%-102.2%), 98.8% (range, 63.2%-102.4%) and 98.8% (range, 62.5%-102.4%), respectively. Treatment compliance was adequate in both arms.
Safety
The collected case report forms of 421 patients (except for 1 patient who withdraw consent) were used for evaluating treatment toxicity (Table 2). Major AEs were hematologic toxicities and infections. Grade (G) 3/4 neutropenia and G 3/4 thrombocytopenia were observed in 97.7% and 8.0% of patients in the R-CHOP arm and 97.1% and 5.3% in the RW-CHOP arm, respectively. G3 febrile neutropenia occurred in 33.8% of the patients in the R-CHOP arm and 22.1% in the RW-CHOP arm.
Toxicity
| Toxicity CTCAE ver. 3.0 . | R-CHOP (N = 213) . | RW-CHOP (N = 208) . | ||
|---|---|---|---|---|
| Any grade (%) . | Grade 3-4 (%) . | Any grade (%) . | Grade 3-4 (%) . | |
| Leukocytes | 99.5 | 94.4 | 99 | 95.2 |
| ANC | 100 | 97.7 | 99 | 97.1 |
| Platelets | 71.4 | 8 | 74 | 5.3 |
| AST | 67.6 | 1.4 | 71.2 | 2.4 |
| ALT | 60.6 | 3.8 | 65.4 | 5.8 |
| Creatinine | 30 | 0.5 | 28.4 | 0 |
| Hyponatremia | 60.1 | 5.2 | 63.9 | 5.3 |
| Hypokalemia | 35.7 | 3.3 | 37 | 1.9 |
| Hyperglycemia | 90 | 3.8 | 89.4 | 5.3 |
| Cardiac ischemia/infarction | 1.4 | 1.4 | 0.5 | 0.5 |
| LV systolic dysfunction | 12.2 | 0.9 | 14.4 | 1.4 |
| Ileus | 3.8 | 0.9 | 1.4 | 1 |
| Febrile neutropenia | — | 33.8 | — | 22.1 |
| Herpes zoster infection | — | 0.9 | — | 1.9 |
| Neuropathy-sensory | 78.4 | 2.3 | 79.8 | 5.8 |
| Neuropathy-motor | 13.1 | 3.3 | 12 | 2.9 |
| Second primary neoplasms | n = 21* | n = 18 | ||
| Toxicity CTCAE ver. 3.0 . | R-CHOP (N = 213) . | RW-CHOP (N = 208) . | ||
|---|---|---|---|---|
| Any grade (%) . | Grade 3-4 (%) . | Any grade (%) . | Grade 3-4 (%) . | |
| Leukocytes | 99.5 | 94.4 | 99 | 95.2 |
| ANC | 100 | 97.7 | 99 | 97.1 |
| Platelets | 71.4 | 8 | 74 | 5.3 |
| AST | 67.6 | 1.4 | 71.2 | 2.4 |
| ALT | 60.6 | 3.8 | 65.4 | 5.8 |
| Creatinine | 30 | 0.5 | 28.4 | 0 |
| Hyponatremia | 60.1 | 5.2 | 63.9 | 5.3 |
| Hypokalemia | 35.7 | 3.3 | 37 | 1.9 |
| Hyperglycemia | 90 | 3.8 | 89.4 | 5.3 |
| Cardiac ischemia/infarction | 1.4 | 1.4 | 0.5 | 0.5 |
| LV systolic dysfunction | 12.2 | 0.9 | 14.4 | 1.4 |
| Ileus | 3.8 | 0.9 | 1.4 | 1 |
| Febrile neutropenia | — | 33.8 | — | 22.1 |
| Herpes zoster infection | — | 0.9 | — | 1.9 |
| Neuropathy-sensory | 78.4 | 2.3 | 79.8 | 5.8 |
| Neuropathy-motor | 13.1 | 3.3 | 12 | 2.9 |
| Second primary neoplasms | n = 21* | n = 18 | ||
—, corresponding grade is not defined in CTCAE; ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; LV, left ventricular; ver., version.
*Including duplicates; 1 patient was diagnosed with papillary adenocarcinoma of thyroid and bladder cancer.
Nonhematologic toxicities were mild and comparable in both arms. The frequencies of severe AEs were 2.3% (95% CI, 0.8%-5.4%) in the R-CHOP arm and 3.8% (95% CI, 1.7%-7.4%) in the RW-CHOP arm, which was not significantly different. A slightly higher incidence of G3/4 herpes zoster infection was observed in the RW-CHOP arm (R-CHOP arm, 0.9%; RW-CHOP arm, 1.9%). HBV reactivation was recognized in 3 patients (R-CHOP arm, 2; RW-CHOP arm, 1). One patient in the RW-CHOP arm developed acute hepatitis by HBV reactivation, but no fulminant hepatitis occurred. One treatment-related death occurred in the RW-CHOP arm because of left ventricular systolic dysfunction 22 days after the eighth course of CHOP. Thirty-nine secondary malignancies (R-CHOP arm, 21; RW-CHOP arm, 18) were also observed. There was no obvious difference between the arms in neoplasm characteristics, and no immunodeficiency-associated lymphoproliferative disorders were observed. No unexpected AEs were seen.
Discussion
This trial, which was planned to confirm the optimal schedule of rituximab when combined with CHOP for patients with untreated DLBCL, did not show superiority of dose-dense rituximab during the early treatment phase in terms of PFS and OS. RW-CHOP was effective, and its treatment adherence and safety profile were very good when it was used as an initial treatment. Although our results suggest that our objective of enhancing the density of rituximab combined with standard chemotherapy was achieved, our attempt to improve PFS using a dose-dense rituximab strategy failed to produce results.
Several recent randomized studies comparing an investigative treatment against R-CHOP failed to demonstrate any benefit to the novel treatment. One of the reasons for this may be that the outcomes obtained from R-CHOP were better than had ever been estimated. The MabThera International Trial (MInT), which was intended for patients with 0 to 1 risk factors according to age-adjusted IPI, is one of the studies that established R-CHOP as the standard of care. The 3-year event-free survival of the R-CHOP arm in the MInT study was 79%.9 The randomized studies that resemble our study in eligibility criteria include R-CHOP-21 vs R-CHOP-14, conducted by the UK National Cancer Research Institute Lymphoma Clinical Study Group, R-CHOP vs obinutuzumab plus CHOP in the GOYA study, R-CHOP vs R-CHOP plus bortezomib by the UK National Cancer Research Institute group and the Schweiz Arbeitsgemeinschaft für Klinische Krebsforschung, and R-CHOP vs dose-adjusted etoposide, predonisolone, vincristine, cyclopyosphamide, doxorubicin and rituximab (EPOCH-R) by the US Alliance. The outcomes of the R-CHOP arm in these studies were 74.8% in 2-year PFS, 66.9% in 3-year PFS, 70.1% in 30-month PFS, and 75.5% in 2-year PFS, respectively,1-3,21 and almost of these results were better than their estimates. Although our study population included many more patients with a lower IPI risk than these studies and resembled the MInT trial, our results compared favorably. Some treatment deviations were included in our work, but the proportion of patients who completed their treatment and relative dose-intensity including rituximab were quite high in both arms, and there was no difference in treatment compliance. We therefore concluded that our results were reliable.
In the rituximab era, it is suggested that male sex is an adverse prognostic factor.22,23 One of the reasons for this is that there is a difference in the blood clearance of rituximab between males and females. The German High-Grade Non-Hodgkin Lymphoma Study Group reported that the clearance of rituximab and aging did not correlate with each other in male patients, but in elderly female patients, there was negative relationship with age and their baseline clearance of rituximab was slower than in males.24,25 They also performed several clinical trials to optimize rituximab dosage and schedule, one of which used the dose-dense and dose-up strategy to increase serum drug concentration. These studies showed that these strategies increased peak serum concentration, although above-threshold exposure times were shorter than with the usual dosage,26,27 and suggested that the outcomes of some male patients might improve when rituximab use is optimized. In their investigation about prolonged rituximab exposure, Pfreundschuh et al28 showed that extended rituximab exposure led to the improved survival of poor prognosis patients. In our study, although dose-dense rituximab during the early treatment period might improve the %CR of all patients and the PFS of male patients, it did not lead to superior survival rates compared with R-CHOP. As one of the reasons why our attempt failed to improve survival, RW-CHOP might lack a strategy for maintaining serum rituximab concentration. Our study is limited by the lack of rituximab pharmacokinetic data and that these findings are just exploratory analyses. There might therefore still be room for rituximab dose and schedule optimization.
RW-CHOP did not show any unexpected toxicity. Although the same extent of the neutropenia, there was a different frequency of febrile neutropenia (FN) between treatment arms. In the R-CHOP arm, a total of 1,657 chemotherapies were prescribed, and 98 FN events were diagnosed. Thirty-five of the 98 cases of FN occurred during the first treatment course. The incidence of FN in each R-CHOP course after the second course was similar. In contrast, a total of 1,549 chemotherapies were prescribed, and 55 cases of FN were diagnosed in the RW-CHOP arm, and 19 of the 55 FN cases occurred during the first treatment course. The frequency of FN was higher in the R-CHOP arm than in the RW-CHOP arm. The incidence was highest during the first treatment course in both arms, but equivalent in incidence during the second course. From these data, we speculated that physicians could probably treat neutropenia of outpatient in the RW-CHOP arm earlier because it required more frequent hospital visits during the early treatment phase than R-CHOP. Imbalances in patient characteristics that the R-CHOP arm contained more patients with unfavorable factors might affected the difference of frequency. However, the true reason for this discrepancy was uncertain. We are going to review granulocyte colony-stimulating factor and prophylactic antibiotic use and when the febrile neutropenia occurred to further understand this relationship.
Past reports warned that dose-dense rituximab combined with dose-dense CHOP increased the risk of Pneumocystis and cytomegalovirus pneumonia because of drug-induced immunodeficiency, and prophylaxis with trimethoprim-sulfamethoxazole and acyclovir was necessary.26 We recommended the prophylactic use of trimethoprim-sulfamethoxazole for both arms but not acyclovir because of medical insurance policy. In the RW-CHOP arm, no pneumonitis including cytomegalovirus pneumonia occurred. Although the frequency of herpes zoster in the RW-CHOP arm was slightly higher than in the R-CHOP arm, it was low in both arms. It is known that lymphoma survivors are at an increased lifetime risk of herpes zoster infection, and careful long-term examination is required to evaluate the immunosuppression caused by dose-dense rituximab.
This study has several limitations. First, there were some imbalances in patient characteristics between the arms, notably stage, lactate dehydrogenase level, the number of patients with extra nodal disease, and IPI score. This was because stratification factors were not readjusted when the protocol was amended to include limited stage and higher IPI patients. However, the fact that survival was equivalent even though the R-CHOP arm contained more patients with unfavorable factors might suggest that dose-dense rituximab does not yield a therapeutic advantage. Second, we were conducting another randomized phase 2 study investigating the efficacy of induction therapy followed by high-dose chemotherapy and upfront autologous stem cell transplantation for DLBCL patients at a high-intermediate or high risk according to IPI (JCOG0908) at the same time and registered it as a priority. This might have led more favorable patients with good prognoses to be enrolled in this study. Third, because we adopted the World Health Organization classification, third edition, as our eligibility criteria, biological marker analyses such as cell-of-origin and the measurement of MYC and BCL2 protein expression were also not performed. The possibility of bias between arms regarding these biomarkers and including subjects who are not suitable for R-CHOP such as double-hit lymphoma cannot be denied. However, the frequency of double-hit lymphoma in all cases of DLBCL is about 5%, and its impact would therefore be small.
In DLBCL patients, limited stage disease can recur despite long-term remission, unlike advanced disease after chemoradiotherapy, which is one of the standard treatments of limited-stage disease.29 The possibility of long-term remission of limited-stage disease is improved with 6 courses of R-CHOP,30 but there is a paucity of data that proves the efficacy of R-CHOP without radiotherapy. Therefore, useful data will be provided when long-term follow-up of our treatment groups is presented.
In summary, when standard CHOP therapy is combined with rituximab, dose-dense rituximab during the early treatment period did not improve the PFS of patients with untreated DLBCL. Although many attempts to outperform R-CHOP have failed, including our trial, standard treatment outperforms expected outcomes even in those patient groups with poor prognoses, such as those with activated B-cell like DLBCL.1,31 In our study, excellent R-CHOP treatment compliance led to a good prognosis. Of course, it is important to keep pursuing new standard treatments and crucial to adhere to current standard treatment, namely R-CHOP, in the interim, as appropriate.
For data, please e-mail the corresponding author at 8jmmd004@is.icc.u-tokai.ac.jp.
Acknowledgments
The authors thank the pathologists (Yoshihiro Matsuno and Naoya Nakamura) for support in the central pathologic review and members of the JCOG Data Center and JCOG Operations Office for support in preparing the paper (Tomohiro Kadota, Tomoko Kataoka, and Keita Sasaki), data management (Yuko Watanabe), and oversight of the study management (Haruhiko Fukuda).
This study was supported in part by the National Cancer Center Research and Development Fund (23-A-16, 23-A-17, 26-A-4, 29-A-3), Grants-in-Aid for Cancer Research (20S-1, 20S-6, 23-A-16, 23-A-17), and Health and Labour Sciences research grants for clinical cancer research (19-20 and 22-14) from the Ministry of Health, Labour, and Welfare.
Authorship
Contribution: K.O., T.K., K. Tobinai, G.O., T. Mizutani, K.Y., S.I., D.M., K.A., M.O., T.H., K. Tsukasaki, and H.N. designed the study; K.O., T.K., G.O., T. Mizutani, K.A., and H.N. analyzed data; and all authors contributed to collection and assembly of data and manuscript writing.
Conflict-of-interest disclosure: K.O. reports receiving honoraria from Kyowa Kirin, Eisai, Chugai Pharma, and Janssen Pharmaceutical. K. Tobinai reports receiving research funding from Chugai Pharma, Kyowa Kirin, Ono Pharmaceutical, Celgene, Janssen Pharmaceutical, Eisai, Mundipharma, Takeda, and AbbVie; receiving honoraria from Zenyaku Kogyo, Eisai, Takeda, Mundipharma, HUYA Bioscience International, Kyowa Kirin, Celgene, Chugai Pharma, Ono Pharmaceutical, Yakult, Daiichi Sankyo, Bristol-Myers Squibb, Meiji Seika Pharma, and Solasia Pharma; and serving on advisory board for Celgene, Zenyaku Kogyo, HUYA Bioscience International, Daiichi Sankyo, Takeda, Mundipharma, Ono Pharmaceutical. N.Y. reports receiving research funding from Ono Pharmaceutical, Celgene, Amgen, and Daiichi-Sankyo. N.F. reports research funding from AbbVie, Bayer, Celgene, Chugai Pharma, Eisai, Gilead Sciences, Janssen Pharmaceutical, Kyowa Kirin, Soleisia Pharma, Takeda, and Ono Pharmacuetical and receiving honoraria from AbbVie, ADC Therapeutics, Celgene, Chugai Pharma, Eisai, Janssen Pharmacuetical, Kyowa Kirin, Mochida, Mundipharma, Nippon Shinyaku, Novartis, Ono Pharmacuetical, Stemline Therapeutics, Takeda, and Zenyaku Kogyo. T. Uchida reports honoraria from Pfizer, Chugai Pharma, Takeda, Janssen Pharmaceutical, Eisai, Celgene, Mundipfarma, Bristol-Myers Squibb, Ono Pharmaceutical, Novertis, Otsuka Pharmaceutical, Nippon Shinyaku, and Kyowa Kirin. K.Y. reports receiving consultant fee from AbbVie, Astra-Zeneca, Celgene, Chugai Pharma, Daiichi Sankyo, Eisai, HUYA Bioscience International, Meiji Seika Pharma, Mundipharma, Ono Pharmaceutical, Stemline Therapeutics, and Takeda; receiving research funding from AbbVie, Astra-Zeneca, Bayer, Celgene, Chugai Pharma, Eisai, IQIVA/Incyte, MSD, Mundipharma, Nippon Shinyaku, Novartis, Ono Pharmaceutical, Otsuka, Solasia Pharma, SymBio, Takeda, Yakult, and Zenyaku Kogyo; and receiving honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Chugai Pharma, Eisai, IQIVA/HUYA, Janssen Pharmaceutical, Kyowa Kirin, Meiji Seika Pharma, Mochida, MSD, Mundipharma, Nippon Shinyaku, Novartis, Ono Pharmaceutical, Otsuka, Pfizer, Sanofi, Sumitomo Dainippon, Takeda, and Yakult. K.M. reports receiving honoraria from Chugai Pharma, Kyowa Kirin, Eisai, SymBio, Celgene, Takeda, Nippon Shinyaku, and Janssen Pharmaceutical. N.T. reports receiving research funding from Kyowa Kirin, Chugai Pharma, Astellas, Takeda, Eisai, Pfizer, and Teijin. S.I. reports receiving consultant fees from Ono Pharmaceutical, Takeda, Janssen Pharmaceutical, Sanofi, AbbVie, and Glaxo SmithKline; receiving research funding from Takeda, Ono Pharmaceutical, Janssen Pharmaceutical, Bristol-Myers Squibb, Sanofi, AbbVie, Chugai Pharma, and Kyowa Kirin; and receiving honoraria from Takeda, Ono Pharmaceutical, Janssen Pharmaceutical, Bristol-Meyers Squibb, Celgene, Daiichi Sankyo, and Sanofi. I.Y. reports receiving research funding from Kyowa Kirin and Chugai Pharma and receiving honoraria from Celgene, Mundipharma, Bristol-Myers Squib, Shire Japan, Janssen Pharmaceutical, Mochida, MSD, Takeda, Taiho, Kyowa Kirin, and Chugai Pharma. S.Y. reports receiving research funding from Chugai Pharma and Kyowa Kirin and honoraria from Eisai and Janssen Pharmaceutical. Y.M. reports receiving research funding from Kyowa Kirin, Astellas, Eisai, Ono Pharmaceutical, Pfizer, Asahi Kasei, MSD, Daiichi-Sankyo, Taisho, Taiho, Takeda, Chugai Pharma, Teijin, Nippon Kayaku, and Mochida. Y.S. reports research funding from Chugai Pharma, Novartis, Bayer, Eisai, Ono Pharmaceutical, Otsuka, Pfizer, Amgen, Daiichi Sankyo, Solasia Pharma, Celgene, and Takeda. K.N. reports receiving honoraria from Kyowa Kirin, Chugai Pharma, Novartis, Celgene, Eisai, Merck Sharp & Dohme, and Bristol-Myer Sqibb. N.D. reports receiving research funding from Japan Agency for Medical Research and Development and having other financial relationships with Pfizer, Chugai Pharma, Astellas, Kyowa Kirin, Otsuka, Celgene, Sysmex, and AbbVie. J.K. reports receiving research funding from Asahi Kasei, Takeda, Pfizer, Shionogi, Chugai Pharma, and Kyowa Kirin and receiving honoraria from Asahi Kasei, Takeda, Pfizer, Chugai Pharma, and Kyowa Kirin. Y.T. reports receiving research funding from Takeda, Chugai Pharma, Kyowa Kirin, Astellas, Ono Pharmaceutical, Bristol-Myers Squibb, Pfizer, TAIHO, and Taisho Toyama and receiving honoraria from Celgene. D.M. reports receiving research funding from Mundipharma, Janssen Pharmaceutical, Takeda, Chugai Pharma, Eisai, Ono Pharmaceutical, Celgene, AbbVie, MSD, Astellas, Amgen, Otsuka, Novartis, Pfizer, Bayer, Zenyaku Kogyo, Solasia, AstraZeneca, Kyowa Kirin, Sanofi, Bristol-Myers Squibb, Daiichi Sankyo, Symbio, and IQVIA and receiving honoraria from Mundipharma, Janssen Pharmaceutical, Takeda, Chugai Pharma, and Eisai. K.I. reports receiving research funding from SymBio, Bayer, AbbVie, and Novartis and receiving honoraria from Takeda, Ono Pharmaceutical, Chugai Pharma, Eizai, Novartis, and Celgene. M.O. reports receiving consultant fees from Berastem, DenovoBiopharma, Celltrion, Teva-Takeda, Daiichi Sankyo, Meiji Seika Pharma, Mundipharma, Eisai, and SymBio and receiving honoraria from Cellgene, Meiji Seika Pharma, and Chugai Pharma. T.Y. reports receiving research funding from Chugai Pharma. T.K. reports receiving consultant fees from Daiich-Sankyo, Ono Pharmaceutical and HUYA Bioscience International; receiving research funding from Daiich-Sankyo, HUYA Bioscience International, Celgene, Chugai Pharma, Byer, and Eizai; and receiving honoraria from Celgene, Chugai Pharma, Byer, Eizai, Kyowa Kirin, Mundipharma, and Takeda. H.N. reports receiving research funding from Chugai Pharma, Celgene, Mundipharma, Bayer, Takeda, Kyowa Kirin, Esai, Ono Pharmaceutical, Zenyaku Kogyo, Solasia Pharma, AstraZeneca, SymBio, IQVIA, Micron, and Nichirei Bioscience and receiving honoraria from Janssen Pharmaceutical, Celgene, Mundipharma, Bayer, Takeda, Kyowa Kirin, Esai, Bristol-Myers Squibb, Ono Pharmaceutical, Zenyaku Kogyo, AstraZeneca, SymBio, and Sanofi. The remaining authors declare no competing financial interests.
A list of the members of the Japan Clinical Oncology Group appears in the supplemental appendix.
Correspondence: Ken Ohmachi, Tokai University School of Medicine, 143 Shimokasuya, Kanagawa 259-1193, Japan; e-mail: 8jmmd004@is.icc.u-tokai.ac.jp.
References
Author notes
The full-text version of this article contains a data supplement.