TO THE EDITOR:
Despite dramatic improvements in survival, multiple myeloma (MM) remains largely incurable, and most patients develop disease that is refractory to available treatment options.1 Use of chimeric antigen receptor T-cell therapy (CART) is a novel approach that is associated with impressive outcomes in heavily pretreated patients. Given the rapid evolution of this treatment paradigm, we assessed the efficacy and toxicity of CART for MM utilizing the most up-to-date results.
Four databases were searched (Web of Science/MEDLINE/PubMed, Embase, and Cochrane Registry of Controlled Trials). An example search strategy is shown in supplemental Table 1. Two independent reviewers (G.R.M., A.R.) screened all studies, and conflict was resolved through mutual discussion. This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations.2
Our search strategy was restricted to include all prospective trials exclusively enrolling ≥2 patients with MM that were published in manuscript or presented in abstract form from 1 January 2013 through 15 November 2020. Furthermore, all abstracts that were presented live at the 62nd American Society of Hematology Annual Meeting were included with most updated information. All other studies, including editorials, case reports, case series, and review articles, were excluded.
The primary outcomes were the pooled response rate for all MM CART, pooled rate of grade 3/4 cytokine release syndrome (CRS), and pooled immune effector cell–associated neurotoxicity syndrome (ICANS). Proportional outcomes were pooled using a random effects model, and the DerSimonian and Laird Method with a correction factor of 0.5 was used. Statistical software Open Meta-Analyst (Brown School of Public Health) was used for calculations. The I2 statistic was used to test for heterogeneity between the studies. The I2 of values of <30%, 30% to 60%, 61% to 75%, and >75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively.3,4 Data were collected by 3 independent reviewers (G.R.M., A.R., and N.B.) and stored using Microsoft Excel. Variables collected include demographic information of participants, information on safety (ICANS, CRS), and efficacy outcomes (response rate, minimal residual disease data, duration of response, progression-free survival [PFS]).
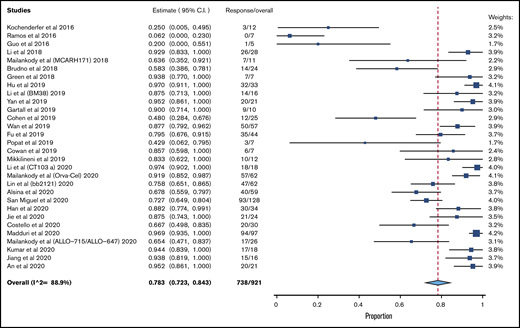
A total of 30 clinical trials that met inclusion criteria was included (supplemental Figure 1). A total of 921 patients was evaluable for efficacy analysis, and 950 patients were available for safety analysis, as pertains to CRS. A total of 781 patients was available for safety analysis of ICANS. Table 1 lists the characteristics/outcomes of these studies. The median prior lines of therapy was 6, based on the 21 studies that reported that data, and 74.4% of patients were triple refractory, among the 5 studies that clearly reported that data.
Characteristics and outcomes of MM CART studies
| Study . | Study name . | Year of most recent report . | Target . | No. patients (efficacy) . | Median prior lines, n . | Grade 3-4 CRS, % . | Grade 3-4 ICANS, % . | ORR, % . | MRD, % . | mDOR, mo . | PFS, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kochenderfer7 | NR | 2016 | BCMA | 12 | NR | NR | NR | 25 | NR | NR | NR |
| Ramos et al8 | KCAR | 2016 | κ light chain | 7 | 5.7 | 0 | 0 | 0 | 14.2 | NR | NR |
| Guo et al9 | NR | 2016 | CD 138 | 5 | NR | NR | NR | 20 | NR | NR | NR |
| Li et al10 | NR | 2018 | BCMA | 28 | NR | 14.3 | NR | 92.9 | NR | NR | NR |
| Mailankody et al11 (MCARH171) | MCARH171 | 2018 | BCMA | 11 | 6 | 20 | 0 | 63.6 | NR | 3.5 | NR |
| Brudno et al12 | NR | 2018 | BCMA | 24 | 9.5 | 25 | 12.5 | 58.3 | 50 | NR | NR |
| Green et al13 | NR | 2018 | BCMA | 7 | 8 | 0 | 0 | 100 | NR | NR | NR |
| Hu et al14 | NR | 2019 | BCMA | 33 | NR | 48.4 | NR | 96.9 | 97.9 | NR | 70.7% at 1 y |
| Li et al15 (BM38) | BM38 | 2019 | BCMA | 16 | NR | 25 | 0 | 87.5 | 87.5 | NR | NR |
| Yan et al16 | NR | 2019 | BCMA/CD19 | 21 | 6 | 4.8 | NR | 95.2 | 80.9 | NR | NR |
| Garfall et al17 | CTL119 | 2019 | BCMA/CD19 | 10 | 3.6 | 0 | 0 | 90 | 50 | NR | NR |
| Cohen et al18 | CAR-BCMA | 2019 | BCMA | 25 | 7 | 32 | 12 | 48 | 20 | 4.1 | NR |
| Wang et al19 | LCAR-B38M | 2019 | BCMA | 57 | NR | 7 | 1 | 87.7 | 68.4 | 22 | 20 |
| Fu et al20 | NR | 2019 | BCMA | 44 | NR | 6 | 0 | 79.5 | 36.3 | NR | 15 |
| Popat et al21 | AUTO2 | 2019 | BCMA, TACI | 7 | 5 | 0 | 0 | 42.9 | NR | NR | NR |
| Cowan et al22 | NR | 2019 | BCMA | 7 | 10 | 0 | 0 | 85.7 | 71.4 | NR | NR |
| Mikkilineni et al23 | FHVH-BCMA-T | 2019 | BCMA | 12 | 6 | 8.3 | 8.3 | 83.3 | NR | NR | NR |
| Li et al24 (CT103 a) | CT103A | 2020 | BCMA | 18 | NR | 39 | 0 | 100 | NR | NR | NR |
| Mailankody et al25 (Orva-Cel) | Orva-cel | 2020 | BCMA | 62 | 6 | 1.6 | 3.2 | 91.93 | NR | NR | NR |
| Lin et al26 (bb2121) | bb2121 | 2020 | BCMA | 62 | NR | 6.5 | 3.2 | 75.8 | 48.4 | 18.1 | 8.8 |
| Alsina et al27 | BB21217 | 2020 | BCMA | 59 | 6 | 4.3 | 4.3 | 67.8 | NR | 6 | NR |
| San Miguel et al28 | Ide-Cel | 2020 | BCMA | 128 | 6 | 5.5 | 3.1 | 72.7 | NR | 10.6 | 8.6 |
| Han et al29 | NR | 2020 | BCMA | 34 | 10 | 2.9 | NR | 88.2 | NR | NR | NR |
| Hao et al30 | CT053 | 2020 | BCMA | 24 | 4.5 | 0 | 4.2 | 87.5 | 70.8 | 21.8 | 18.8 |
| Costello et al31 | p-BCMA-101 | 2020 | BCMA | 30 | 7 | 0 | 3.6 | 66.7 | NR | NR | NR |
| Madduri et al32 | Cilta-cel | 2020 | BCMA | 97 | 6 | 4.1 | 10.3 | 96.9 | 50.5 | NR | NR |
| Mailankody et al33 (ALLO-715/ALLO-647) | ALLO-715/ALLO-647 | 2020 | BCMA | 26 | 5 | 0 | 0 | 65.4 | NR | NR | NR |
| Kumar et al34 | CT053 | 2020 | BCMA | 18 | 5 | 0 | 0 | 94.4 | 61.1 | NR | NR |
| Jiang et al35 | GC012F | 2020 | BCMA/CD19 | 16 | 5 | 11.2 | 0 | 93.8 | 68.8 | NR | NR |
| An et al36 | C-CAR088 | 2020 | BCMA | 21 | 4 | 4.7 | 0 | 95.2 | NR | NR | NR |
| Study . | Study name . | Year of most recent report . | Target . | No. patients (efficacy) . | Median prior lines, n . | Grade 3-4 CRS, % . | Grade 3-4 ICANS, % . | ORR, % . | MRD, % . | mDOR, mo . | PFS, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kochenderfer7 | NR | 2016 | BCMA | 12 | NR | NR | NR | 25 | NR | NR | NR |
| Ramos et al8 | KCAR | 2016 | κ light chain | 7 | 5.7 | 0 | 0 | 0 | 14.2 | NR | NR |
| Guo et al9 | NR | 2016 | CD 138 | 5 | NR | NR | NR | 20 | NR | NR | NR |
| Li et al10 | NR | 2018 | BCMA | 28 | NR | 14.3 | NR | 92.9 | NR | NR | NR |
| Mailankody et al11 (MCARH171) | MCARH171 | 2018 | BCMA | 11 | 6 | 20 | 0 | 63.6 | NR | 3.5 | NR |
| Brudno et al12 | NR | 2018 | BCMA | 24 | 9.5 | 25 | 12.5 | 58.3 | 50 | NR | NR |
| Green et al13 | NR | 2018 | BCMA | 7 | 8 | 0 | 0 | 100 | NR | NR | NR |
| Hu et al14 | NR | 2019 | BCMA | 33 | NR | 48.4 | NR | 96.9 | 97.9 | NR | 70.7% at 1 y |
| Li et al15 (BM38) | BM38 | 2019 | BCMA | 16 | NR | 25 | 0 | 87.5 | 87.5 | NR | NR |
| Yan et al16 | NR | 2019 | BCMA/CD19 | 21 | 6 | 4.8 | NR | 95.2 | 80.9 | NR | NR |
| Garfall et al17 | CTL119 | 2019 | BCMA/CD19 | 10 | 3.6 | 0 | 0 | 90 | 50 | NR | NR |
| Cohen et al18 | CAR-BCMA | 2019 | BCMA | 25 | 7 | 32 | 12 | 48 | 20 | 4.1 | NR |
| Wang et al19 | LCAR-B38M | 2019 | BCMA | 57 | NR | 7 | 1 | 87.7 | 68.4 | 22 | 20 |
| Fu et al20 | NR | 2019 | BCMA | 44 | NR | 6 | 0 | 79.5 | 36.3 | NR | 15 |
| Popat et al21 | AUTO2 | 2019 | BCMA, TACI | 7 | 5 | 0 | 0 | 42.9 | NR | NR | NR |
| Cowan et al22 | NR | 2019 | BCMA | 7 | 10 | 0 | 0 | 85.7 | 71.4 | NR | NR |
| Mikkilineni et al23 | FHVH-BCMA-T | 2019 | BCMA | 12 | 6 | 8.3 | 8.3 | 83.3 | NR | NR | NR |
| Li et al24 (CT103 a) | CT103A | 2020 | BCMA | 18 | NR | 39 | 0 | 100 | NR | NR | NR |
| Mailankody et al25 (Orva-Cel) | Orva-cel | 2020 | BCMA | 62 | 6 | 1.6 | 3.2 | 91.93 | NR | NR | NR |
| Lin et al26 (bb2121) | bb2121 | 2020 | BCMA | 62 | NR | 6.5 | 3.2 | 75.8 | 48.4 | 18.1 | 8.8 |
| Alsina et al27 | BB21217 | 2020 | BCMA | 59 | 6 | 4.3 | 4.3 | 67.8 | NR | 6 | NR |
| San Miguel et al28 | Ide-Cel | 2020 | BCMA | 128 | 6 | 5.5 | 3.1 | 72.7 | NR | 10.6 | 8.6 |
| Han et al29 | NR | 2020 | BCMA | 34 | 10 | 2.9 | NR | 88.2 | NR | NR | NR |
| Hao et al30 | CT053 | 2020 | BCMA | 24 | 4.5 | 0 | 4.2 | 87.5 | 70.8 | 21.8 | 18.8 |
| Costello et al31 | p-BCMA-101 | 2020 | BCMA | 30 | 7 | 0 | 3.6 | 66.7 | NR | NR | NR |
| Madduri et al32 | Cilta-cel | 2020 | BCMA | 97 | 6 | 4.1 | 10.3 | 96.9 | 50.5 | NR | NR |
| Mailankody et al33 (ALLO-715/ALLO-647) | ALLO-715/ALLO-647 | 2020 | BCMA | 26 | 5 | 0 | 0 | 65.4 | NR | NR | NR |
| Kumar et al34 | CT053 | 2020 | BCMA | 18 | 5 | 0 | 0 | 94.4 | 61.1 | NR | NR |
| Jiang et al35 | GC012F | 2020 | BCMA/CD19 | 16 | 5 | 11.2 | 0 | 93.8 | 68.8 | NR | NR |
| An et al36 | C-CAR088 | 2020 | BCMA | 21 | 4 | 4.7 | 0 | 95.2 | NR | NR | NR |
mDOR, median duration of response; MRD, minimal residual disease; NR, not reported; ORR, overall response rate.
The pooled response rate was 78.3% (95% confidence interval [CI], 72.3-84.3; I2, 88.9) (Figure 1). The pooled grade 3/4 or higher CRS rate was 6.4% (95% CI, 4.1-8.8; I2, 62.6) (supplemental Figure 2), and the pooled grade 3/4 ICANS rate was 3.5% (95% CI, 2.2-4.9; I2, 0) (supplemental Figure 3). The risk of bias is reported in supplemental Table 2.
Because the vast majority of studies used BCMA as the sole target, a subgroup analysis of efficacy was done exclusively for BCMA CART. The pooled response rate for these 24 studies was 81.9% (95% CI, 76.6-87.7; I2, 84.0). A total of 13 studies had a median prior lines of treatment ≥ 6. In these studies, the pooled response rate was 79.6% (95% CI, 71.3-87.9; I2, 88.6%).
Our study is the most current and comprehensive meta-analysis of CART therapies in MM, with 950 patients included. With a pooled response rate of 78% in heavily pretreated patients, these results are promising. Progression-free survival (PFS) has not been reported for the majority of studies as a result of the short duration of follow-up or it was inconsistently reported for different dosing strategies or for different end points (eg, 9-month PFS, 6-month PFS), precluding a quantitative synthesis. When reported, the median PFS ranged from 8 to 20 months, which is significantly greater than currently available treatments for this patient population. The use of allogeneic products that are currently being evaluated, such as ALLO-715/ALLO-647, as well as products that are easily administered in an outpatient setting (p-BCMA-101), may allow these treatments to reach a wider population. Although the vast majority of constructs have targeted BCMA, other targets under consideration include NKG2D, SLAMF7, and CD229.5
Our analysis has several limitations. We used per-protocol analysis, as reported by the individual studies; hence, patients who progress while awaiting products are excluded from analysis. Thus, our response rate likely overestimates the intention-to-treat response rate, should these products be used off-protocol in a broader population. Conversely, we analyzed all doses used in dose-escalation studies, and it is possible that the use of higher doses in subsequent studies leads to higher response rates. It also must be noted that different manufacturing protocols can be used within a study, leading to different response rates and heterogeneity within a study. Because of the limited number of studies having a sufficiently long follow-up to report on median duration of follow-up or a median PFS, a composite outcome was not computed for those variables. The I2 statistic in our study indicates significant heterogeneity for efficacy outcomes, likely owing to the inclusion of several studies with small sample sizes and variability in observed efficacy.
In summary, CART for MM appears to be a promising therapy with a high response rate and comparatively low rates of toxicity compared with CD19-targeted therapy.6 Its use in earlier lines in less pretreated populations, as well as newer constructs with more durable responses, is expected to further improve efficacy.
Data sharing requests should be sent to Ghulam Rehman Mohyuddin (rehmanm@gmail.com).
Acknowledgment:
The authors thank Lee Wades for assistance with the literature search.
Contribution: G.R.M. conceived the study, performed statistics, and wrote the manuscript; A.R. and N.B. assisted with the literature search and data collection and revised the manuscript; M.A. conceived the search strategy and cross-checked the statistical calculations; and B.M., D.W.S., and S.K.K. provided critical input and extensively revised the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: D.W.S. has acted as a consultant for Janssen Pharmaceuticals, SkylinDx, GlaxoSmithKline, Legend Biotech, Amgen, and Celgene. S.K.K. has received institutional research funding for clinical trials from Celgene, Takeda, Janssen Pharmaceuticals, Bristol Myers Squibb, KITE, Merck, AbbVie, Medimmune, Novartis, Roche-Genentech, Amgen, TeneoBio, and CARsgen Therapeutics; has acted as a consultant for and/or served on Advisory Boards (with no personal payment) for Celgene, Takeda, Janssen Pharmaceuticals, AbbVie, Genentech, Amgen, and Molecular Partner; and has acted as a consultant for and/or served on Advisory Boards (with personal payment) for Oncopeptides, GeneCentrix, and Cellectar. The remaining authors declare no competing financial interests.
Correspondence: Ghulam Rehman Mohyuddin, Kansas University Medical Center, 2350 Shawnee Mission Parkway, Westwood, KS 66160; e-mail: rehmanm@gmail.com.
References
Author notes
The full-text version of this article contains a data supplement.