TO THE EDITOR:
The red blood cell distribution width (RDW) is a marker that describes the degree of heterogeneity of erythrocyte volume. Because it is part of the routine automated output of blood cell counters, the RDW is included in the diagnostic work-up of most hematologic diseases and is easily and inexpensively assessed. Red blood cells (RBCs) typically decrease in cellular volume across their lifespan, which is why a delayed clearance of older erythrocytes leads to higher RDW levels.1 Subsequently, a higher RDW mirrors a dysregulated erythrocyte homeostasis with either impaired erythropoiesis (including reticulocytosis and macrocytosis; eg, due to toxic effects) or abnormal (prolonged) RBC survival. Although, historically, the clinical meaning of the RDW was limited to the differential diagnosis of anemia, a wider applicability recently became evident. Increased RDW levels are associated with a higher mortality in healthy people, which has been linked to oxidative stress, poor nutritional status, and older age.2 Data also suggest that a high RDW defines a proinflammatory state,3 leading to a higher incidence of cardiovascular diseases, including heart failure, coronary heart disease, and cardiac mortality, as well as a greater likelihood of developing a variety of malignancies.4
The presence of clonal hematopoiesis of indeterminate potential (CHIP) in older individuals is associated with a higher risk for developing a myeloid neoplasm and a higher RDW (>14.5%) at CHIP detection.5 In patients with unexplained cytopenias, a high RDW independently predicted the diagnosis of a myelodysplastic syndrome (MDS)6 and discriminated blood samples from healthy individuals and from patients with MDS.7 Additionally, in healthy individuals, a higher RDW is associated with a higher risk for developing an acute myeloid leukemia (AML) several years later.4,8 However, no study has assessed the clinical value of RDW levels in patients diagnosed with AML.
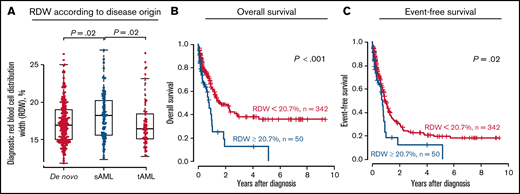
We analyzed a cohort of 294 patients newly diagnosed with AML (median age at diagnosis, 60.6 years; range, 14.3-76.5). Details on therapies and patients’ characteristics are given in supplemental Material and supplemental Table 1, respectively. The RDW was measured at diagnosis in all patients before the start of cytoreductive therapies; it was above the upper limit of normal (>15%) in 73% of patients (n = 216). The RDW, regarded as a continuous parameter, was significantly higher in patients harboring an AML developing from an antecedent myeloid disorder (MDS, myeloproliferative neoplasm [MPN], or MDS/MPN) than in patients with de novo (P = .01) or treatment-related AML (after lymphatic or solid neoplasm, P = .02; Figure 1A). We also observed higher RDW levels in patients harboring gene mutations linked to AML of secondary origin.9,10 Compared with patients with a wild-type mutation status, RDW levels were significantly higher in patients with a JAK2 mutation (P = .03), ASXL1 mutation (P = .004), or spliceosome mutation (P = .03, comprising SF3B1, SRSF2, U2AF1, and ZRSR2; Figure 1B), which was particularly driven by SRSF2 mutations (P = .002; supplemental Figure 1).
Associations and outcomes according to RDW levels at AML diagnosis in patients treated with allogeneic HSCT (n = 294). Association with disease origin (A) and mutational status of genes known to associate with secondary disease (B). CIR (C) NRM (D), OS (E), and EFS (F) applying a 20.7% cut-point (high vs low). mut, mutant; sAML, secondary AML; tAML, treatment-related AML; wt, wild-type.
Associations and outcomes according to RDW levels at AML diagnosis in patients treated with allogeneic HSCT (n = 294). Association with disease origin (A) and mutational status of genes known to associate with secondary disease (B). CIR (C) NRM (D), OS (E), and EFS (F) applying a 20.7% cut-point (high vs low). mut, mutant; sAML, secondary AML; tAML, treatment-related AML; wt, wild-type.
All patients underwent an allogeneic hematopoietic stem cell transplantation (HSCT) at the University Hospital Leipzig while in first (54%) or second (9%) complete remission (CR) or CR with incomplete peripheral recovery (18%) or with active disease (19%). The collection and analysis of patient samples were approved by the university's institutional review board and were performed in accordance with the Declaration of Helsinki.
With regard to the prognostic relevance of a myeloid neoplasm at diagnosis, higher-than-normal RDW levels have been linked to worse event-free survival (EFS) in chronic myeloid leukemia (CML)11 and shorter overall survival (OS) in patients with MDS.12,13 In our study, we did not observe any prognostic significance of a RDW above the upper limit of normal range (ie, 15%) with regard to the cumulative incidence of relapse (CIR; P = .65), nonrelapse mortality (NRM; P = .49), OS (P = .60), or EFS (P = .90; supplemental Figure 2). However, when introducing an optimal cut-point derived by receiver operator characteristics, a higher RDW (>20.7%) was associated with a significantly higher NRM (P = .02; Figure 1D), which also translated into shorter OS (P = .009; Figure 1E) and a trend for shorter EFS (P = .07; Figure 1F), but similar CIR (P = .96; Figure 1C). In multivariate analyses, the RDW retained its prognostic significance for a higher NRM after adjustment for age at diagnosis, whereas the Eastern Cooperative Oncology Group Performance Status at HSCT and the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) did not remain in the final model (supplemental Table 2). Similar results were obtained when we restricted our analyses to patients transplanted while in morphologic remission (CIR, P = .43; NRM, P = .05; OS, P < .001; EFS, P = .01; supplemental Figure 3). Importantly, the RDW was not significantly associated with a particular cause of death (P = .29; supplemental Table 1). When adapting the optimal cut-point, a high RDW again was associated with secondary AML (P = .05; supplemental Table 1) and mutations of genes associated with secondary AML origin, such as JAK2 (P = .05), ASXL1 (P = .02), and, by trend, SRSF2 (P = .09). We also observed a trend for a lower incidence of NPM1 mutations (P = .06), which are usually enriched in de novo AML. In patients with MDS, a high RDW was associated with low hemoglobin levels and higher neutrophils,13 whereas it was associated with female sex, a higher white blood cell count, higher blast percentages, and lower hemoglobin levels in patients with CML.11 High RDW was also associated with lower hemoglobin levels (P = .002) in this AML cohort. Similar to data in MDS,12,13 RDW levels were not associated with chromosomal abnormalities or disease risk, according to the ELN2017 risk stratification. Despite the described association of a high RDW with a higher cardiovascular and inflammatory risk in our study, the HCT-CI risk score at HSCT (P = .77) and the incidence of acute or chronic graft-versus-host disease (P = 1.0 and P = .63, respectively) did not differ according to RDW levels in patients with AML. Because our transplant cohort may have been biased toward higher-risk AML (including secondary and therapy-related AMLs) and to verify our results, irrespective of the consolidating therapy, we analyzed a second AML cohort treated at the University Hospital Dresden (n = 392; for details see supplemental Material and supplemental Table 3); 46% of patients received chemotherapy alone, and patients who underwent allogeneic HSCT were censored at the time of HSCT. In this validation cohort, RDW levels at diagnosis were also higher in patients with secondary AML than in patients with de novo AML (P = .02) or treatment-related AML (P = .02; Figure 2A). RDW levels at diagnosis were above the upper limit of normal in most patients (79%). When introducing the 20.7% optimal cut-point established in the primary patient set, validation patients with a high RDW at diagnosis had significantly shorter OS (P < .001; Figure 2B) and shorter EFS (P = .02; Figure 2C). Applying this cutoff, the validation patients with a high RDW also had lower hemoglobin levels at diagnosis (P = .001), a trend toward more secondary AML (P = .07), and were less likely to harbor a NPM1 mutation (P = .05). Similar to the primary set, no association was observed between the RDW and ELN2017 disease risk (P = .25).
Associations and outcome according to RDW levels at AML diagnosis in the validation set (n = 392). (A) Association with disease origin. OS (B) and EFS (C) applying a 20.7% cut-point (high vs low). sAML, secondary AML; tAML, treatment-related AML.
Associations and outcome according to RDW levels at AML diagnosis in the validation set (n = 392). (A) Association with disease origin. OS (B) and EFS (C) applying a 20.7% cut-point (high vs low). sAML, secondary AML; tAML, treatment-related AML.
In conclusion, our study is the first to evaluate the RDW in newly diagnosed patients with AML. Patients with secondary AML or secondary AML–associated gene mutations had higher diagnostic RDW levels. The presence of a high diagnostic RDW identifies patients with AML with worse outcomes, irrespective of the ELN2017 risk classification or the applied consolidation treatment, which were attributed to a higher treatment-associated mortality. This is consistent with data in healthy individuals; a high RDW is associated with the onset of metabolic and cardiovascular diseases,4 and a high RDW predicted anthracycline-induced cardiotoxicity in patients with cancer.14 Subsequently, the RDW, as a cost-effective and fast-to-assess parameter, allows the identification of patients who are at higher risk for treatment-related morbidity and mortality and who may benefit from less-intensive therapy regimens in the future.
Acknowledgments: The authors thank Christel Müller, Daniela Bretschneider, Evelin Hennig, Sabine Leiblein, Martina Pleß, Ulrike Bergmann, Janet Bogardt, Annette Jilo, and Dagmar Cron for help with cytogenetic, morphologic, and immunological analyses, as well as Julia Schulz, Christine Günther, Scarlett Schwabe, Ines Kovacs, and Kathrin Wildenberger for their help with sample processing.
Contribution: V.V., M.J., and S.S. designed and analyzed the study; M.J., D. Brauer, and D. Backhaus carried out the clinical and laboratory-based research; L.R., K.S., M.A.R., C.R., and M.B. cared for patients and provided the validation set; V.V., M.J., and S.S. performed statistical analyses; and G.-N.F., D.N., U.P., C.R., M.B., M.J., and S.S. provided administrative support; and all authors contributed to the writing the manuscript and agreed on the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madlen Jentzsch, Medical Clinic and Policlinic 1, Hematology, Cellular Therapy, and Hemostaseology, University of Leipzig Medical Center, Liebigstraße 22, Haus 7, Leipzig 04103, Germany; e-mail: madlen.jentzsch@medizin.uni-leipzig.de.
References
Author notes
S.S. and M.J. contributed equally to this study and are joint senior authors.
Requests for data sharing may be submitted to Madlen Jentzsch (madlen.jentzsch@medizin.uni-leipzig.de).
The full-text version of this article contains a data supplement.