Key Points
Postchemotherapy, of 100 CRMRD+ patients, 42% remained progression free at 1 year (spontaneous CRMRD− or low-level NPM1mut persistence).
Relapse is associated with FLT3-ITD and poor NPM1mut MRD response after chemotherapy. MRD-directed preemptive therapy may prolong remission.
Abstract
Monitoring of NPM1 mutant (NPM1mut) measurable residual disease (MRD) in acute myeloid leukemia (AML) has an established role in patients who are treated with intensive chemotherapy. The European LeukemiaNet has defined molecular persistence at low copy number (MP-LCN) as an MRD transcript level <1% to 2% with a <1-log change between any 2 positive samples collected after the end of treatment (EOT). Because the clinical impact of MP-LCN is unknown, we sought to characterize outcomes in patients with persistent NPM1mut MRD after EOT and identify factors associated with disease progression. Consecutive patients with newly diagnosed NPM1mut AML who received ≥2 cycles of intensive chemotherapy were included if bone marrow was NPM1mut MRD positive at the EOT, and they were not transplanted in first complete remission. One hundred patients were followed for a median of 23.5 months; 42% remained free of progression at 1 year, either spontaneously achieving complete molecular remission (CRMRD−; 30%) or retaining a low-level NPM1mut transcript (12% for ≥12 months and 9% at last follow-up). Forty percent met the criteria for MP-LCN. Preemptive salvage therapy significantly prolonged relapse-free survival. Risk factors associated with disease progression were concurrent FLT3-internal tandem duplication at diagnosis and suboptimal MRD response (NPM1mut reduction <4.4-log) at EOT.
Introduction
Acute myeloid leukemia (AML) with mutated NPM1 (NPM1mut) is the most common subtype of AML with recurrent genetic abnormalities.1 Monitoring NPM1mut measurable residual disease (MRD) has an established role in the evaluation of patients after intensive chemotherapy2,3 and is an emerging regulatory approval end point.4 Approximately 25% to 50% of patients with NPM1mut have persistent MRD at the end of treatment (EOT), which is associated with a higher risk for relapse.2,5,6 The European LeukemiaNet (ELN) has defined molecular persistence at low copy number (MP-LCN) as MRD positivity in complete remission (CR) with <1000 to 2000 transcripts per 105 copies of ABL and a relative increase <1-log between 2 positive samples collected after EOT.7 The UK National Cancer Research Institute working group reported the impact of pretransplant NPM1mut MRD on relapse risk after allogeneic hematopoietic stem cell transplantation (HSCT);8 however, the clinical fate of patients with persistent NPM1mut not undergoing transplantation is unknown. Therefore, we sought to characterize the clinical impact of NPM1mut MRD in patients at EOT and identify the factors associated with disease progression.
Methods
Consecutive patients with newly diagnosed NPM1mut AML were included if they had received ≥2 cycles of intensive chemotherapy, NPM1mut MRD was present in the bone marrow at EOT, and the patient was not planned for HSCT in the first CR. Patients in the United Kingdom were prospectively enrolled in the National Cancer Research Institute AML17 study (2009-2014) and AML19 study (2017 to June of 2019) (see supplemental Figure 1 for consort diagram). Australian patients were retrospectively identified (2015-2020). Bone marrow samples were analyzed by quantitative reverse transcription polymerase chain reaction, as previously described,2 with the copy numbers expressed per 105 copies of ABL. The study was approved by the Alfred Health (88/20) and Wales (14/WA/1056) research ethics committee and was conducted in accordance with the Declaration of Helsinki. ELN MRD recommended definitions7 of molecular progression or relapse (collectively termed molecular failure), MP-LCN, and complete molecular remission (CRMRD−) were used. The ELN criteria for molecular failure were not strictly met in 2 subjects: 1 for molecular progression not reaching a ≥1-log10 increase, and another had molecular relapse without a confirmation sample. Survival estimates were calculated from EOT to death (overall survival [OS]), and/or morphologic relapse (relapse-free survival [RFS]), and/or molecular failure (event-free survival [EFS]). The Cox model was used for univariate and multivariate analyses.
Results and discussion
A total of 100 patients had NPM1mut MRD detected at a median of 36 days (range, 19-90) from the last course of chemotherapy (characteristics are summarized in supplemental Figure 2 and supplemental Table 1). Median age was 50 years, 85% had a normal karyotype, and 39% had concurrent FLT3-internal tandem duplication (ITD) at diagnosis (allelic ratio ≥ 0.5 in 17%). At EOT, the median NPM1mut level was 13 copies (range, 0.3-20 756; 5 patients had levels > 2000), and median reduction from baseline was 4.5-log (range, 1.8-6.0; baseline data were missing in 7 patients). In this study cohort, MRD levels at EOT were comparable, irrespective of the number of chemotherapy courses completed (range, 2-5) (Supplemental Figure 3). Patients with longer time to EOT MRD assessment had longer EFS and RFS (Supplemental Figure 4).
Patients were followed for a median of 23.5 months: 29 patients died, including 2 after preemptive intensive salvage. A total of 41 patients experienced morphologic relapse, including 16 without detection of preceding molecular failure: 8 had MRD testing within 90 days of relapse (range, 6-73 days), 3 were tested after a >90 day-interval; 1 had extramedullary disease progression; 1 had NPM1 wild-type relapse; and 3 were not monitored. Thirty-nine (40% of 97 monitored) had subsequent MRD levels meeting the criteria for MP-LCN, with a 2-year EFS of 58% (supplemental Figure 5).
To study the clinical impact of persistent NPM1mut MRD detected at the EOT, we studied the fate of patients at 3-month intervals. By the first landmark analysis at 3 months, 9% of patients had experienced morphologic relapse, 27% had molecular failure, and 15% spontaneously achieved sustained CRMRD− (Figure 1). By the 12-month landmark, 15% had morphologically relapsed, 43% had molecular failure, 30% had CRMRD−, and 12% had MP-LCN (Figure 1). At the final follow-up, 9 patients (9%) had MP-LCN persisting for a median of 14 months (range, 9-32 months) and survived for a median of 20.5 months (range, 9-35 months). From study entry, 31 patients (32%) spontaneously achieved CRMRD− that lasted for >6 months, with the majority (81%) achieving CRMRD− within the first 6 months and only 1 patient subsequently progressing during the follow-up period. MP-LCN is a well characterized phenomenon in core-binding factor AML.9,10 Persistence of NPM1mut transcripts above 1000 to 2000 copies per 105ABL is associated with higher risk of disease relapse,5,6 leading to the adoption of this threshold by the ELN.7 We were unable to confirm this threshold in our study because patients received preemptive therapy prior to reaching this threshold. However, 10 patients with molecular failure (>1-log increase) who did not receive preemptive therapy all relapsed. Of the patients who remained event-free at last follow-up (n = 39), the highest recorded NPM1mut MRD level was only 90 copies. Paired peripheral blood and bone marrow sampling was not performed in this study. Therefore, the clinical relevance of MP-LCN in peripheral blood was not able to be addressed.
Clinical impact of patients with detectable NPM1mut MRD at completion of chemotherapy. Conversion from initial state (molecular persistence) to different outcomes censored after the first event, either sustained CRMRD−, molecular failure, or morphologic relapse without preceding molecular failure. The proportion of patients in each category at specified time points are listed in the table below.
Clinical impact of patients with detectable NPM1mut MRD at completion of chemotherapy. Conversion from initial state (molecular persistence) to different outcomes censored after the first event, either sustained CRMRD−, molecular failure, or morphologic relapse without preceding molecular failure. The proportion of patients in each category at specified time points are listed in the table below.
Among the 43 patients (44%) who experienced molecular failure within the first 12 months, 33 (77%) received preemptive salvage therapy prior to morphologic relapse, resulting in a significantly prolonged RFS compared to those not receiving preemptive therapy (median, 10.6 vs 0.7 months) (supplemental Figure 6). Time to molecular failure from EOT was similar between patients receiving or not receiving preemptive therapy (median, 64 vs 99 days).
Median time to initiation of preemptive therapy after molecular failure was 27 days (range, 9-149; supplemental Figure 7A), with therapy consisting of FLAG-based chemotherapy (n = 13), venetoclax plus low-dose cytarabine (n = 11), immediate HSCT (n = 5), or others (supplemental Figure 2). Of 10 patients not salvaged until morphologic relapse, the median interval between molecular failure and morphologic relapse was 21 days (range, 11-89; supplemental Figure 7B; salvage therapy is summarized in supplemental Figure 2). Various MRD-directed preemptive strategies have been used, ranging from azacitidine to intensive salvage chemotherapy8,11 ; we previously reported the promising role of venetoclax-based low-intensity therapy in eliminating NPM1mut MRD in 11 of 12 (92%) patients treated.12 The survival benefit of preemptive therapy requires future prospective evaluation.
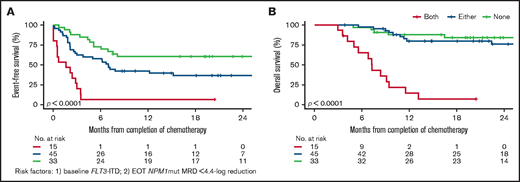
Next, we examined variables associated with molecular failure, morphologic relapse, and death (supplemental Table 2). In univariate analyses, variables associated (P < .1) with molecular failure were white cell count and FLT3-ITD at diagnosis, chemotherapy courses received, and EOT NPM1mut MRD levels. In multivariate analyses (and confirmed by backward selection), only baseline FLT3-ITD and EOT NPM1mut reduction retained significance; an optimal NPM1mut reduction threshold of 4.4-log between baseline and EOT was determined by maximally selected rank statistics.13 Most patients with both adverse factors (16%) relapsed within 4 months (median, 1.4 months), and few survived beyond 12 months (median, 7.3 months). Patients with 0 or 1 risk factor had a 1-year EFS of 42% to 60% and an OS of 80% to 88% (Figure 2). The prognostic value of NPM1mut MRD after induction chemotherapy was reported previously, with MRD still detectable in peripheral blood after 2 induction cycles2 and a <4-log reduction postinduction3 associated with 3-year relapse risks of 82% and 66%, respectively. In the subset analyses of the UK cohort, both adverse factors remained significant, in addition to the peripheral blood MRD status after course 2 (data not shown).
Kaplan-Meier survival curves. (A) Event-free survival and (B) overall survival according to FLT3-ITD status at diagnosis and at EOT NPM1mut reduction (4.4-log) from baseline.
Kaplan-Meier survival curves. (A) Event-free survival and (B) overall survival according to FLT3-ITD status at diagnosis and at EOT NPM1mut reduction (4.4-log) from baseline.
A sensitivity analysis revealed a similar MRD log reduction at EOT between the UK and Australian cohorts (median, 4.6 vs 4.4 log), standard vs high-dose cytarabine induction (4.5 vs 4.8-log), and treatment or not with gemtuzumab ozogamicin (4.8 vs 4.5 log). Among patients with positive FLT3-ITD at diagnosis, a trend for greater MRD reduction at EOT was observed among those who received midostaurin (5.1 vs 4.4 log, P = .18), but conversion to CRMRD− during the maintenance phase did not differ (36% vs 35%).
In conclusion, patients with NPM1mut MRD positivity after completing intensive chemotherapy and not undergoing HSCT in first remission have a variable course, with a substantial fraction (42%) remaining relapse-free at 1 year, either spontaneously achieving CRMRD− (30%) or retaining low-level transcript in the bone marrow for ≥12 months (12%). Risk factors associated with subsequent disease progression include concurrent FLT3-ITD at diagnosis and suboptimal MRD response at the EOT. This information will be of value to clinicians using NPM1mut MRD to make EOT transplant decisions. The role of preemptive treatment role in the management of molecular failure remains to be determined.
Acknowledgments
A.H.W. received Medical Research Future Fund, Leukemia and Lymphoma Society (SCOR Strasser), National Health and Medical Research Council. R.D. and N.H.R. received Cancer Research UK, Blood Cancer UK, and the UK National Institute for Health Research.
Authorship
Contribution: I.S.T., R.D., A.I., N.H.R., and A.H.W. designed research, analyzed data, and wrote the manuscript and C.H.K., J.A.K., N.T., K.M.S., A. Tedjaseputra, J.P.R., C.S.G., E.A., J.S., D.K.H., A.B., N.E.P., M.L.S., C.J.H., A. Thomas, and A.F.G. contributed patients or analytical tools, interpreted data, and approved the final version of the manuscript.
Conflict-of-interest disclosure: I.S.T. has served on speaker’s bureaus for Amgen and Servier and has acted as a consultant for Pfizer and Servier. R.D. has served on advisory boards for AbbVie, Jazz Pharmaceuticals, MENARINI, Novartis, and Pfizer; has received institutional research funding from AbbVie, Amgen; has served on speaker’s bureaus for Astellas and Novartis; and has acted as a consultant for AbbVie, Astellas, Jazz Pharmaceuticals, and Pfizer. C.S.G. has served on the advisory board for AbbVie. N.H.R. has served on advisory boards for Astellas and Pfizer; has received institutional research funding from Jazz Pharmaceuticals and Pfizer; and has served on speaker’s bureaus for Jazz Pharmaceuticals and Novartis. A.H.W. has served on advisory boards for Novartis, Janssen, Amgen, Roche, Pfizer, AbbVie, Servier, Celgene - Bristol Myers Squibb, MacroGenics, Agios, and Gilead; has received institutional research funding from Novartis, AbbVie, Servier, Celgene - Bristol Myers Squibb, Astra Zeneca, and Amgen; has served on speaker’s bureaus for AbbVie, Novartis, and Bristol Myers Squibb; and has received royalty payments from the Walter and Eliza Hall Institute of Medical Research related to venetoclax. The remaining authors declare no competing financial interests.
Correspondence: Andrew H. Wei, Blood Cells and Blood Cancer, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria 3052, Australia; e-mail: wei.a@wehi.edu.au.
References
Author notes
I.S.T., R.D., and A.I. contributed equally to this study.
N.H.R. and A.H.W. contributed equally to this study.
Data sharing requests should be sent to Andrew H. Wei (wei.a@wehi.edu.au).
The full-text version of this article contains a data supplement.