TO THE EDITOR:
To accurately evaluate the benefit of new cancer treatments, clinical trials must assess health-related quality of life (HRQOL) measures, as well as safety and antitumor efficacy. HRQOL measures reflect the physical and mental health of patients and are particularly important in hematological malignancies, in which some interventions have modest survival benefits but significant toxicities. Thus, studying how various hematologic malignancies and their treatment affect HRQOL is crucial to determining the best management strategies.1 Here, we analyzed the extent to which the field appreciates the value of HRQOL in pivotal clinical trials by calculating how often trials for drugs to treat hematological malignancies that resulted in US Food and Drug Administration (FDA) approval assessed patient QOL.
We obtained data on HRQOL from study protocols (https://clinicaltrials.gov/) and publicly available product labeling at Drugs@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). We analyzed drugs approved between January 2016 and May 2020 to reflect the most recent hematologic malignancies drug approvals. The studied hematologic malignancies included multiple myeloma, leukemias, non-Hodgkin lymphomas, Hodgkin lymphoma, and myelodysplastic syndrome. We reviewed primary, secondary, and exploratory outcome measures in each protocol. If any QOL measurement was included as part of the primary, secondary, or exploratory outcomes in the study protocol, we deemed this study to assess HRQOL. Only 69% of clinical trials had some results reported at clinicaltrials.gov. Therefore, for the studies that collected data on HRQOL, we systemically searched of PubMed, Cochrane Central Register of Controlled Trials, Scopus, and the bibliographies of the relevant articles using the National Clinical Trial number, study title/subtitle, authors names, and/or studied drug/s to ascertain whether the published studies that supported FDA drug approval reported QOL measures. To assess reporting of HRQOL, we analyzed all publications available by 10 February 2021 (our data analysis cutoff point). Each drug approval had at least 1 available publication or abstract presentation. The frequency of HRQOL assessment and subsequent reporting of HRQOL measures were analyzed.
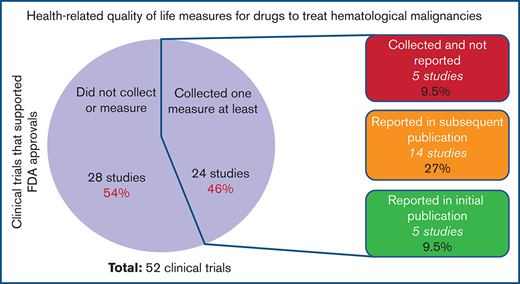
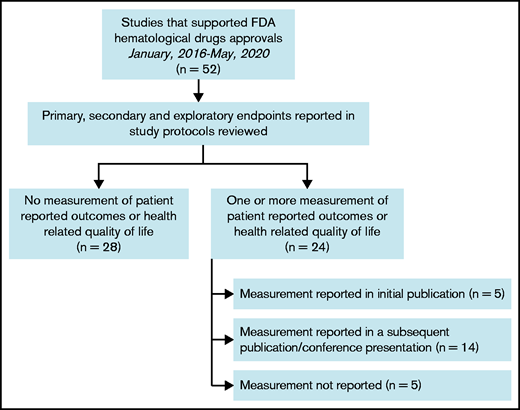
We examined a total of 52 clinical trials conducted in the studied period. These trials enrolled 16 246 patients, supporting 49 drug approvals. Only 24/52 (46%) of the clinical trials included at least 1 HRQOL metric as part of their end points. Of those trials, only 19 (79%) provided data on their measured HRQOL parameters (Figure 1). Of the clinical trials that reported measured HRQOL metrics, 26% (5/19) included them within the initial publication, whereas 74% (14/19) reported them in a different publication or at a conference or meeting (Table 1). In leukemias, chronic myeloid leukemia and acute lymphoblastic leukemia collected and reported HRQOL in 75% of clinical trials, whereas none of the trials supporting acute myeloid leukemia drug approvals collected HRQOL metrics. In multiple myeloma, 57% of clinical trials collected and reported an HRQOL metric. In lymphomas, only T-cell lymphoma trials collected and reported HRQOL metrics more than half of the time. Most clinical trials that collected HRQOL metrics used either 1 metric (38%) or 2 metrics (54%); only 2 clinical trials (8%) used 3 metrics. The most commonly used metrics were the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30) and the EuroQol 5 Dimension 5 survey. There was no statistically significant trend for improved collection or reporting of HRQOL over time. The trials that collected at least 1 HRQOL metric but did not report it (n = 5) tended to be more recent (2 trials in 2018, 1 trial in 2019, and 2 trials in 2020).
Frequencies of analyzed clinical trials
| Disease . | No. of trials . | No. of patients . | Range of patient numbers enrolled in all studies . | No. (%) of study protocols that assessed PRO and/or HRQOL . | No. (%) of reported PRO/HRQOL . | PRO or HRQOL measure/s used by clinical trials . |
|---|---|---|---|---|---|---|
| Leukemia | 26 | 7262 | 34-717 | 10 (38) | 8 (31) | Per disease |
| Chronic lymphoid | 8 | 3094 | 106-535 | 4 (50) | 2 (25) | FACT-Leu, EORTC QLQ-C30, EQ-5D-3L, QLQ-CLL16 |
| Chronic myeloid | 4 | 854 | 51-487 | 3 (75) | 3 (75) | FACT-Leu, EORTC QLQ-C30, EQ-5D-3L |
| Acute lymphoid | 4 | 922 | 75-405 | 3 (75) | 3 (75) | PedsQL, EORTC QLQ-C30, EQ-5D |
| Acute myeloid | 9 | 2312 | 34-717 | 0 (0) | 0 (0) | NA |
| Hairy cell | 1 | 80 | NA | 0 (0) | 0 (0) | NA |
| Lymphoma | 18 | 5212 | 80-1334 | 9 (50) | 7 (39) | Per disease |
| Hodgkin | 3 | 1639 | 95-1334 | 2 (66) | 1 (33) | EORTC QLQ-C30 |
| Diffuse large B cell | 3 | 281 | 80-108 | 1 (33) | 1(33) | FACT-Lym, SF-36 |
| Mantle cell | 2 | 210 | 86-124 | 1 (50) | 1 (50) | EORTC QLQ-C30 |
| Follicular | 6 | 2300 | 83-1202 | 3 (50) | 2 (33) | FACT-Lym, EQ-5D |
| Primary mediastinal B cell | 1 | 53 | NA | 0 (0) | 0 (0) | NA |
| T cell | 3 | 729 | 131-372 | 2 (66) | 2 (66) | FACT-G, EQ-5D, Skindex-29 |
| Multiple myeloma | 7 | 3543 | 122-1085 | 4 (57) | 4 (57) | EORTC QLQ-C30, EQ-5D, FACT-MM, QLQ-MY20 |
| Myelodysplastic syndrome | 1 | 229 | NA | 1 (100) | 0 (0) | EORTC QLQ-C30 |
| Total | 52 | 16,246 | 34-1334 | 24 (46) | 19 (37) | Per disease |
| Disease . | No. of trials . | No. of patients . | Range of patient numbers enrolled in all studies . | No. (%) of study protocols that assessed PRO and/or HRQOL . | No. (%) of reported PRO/HRQOL . | PRO or HRQOL measure/s used by clinical trials . |
|---|---|---|---|---|---|---|
| Leukemia | 26 | 7262 | 34-717 | 10 (38) | 8 (31) | Per disease |
| Chronic lymphoid | 8 | 3094 | 106-535 | 4 (50) | 2 (25) | FACT-Leu, EORTC QLQ-C30, EQ-5D-3L, QLQ-CLL16 |
| Chronic myeloid | 4 | 854 | 51-487 | 3 (75) | 3 (75) | FACT-Leu, EORTC QLQ-C30, EQ-5D-3L |
| Acute lymphoid | 4 | 922 | 75-405 | 3 (75) | 3 (75) | PedsQL, EORTC QLQ-C30, EQ-5D |
| Acute myeloid | 9 | 2312 | 34-717 | 0 (0) | 0 (0) | NA |
| Hairy cell | 1 | 80 | NA | 0 (0) | 0 (0) | NA |
| Lymphoma | 18 | 5212 | 80-1334 | 9 (50) | 7 (39) | Per disease |
| Hodgkin | 3 | 1639 | 95-1334 | 2 (66) | 1 (33) | EORTC QLQ-C30 |
| Diffuse large B cell | 3 | 281 | 80-108 | 1 (33) | 1(33) | FACT-Lym, SF-36 |
| Mantle cell | 2 | 210 | 86-124 | 1 (50) | 1 (50) | EORTC QLQ-C30 |
| Follicular | 6 | 2300 | 83-1202 | 3 (50) | 2 (33) | FACT-Lym, EQ-5D |
| Primary mediastinal B cell | 1 | 53 | NA | 0 (0) | 0 (0) | NA |
| T cell | 3 | 729 | 131-372 | 2 (66) | 2 (66) | FACT-G, EQ-5D, Skindex-29 |
| Multiple myeloma | 7 | 3543 | 122-1085 | 4 (57) | 4 (57) | EORTC QLQ-C30, EQ-5D, FACT-MM, QLQ-MY20 |
| Myelodysplastic syndrome | 1 | 229 | NA | 1 (100) | 0 (0) | EORTC QLQ-C30 |
| Total | 52 | 16,246 | 34-1334 | 24 (46) | 19 (37) | Per disease |
EQ-5D, EuroQol 5 Dimension 5; FACT-G, Functional Assessment of Cancer Therapy-General; FACT-Leu, Functional Assessment of Cancer Therapy-Leukemia; FACT-Lym, Functional Assessment of Cancer Therapy-Lymphoma; FACT-MM, Functional Assessment of Cancer Therapy-Multiple Myeloma; NA, not applicable; PedsQL, Pediatric Quality of Life Inventory; QLQ-CLL16, Quality of Life Questionnaire-Chronic Lymphocytic Leukemia Module; QLQ-MY20, Quality of Life Questionnaire-Multiple Myeloma Module; SF-36, Short Form 36.
Patient-reported outcomes (PROs), including HRQOL measures, are becoming an integral part of patient assessment in oncology clinical research and clinical practice.2 The use of HRQOL tools can facilitate the assessment of outcomes that are important to patients. Such measures can help capture patient experiences outside of their clinical visits and the impact of studied drugs on their daily life. These data will help patients and their physicians to choose the optimal available treatment. Common types of PRO measures include rating scales and counts of events. The FDA published guidance describing how to implement PROs. Though helpful in describing the process of PRO implementation, the guidance does not establish legally enforceable responsibilities.3 In 2020, the FDA Oncology Center of Excellence launched Project Patient Voice, a pilot project collecting patient-reported symptom data from clinical trials supporting FDA approval for certain cancer drugs.4 The goal of this project is to enable patients and their doctors to make better decisions because these symptom data are not usually included on drug labels but can provide valuable additional information.
A previous report indicated lower functioning scores and higher symptom burden scores in hematological malignancy survivors compared with population controls, suggesting HRQOL is especially important in this patient cohort.5 Our analysis revealed that HRQOL measures have been understudied in clinical trials leading to FDA drug approval in hematological malignancies. Fewer than one-half of the 52 studies we analyzed measured HRQOL, and most of those did not report the results of HRQOL measures in the initial publication. This problem is relevant to oncology more broadly because only a minority of new oncology drugs authorized by the European Medicines Agency from 2009 to 2013 were approved with evidence of improved QOL.6 Indeed, most oncology studies published in 3 high-impact journals were found to assess QOL only during the intervention phase, with a minority (3.4%) of studies assessing QOL until time of death.7
Despite the limited number of well-developed, disease-specific instruments to measure HRQOL, multiple validated tools are available. The EORTC QLQ-C308 and the Functional Assessment of Cancer Therapy-General questionnaires are frequently used. The EORTC QLQ-C30 is designed to use in multiple cancers and is intended to be supplemented by cancer-specific questionnaire modules or supplements. For example, the EORTC QLQ-MY20 is a cancer-specific questionnaire used in multiple myeloma. Hematological malignancy-specific tools to evaluate HRQOL in clinical trials are being developed.9,10 The FACT-Leukemia is a newly validated 27-question tool that can be used in acute or chronic leukemia patients regardless of eligibility for intensive therapy.10 The availability of validated disease-specific validated tools will help to standardize the process of QOL reporting.
The lack of collection and reporting of HRQOL measures in hematological malignancies may be related to an underappreciation of their significance or that they are not required for drug approval. However, broader availability of HRQOL will enable patients to choose the best therapy for their treatment goals as well as their lifestyle.
One caveat to this study is that we included only clinical trials that led to FDA approval because they represent the drugs with the best current evidence of activity. HRQOL results from more recent studies may need more time to be analyzed and may be reported later in separate publications. Nonetheless, because clinical trials have changed the treatment paradigm for most hematological malignancies, more frequent assessment and reporting of HRQOL in these diseases is needed. Overall, more robust and reliable HRQOL data will improve patient care; therefore, we believe clinical trials should be required to collect HRQOL data and report them as part of the FDA approval application.
Acknowledgments
S.A.H. reports salary support by the National Institutes of Health National Heart, Lung, and Blood Institute (grant 5T32HL092332-18). H.E.H. receives research support from the National Institutes of Health National Cancer Institute (grants P50CA126752 and P01CA094237), Stand Up To Cancer (SU2C)/American Association for Cancer Research (AACR) (grant 604817), Meg Vosburg T-Cell Lymphoma Dream Team, and the Leukemia and Lymphoma Society. SU2C is a program of the Entertainment Industry Foundation administered by the AACR.
Authorship
Contribution: S.A.H. designed research, performed research, contributed vital new reagents or analytical tools, analyzed data, and wrote the paper; and R.T.K., G.C., H.E.H., and C.A.R. analyzed data and wrote the paper.
Conflict-of-interest disclosure: H.E.H. is a cofounder with equity in Allovir and Marker Therapeutics; has served on advisory boards for Novartis, Gilead, Tessa Therapeutics, PACT Pharma, and Mesoblast; and received research support from Tessa Therapeutics and Kuur Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Samer Al Hadidi, Myeloma Center, Winthrop P. Rockefeller Cancer Institute University of Arkansas for Medical Sciences, 4301 W. Markham St. Little Rock, AR 72205; email: salhadidi@uams.edu.
References
Author notes
For data sharing, contact the corresponding author: salhadidi@uams.edu.
Portions of this study were presented at the American Society of Clinical Oncology Quality Care Symposium (virtual meeting), 9-10 October 2020, and the Society of Hematologic Oncology Annual Meeting, 9-12 September 2020 (virtual meeting).