TO THE EDITOR:
We read with interest the recent article by Zamora et al describing that letermovir prophylaxis following allogeneic hematopoietic stem cell transplantation (allo-HSCT) may be associated with delayed reconstitution of cytomegalovirus (CMV)-specific T-cell immunity.1 They hypothesize that this may be due to decreased exposure to CMV antigen. CMV infection is a cause of significant morbidity and mortality following allo-HSCT where an absolute and functional deficiency of CMV-reactive T lymphocytes can lead to the development of CMV disease.2 In the pivotal phase 3 randomized clinical trial by Marty et al,3 letermovir prophylaxis given for 14 weeks following transplantation was shown to reduce the risk of clinically significant CMV infection (as defined as CMV disease or CMV viremia leading to preemptive therapy) by week 24 when compared with placebo. This led to UK National Health Service approval of the use of letermovir therapy in July 2019 as prophylaxis against CMV for the first 100 days posttransplant in CMV-seropositive allo-HSCT recipients. Although many patients in the United Kingdom receive alemtuzumab as graft-versus-host disease (GVHD) prophylaxis, only 12 patients (3.2%) in the letermovir group and 11 patients (5.7%) in the placebo group received alemtuzumab in the phase 3 clinical trial.3 In view of these small numbers, it remains unclear whether letermovir prophylaxis is effective at reducing the risk of clinically significant CMV infection in this group and whether that benefit remains once the letermovir therapy is ceased. Alemtuzumab-containing regimens are associated with both delayed CMV-specific immune reconstitution and relatively high CMV reactivation rates.4,5 It is therefore possible that these patients would be at particularly high risk of CMV infection following cessation of letermovir at day 100, particularly if decreased CMV antigen exposure results in further delayed CMV-specific immune reconstitution as hypothesized by Zamora et al.
In an attempt to address these issues, we performed a United Kingdom–based multicenter retrospective study of allogeneic HSCT recipients who received alemtuzumab as GVHD prophylaxis and letermovir for the first 100 days posttransplant. Twelve transplant centers contributed data on patients transplanted between July 2019 and August 2020. All transplant recipients were CMV seropositive with a requirement for at least 50 days of follow-up or until death if this was earlier. We used a historical comparator cohort of 234 consecutive CMV-seropositive recipients who received alemtuzumab-based T-cell–depleted allo-HSCT at our institution between the dates of January 2006 and February 2017 and did not receive letermovir prophylaxis. All patients had consented to collection of baseline data regarding the collected parameters for registry reporting. Statistical analyses were performed using NCSS 2020, and a P value <.05 was considered statistically significant. Cumulative incidences were calculated for CMV detection and clinically significant CMV infection rates, with death without infection or clinically significant CMV infection, respectively, being the competing risks.
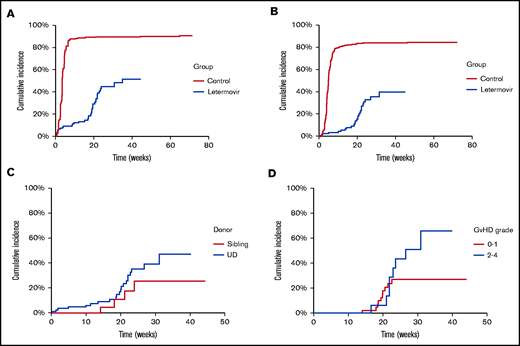
Patient characteristics are shown in Table 1. The majority (102/110, 92.7%) received letermovir at a dose of 240 mg once daily because of concomitant ciclosporin therapy. Compared with the comparator group, there was a significant decrease in the cumulative incidence of detection of a quantifiable CMV viral load by week 40 (51.5% [39.8% to 66.6%] vs 90.3% [86.6% to 94.2%]; P value <.0001; Figure 1A). The temporal kinetic of detection in the letermovir group mirrored that in the registration study, with a cumulative incidence of 13.2% (8.1% to 21.6%) at week 14 and 44.7% (34.3% to 58.3%) at week 24. The cumulative incidence of clinically significant CMV infection was 6.8% (3.3% to 14.0%) at week 14 and 33.0% (23.3% to 46.7%) at week 24 (Figure 1B) compared with 81.6% (76.8% to 86.7%) and 83.8% (79.2% to 88.6%), respectively, in the comparator cohort (P value <.0001), with no statistically significant difference according to donor type (Figure 1C). By comparison, the incidence proportion was 7.7% at week 14 and 17.5% at week 24 in the registration study (with a Kaplan-Meier event rate of 18.9% [14.4% to 23.5%] at week 24). It is notable that although patients receiving alemtuzumab were considered “low risk” in the registration study, their outcomes more closely approximated those of the predefined “high-risk” cohort in terms of the increased incidence between week 14 and week 24, which was noted to relate closely to GVHD and glucocorticoid use in that cohort. Relatively few patients receiving alemtuzumab develop higher grades of GVHD. An exploratory analysis of development of clinically significant CMV infection after week 14 according to GVHD status (Figure 1D) illustrates that the main driver of the late increase is within the larger cohort with grade 0 to 1 GVHD and more likely associated with delayed immune reconstitution rather than enhanced immune suppression. Numbers in the grade 2 to 4 GVHD group limit definitive interpretation with no statistically significant difference between the curves, although it remains probable that this group does particularly poorly because of the combination of both delayed immune reconstitution and enhanced immune suppression.
Patient characteristics
| . | Letermovir group . | Comparator group (no letermovir) . |
|---|---|---|
| No. of patients | 110 | 234 |
| Median follow-up (d) | 131 (3-311) | 688 (21-4158) |
| Median age (y) | 59 (15-73) | 49 (19-68) |
| Sex | ||
| Female | 46 | 96 |
| Male | 64 | 138 |
| CMV status | ||
| Pos/Pos | 81 | 193 |
| Pos/Neg | 29 | 41 |
| Diagnosis | ||
| ALL | 9 | 24 |
| AML/MDS | 66 | 90 |
| Lymphoma | 15 | 108 |
| CLL/PLL | 2 | 7 |
| Aplastic anemia | 8 | 0 |
| MPD/myelofibrosis | 0 | 3 |
| CML/CMML | 8 | 1 |
| Immunodeficiency syndrome | 2 | 1 |
| Donor | ||
| Sibling | 25 | 104 |
| MUD | 63 | 81 |
| MMUD | 22 | 49 |
| Conditioning regimen | ||
| Myeloablative | 10 | 42 |
| Reduced intensity | 100 | 192 |
| Maximum GVHD grade | ||
| 0 to 1 | 79 | 170 |
| 2 | 20 | 44 |
| 3 to 4 | 7 | 19 |
| Unknown | 4 | 1 |
| . | Letermovir group . | Comparator group (no letermovir) . |
|---|---|---|
| No. of patients | 110 | 234 |
| Median follow-up (d) | 131 (3-311) | 688 (21-4158) |
| Median age (y) | 59 (15-73) | 49 (19-68) |
| Sex | ||
| Female | 46 | 96 |
| Male | 64 | 138 |
| CMV status | ||
| Pos/Pos | 81 | 193 |
| Pos/Neg | 29 | 41 |
| Diagnosis | ||
| ALL | 9 | 24 |
| AML/MDS | 66 | 90 |
| Lymphoma | 15 | 108 |
| CLL/PLL | 2 | 7 |
| Aplastic anemia | 8 | 0 |
| MPD/myelofibrosis | 0 | 3 |
| CML/CMML | 8 | 1 |
| Immunodeficiency syndrome | 2 | 1 |
| Donor | ||
| Sibling | 25 | 104 |
| MUD | 63 | 81 |
| MMUD | 22 | 49 |
| Conditioning regimen | ||
| Myeloablative | 10 | 42 |
| Reduced intensity | 100 | 192 |
| Maximum GVHD grade | ||
| 0 to 1 | 79 | 170 |
| 2 | 20 | 44 |
| 3 to 4 | 7 | 19 |
| Unknown | 4 | 1 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; MMUD, mismatched unrelated donor; MUD, matched unrelated donor; Neg, Negative; PLL, prolymphocytic leukemia; Pos, positive.
(A) Cumulative incidence of detection of quantifiable CMV according to use of letermovir as prophylaxis. (B) Cumulative incidence of clinically significant CMV infection. (C) Cumulative incidence of preemptive CMV therapy in patients who received letermovir by donor type. (D) Cumulative incidence of clinically significant CMV infection in patients receiving letermovir with at least 14 weeks follow-up according to GVHD severity. UD, unrelated donor.
(A) Cumulative incidence of detection of quantifiable CMV according to use of letermovir as prophylaxis. (B) Cumulative incidence of clinically significant CMV infection. (C) Cumulative incidence of preemptive CMV therapy in patients who received letermovir by donor type. (D) Cumulative incidence of clinically significant CMV infection in patients receiving letermovir with at least 14 weeks follow-up according to GVHD severity. UD, unrelated donor.
Despite the relatively high incidence of late CMV events in the alemtuzumab cohort, the high levels of viremia and need for preemptive therapy without letermovir prophylaxis means that the potential therapeutic benefit and cost-effectiveness of letermovir is, however, perhaps even greater in the setting of transplants incorporating alemtuzumab. The treatment difference (letermovir-placebo) in terms of clinically significant CMV infection through week 24 was estimated at −23.5% in the registration study, but likely approaches double that in the setting of alemtuzumab-based transplants (−50.8% in our analysis). Even considering that clinically significant CMV events continued to occur after week 24 in our study, reaching a level of 39.9% by week 32, the treatment difference likely exceeds 40%. Thus, despite any additional impact letermovir may have to that of alemtuzumab-mediated T-cell depletion on slowing immune reconstitution, overall clinical benefit appears to be maintained in this group. Our data raise the question of how much additional benefit would be gained by prolonging letermovir prophylaxis in these patients. The current extension study (MK-8228-040, #NCT03930615) evaluating prolongation of prophylaxis to day 200 following allo-HSCT includes patients receiving alemtuzumab and will help to address this issue. Whether letermovir significantly delays immune reconstitution still further as indicated by the observation of Zamora et al, resulting in temporal displacement of a significant ongoing risk to later time points when patient follow-up is generally less frequent, will be an important factor in determining its optimal deployment. The evaluation of T-cell reconstitution and CMV-specific immunity parameters should be incorporated into future prospective studies as they will be critical for a deeper understanding of the issues raised and potential refinement of therapeutic strategies. We await the results of prospective clinical trials investigating this issue with interest.
Acknowledgment: The authors thank Shu Wong for her contribution to the data collection.
Contribution: M.A.V.M. and K.S.P. designed and performed the research, analyzed the data, and wrote the paper; V.M., K.J.T., E.T., A.J.C.B., A.P., R.L., K.O., A.P., E.N., J.A.S., J.B., A.K., M.H.G., G.E., S.L., E.H., N.D., J.P., P.C., B.C., and A.P. performed the research, supplied the data, and reviewed the paper.
Conflict-of-interest disclosure: V.M. received research grants from MSD/Gilead and speakership from MSD, Gilead, Pfizer, Novartis, Jazz, Incyte. E.T. declares advisory boards for Celgene, Gilead/Kite, Jazz, Novartis, Pfizer and MSD, speaker fees for Gilead/Kite, Janssen, Jazz, Novartis, and Pfizer and consultancy for educational materials for Gilead/Kite. A.P. declares advisory board for MSD. R.L. received educational grants and speaker fees for MSD, Chugai Pharma, Astellas, Gilead, and Sanofi. E.N. declares advisory boards for Kite/Gilead, Novartis, BMS/Celgene, and Pfizer and conference support for Kite/Gilead, Novartis, and speaker fees for Kite/Gilead. J.A.S. declares honoraria for educational events supported by Jazz, Mallinckrodt, Gilead, Janssen, and Actelion, advisory board participation for Medac, and IDMC membership for a Kiadis Pharma supported clinical trial, none of which are directly related to this study. E.H. received honoraria from MSD. K.S.P. received speaker fees from Merck. The remaining authors declare no competing financial interests.
Correspondence: Maria A. V. Marzolini, Department of Haematology, UCL Cancer Institute, University College London, London. United Kingdom e-mail: m.marzolini@ucl.ac.uk.
References
Author notes
Method for sharing data will be by e-mails to the corresponding author: m.marzolini@ucl.ac.uk.