Key Points
Cardiac abnormalities in children and young adults with sickle cell anemia are common.
Timely initiation of treatment with hydroxyurea may lead to reverse cardiac remodeling and improvement in these cardiac abnormalities.
Abstract
Cardiac abnormalities such as left ventricular hypertrophy, left ventricular dilation, and pulmonary hypertension in sickle cell anemia have been previously described. Hydroxyurea, a disease-modifying therapy for sickle cell anemia, has been used for several decades. Longitudinal assessment of echocardiographic abnormalities in children and young adults with sickle cell anemia receiving hydroxyurea therapy is lacking. The goal of this retrospective study was to determine the prevalence of echocardiographic abnormalities in children and young adults with sickle cell anemia and to examine the effects of hydroxyurea on reverse cardiac remodeling. We reviewed the records of patients with sickle cell anemia who underwent routine cardiac screening at Cohen Children’s Medical Center between 2010 and 2017, followed by retrospective longitudinal analysis of echocardiograms performed on patients receiving treatment with hydroxyurea. Data on a total of 100 patients with sickle cell anemia were analyzed; 60 (60%) were being treated with hydroxyurea. Twenty-five (41.6%) of the patients on hydroxyurea had been treated for <1 year; these patients had a significantly greater prevalence of left ventricular dilation compared with those who had been on treatment for >1 year. Serial echocardiograms of patients receiving hydroxyurea were then analyzed. Left ventricular dilation and hypertrophy improved significantly with hydroxyurea treatment. In addition, the left ventricular volume and mass correlated negatively with duration of treatment with hydroxyurea. Our study provides evidence that prolonged hydroxyurea therapy may lead to reverse cardiac remodeling. Future studies should attempt to follow up this patient cohort for a longer duration.
Introduction
Sickle cell anemia (SCA) is an au tosomal recessive Mendelian disease affecting ∼1 in 500 African-American subjects and 1 in 1200 Hispanic-American subjects.1-4 There are ∼100 000 individuals living with SCA in the United States, and the global burden of the disease is even greater. Cardiac complications are a common feature of SCA and are thought to be an important cause of the morbidity and mortality associated with this disease. The chronic anemia of SCA results in an increase in cardiac output by increasing stroke volume with only a minimal increase in heart rate. The dilated left ventricle adapts to the increased wall stress by developing eccentric hypertrophy that allows the left ventricle to adapt to chronic volume overload by initially preserving diastolic compliance.5 Over time, progressive dilation leads to increased wall stress and an increase in left ventricular (LV) mass.6
Children and adults with SCA are known to have a higher incidence of cardiac disease such as pulmonary hypertension, LV hypertrophy, LV dilation, and diastolic dysfunction.6-8 Many studies have shown that LV systolic function as assessed by shortening fraction (LVSF) and ejection fraction (LVEF) is preserved in most children with SCA.9 However, practice guidelines are unclear as to whether screening echocardiography is indicated in asymptomatic children, when it should be initiated, and how frequently it should be performed.10
Hydroxyurea (HU) is one of the approved pharmacologic therapies for SCA. HU augments fetal hemoglobin (Hb) production, prevents sickling, and improves Hb level. The full mechanism of action of HU is not completely understood, but it also helps with red blood cell hydration and decreases neutrophils and platelets, which also helps to improve blood flow and decrease sickling.11 Important factors in the decision to start HU include age, genotype, and clinical disease severity.12 According to a 2014 expert panel report sponsored by the National Heart, Lung, and Blood Institute on the evidence-based management of SCA, HU therapy should be offered to all children as young as 9 months of age, regardless of symptoms.13 Longitudinal studies monitoring children and young adults receiving HU to determine if such SCA-modifying therapy is effective at preventing or improving progression of echocardiographic abnormalities are lacking.
Methods
Children and young adults with SCA followed up at Cohen Children’s Medical Center Pediatric Sickle Cell Program are routinely referred to pediatric cardiology at 5 years of age for an initial evaluation (including an echocardiogram), and this evaluation is repeated every other year unless otherwise indicated. The current study is a single-center, retrospective chart review of patients with SCA (HbSS and HbS-β° thalassemia genotypes) who underwent routine cardiac screening at Cohen Children’s Medical Center between 2010 and 2017. Before 2014, the indications for initiating treatment with HU at our institution were severe anemia (Hb level <7.0 g/dL), recurrent vaso-occlusive events, or recurrent acute chest syndrome. Subsequently, HU therapy was offered to all children older than 9 months of age, regardless of symptoms.
For patients on treatment with HU, a retrospective longitudinal analysis of echocardiograms obtained before and after initiation of therapy was performed. The most recent echocardiogram before initiation of HU was regarded as the “baseline” echocardiogram. Echocardiograms performed while on HU were regarded as “post-HU” echocardiograms. To be considered on treatment with HU at the time of echocardiogram, the patient had to have been taking HU for at least 6 months. Exclusion criteria included sickle cell genotypes other than HbSS or HbS-β° thalassemia, any structural cardiac disease other than a patent foramen ovale, patients on chronic transfusion therapy, patients receiving therapy with cardiac-modifying agents (eg, β-blocker, angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker), and echocardiograms performed during a hospital admission or emergency department visit. This study was approved by the institutional review board of Cohen Children’s Medical Center with a waiver of informed consent.
Information was obtained from electronic medical records and the transthoracic echocardiogram database. Clinical data included age, sex, sickle cell genotype, and the date of initiation of HU. For each echocardiogram performed, laboratory data obtained up to 6 months before or after the echocardiogram were collected. The laboratory data included white blood cell count, Hb, mean corpuscular volume, platelet count, and percent HbF. All echocardiograms were performed and interpreted according to the American Society of Echocardiography guidelines.14,15 Transthoracic echocardiogram data included measures of: (1) LV size: M-mode LV end-systolic diameter (LVESd), LV end-diastolic diameter (LVEDd), and LV end-diastolic volume (LVEDv) by the 5/6 × area × length (5/6AL) method16 ; (2) LV mass: calculated by using the 5/6AL method; and (3) LV systolic function: LV ejection fraction (LVEF) according to the 5/6AL method and M-mode fractional shortening (LVSF). To standardize values for body surface area, z-scores, which represent the degree of deviation from the mean, were reported when available. Lastly, right ventricular pressure was estimated by tricuspid valve regurgitation velocity (TR) if an appropriate tracing was present. The following were considered abnormal: a TR ≥2.5 m/s; z-score ≥2 for LVESd, LVEDd, and LV mass; and a z-score less than or equal to –2 for LVSF and LVEF. The left ventricle was considered dilated if either the LVEDd or LVEDv z-score was ≥2. More detailed measures of LV diastolic function (including left atrial volume) were not included because these were only available in the more recent echocardiograms and would not provide complete longitudinal assessment. All these echocardiographic measurements were performed by a single reader, with ∼30% (50 of 169) of echocardiograms being interpreted by an additional reader for interrater agreement analysis.
Analysis was performed by using descriptive statistics with frequency distribution for categorical variables and mean, median, and standard deviations for continuous variables. Comparisons of categorical variables were made by using the Fisher’s exact test; comparisons of continuous variables were made by using the Mann-Whitney rank sum test and Spearman correlation analysis, where appropriate. A multivariate mixed model was used to determine whether there was a change in echocardiographic and hematologic parameters over time. Of note, age was included in all the multivariate analyses. Scatter plot was used to visualize the distribution of each echocardiographic measurement over time. In addition, Cohen’s κ and Gwet’s AC1 analyses were performed to examine interrater agreement on echocardiographic measurements.
Results
This retrospective cross-sectional analysis included data from 100 children and young adults with SCA (Table 1). The mean age was 12 ± 4.9 years (range, 3-22 years), and 58 (58%) were male. Overall, 50 patients (50%) had a dilated left ventricle based on an LVEDd and/or LVEDv z-score ≥2. The LV systolic function was preserved in most patients, with only 3% having abnormal LVSF and 4% having abnormal LVEF. Thirty-two patients (32%) had abnormal TR of ≥2.5 m/s.
Clinical characteristics of study population (N = 100)
| Characteristic . | Mean ± SD or N . | Range or % . |
|---|---|---|
| Age (y) | 12 ± 4.9 | 3 to 22 |
| Male sex | 58 | 58 |
| Genotype | ||
| HbSS | 95 | 95 |
| HbSβ0 | 5 | 5 |
| On HU | 60 | 60 |
| Hb (g/dL) | 9.28 ± 1.20 | 5.40 to 13 |
| MCV (fL) | 86 ± 11 | 61 to 117 |
| Platelet count (K/µL) | 365 ± 130 | 110 to 695 |
| HbF (%) | 8.10 ± 8.40 | 1 to 32 |
| LVEDd z-score | 1.85 ± 1.27 | −1.83 to 4.47 |
| LVESd z-score | 1.06 ± 1.19 | −1.2 to 3.43 |
| LV volume z-score | 2.03 ± 1.44 | −1.80 to 5.70 |
| LV mass z-score | 0.87 ± 1.55 | −2.50 to 5.90 |
| LV mass index (g/ht2.7) | 42.1 ± 10.64 | 17 to 69.80 |
| Dilated left ventricle | 50 | 50 |
| LVSF (%) | 37 ± 17 | 26 to 48 |
| <28% | 3 | 3 |
| LVEF (%) | 62 ± 29 | 45 to 75 |
| <55% | 4 | 4 |
| TR (m/s) | 2.30 ± 0.20 | 1.70-2.80 |
| ≥2.5 m/s | 32 | 32 |
| Characteristic . | Mean ± SD or N . | Range or % . |
|---|---|---|
| Age (y) | 12 ± 4.9 | 3 to 22 |
| Male sex | 58 | 58 |
| Genotype | ||
| HbSS | 95 | 95 |
| HbSβ0 | 5 | 5 |
| On HU | 60 | 60 |
| Hb (g/dL) | 9.28 ± 1.20 | 5.40 to 13 |
| MCV (fL) | 86 ± 11 | 61 to 117 |
| Platelet count (K/µL) | 365 ± 130 | 110 to 695 |
| HbF (%) | 8.10 ± 8.40 | 1 to 32 |
| LVEDd z-score | 1.85 ± 1.27 | −1.83 to 4.47 |
| LVESd z-score | 1.06 ± 1.19 | −1.2 to 3.43 |
| LV volume z-score | 2.03 ± 1.44 | −1.80 to 5.70 |
| LV mass z-score | 0.87 ± 1.55 | −2.50 to 5.90 |
| LV mass index (g/ht2.7) | 42.1 ± 10.64 | 17 to 69.80 |
| Dilated left ventricle | 50 | 50 |
| LVSF (%) | 37 ± 17 | 26 to 48 |
| <28% | 3 | 3 |
| LVEF (%) | 62 ± 29 | 45 to 75 |
| <55% | 4 | 4 |
| TR (m/s) | 2.30 ± 0.20 | 1.70-2.80 |
| ≥2.5 m/s | 32 | 32 |
MCV, mean corpuscular volume; SD, standard deviation.
Sixty patients were on treatment with HU. There was no difference in LV size, LV mass, and TR between the patients who were on HU and those who were not on HU when comparing their most recent echocardiograms (Table 2). Among the HU treatment group, the age at initiation of HU was statistically positively associated with LV mass (P = .04) and TR jet velocity (P = .02).
Patients on HU vs not on HU
| . | HU (n = 60) . | No HU (n = 40) . | P . |
|---|---|---|---|
| Genotype | |||
| HbSS | 59 (98%) | 36 (90%) | .15 |
| HbSβ0 | 1 (2%) | 4 (10%) | .28 |
| Age, y | 11 ± 3.7 | 12 ± 2.3 | .57 |
| Hb, g/dL | 9.43 ± 1.30 | 8.60 ± 2.40 | .03 |
| MCV, fL | 92.10 ± 12.10 | 82.50 ± 21.20 | .003 |
| Platelet count, K/µL | 367 ± 134 | 317 ± 145 | .08 |
| HbF, % | 9.70 ± 8.60 | 5.20 ± 7.40 | .008 |
| LVEDd z-score | 1.85 ± 1.20 | 1.85 ± 1.10 | 1.00 |
| LVESd z-score | 1.06 ± 1.27 | 1.03 ± 1.17 | 1.00 |
| LV volume z-score | 2.10 ± 1.40 | 1.92 ± 1.40 | .53 |
| LV mass z-score | 0.82 ± 1.50 | 0.93 ± 1.62 | .73 |
| LV mass index, g/ht2.7 | 42 ± 10 | 42 ± 10 | 1.00 |
| LVSF, % | 37 ± 4 | 37 ± 4 | .69 |
| LVEF, % | 61 ± 5 | 62 ± 5 | .26 |
| TR, m/s | 2.30 ± 0.20 | 2.40 ± 0.30 | .95 |
| . | HU (n = 60) . | No HU (n = 40) . | P . |
|---|---|---|---|
| Genotype | |||
| HbSS | 59 (98%) | 36 (90%) | .15 |
| HbSβ0 | 1 (2%) | 4 (10%) | .28 |
| Age, y | 11 ± 3.7 | 12 ± 2.3 | .57 |
| Hb, g/dL | 9.43 ± 1.30 | 8.60 ± 2.40 | .03 |
| MCV, fL | 92.10 ± 12.10 | 82.50 ± 21.20 | .003 |
| Platelet count, K/µL | 367 ± 134 | 317 ± 145 | .08 |
| HbF, % | 9.70 ± 8.60 | 5.20 ± 7.40 | .008 |
| LVEDd z-score | 1.85 ± 1.20 | 1.85 ± 1.10 | 1.00 |
| LVESd z-score | 1.06 ± 1.27 | 1.03 ± 1.17 | 1.00 |
| LV volume z-score | 2.10 ± 1.40 | 1.92 ± 1.40 | .53 |
| LV mass z-score | 0.82 ± 1.50 | 0.93 ± 1.62 | .73 |
| LV mass index, g/ht2.7 | 42 ± 10 | 42 ± 10 | 1.00 |
| LVSF, % | 37 ± 4 | 37 ± 4 | .69 |
| LVEF, % | 61 ± 5 | 62 ± 5 | .26 |
| TR, m/s | 2.30 ± 0.20 | 2.40 ± 0.30 | .95 |
Data are expressed as mean ± standard deviation unless otherwise indicated. MCV, mean corpuscular volume.
When the duration of HU treatment was analyzed, patients who had been on treatment for <1 year had a significantly greater prevalence of LV dilation compared with those who had been on treatment for >1 year according to their most recent echocardiogram (Table 3). The mean LVEDd z-score was 2.24 ± 1.3 for patients who had been on HU for <1 year compared with 1.57 ± 1.1 for patients on HU for >1 year (P = .04). The mean LVEDv z-score was 2.62 ± 1.5 for patients who had been on HU for <1 year compared with 1.72 ± 1.2 for patients on HU for >1 year (P = .02). There was no difference in TR between these 2 treatment groups.
HU treatment <1 year vs >1 year
| . | HU <1 y (n = 25) . | HU >1 year (n = 35) . | P . |
|---|---|---|---|
| Age, y | 12 ± 4.2 | 13 ± 3.7 | .42 |
| Hb, g/dL | 9.23 ± 3.50 | 9.54 ± 2.90 | .36 |
| MCV, fL | 87.30 ± 10.80 | 95.50 ± 11.80 | .09 |
| Platelet count, K/µL | 365 ± 122 | 368 ± 144 | .92 |
| HbF, % | 7.80 ± 6.20 | 11 ± 9.40 | .03 |
| LVEDd z-score | 2.24 ± 1.30 | 1.57 ± 1.10 | .04 |
| LVESd z-score | 1.28 ± 1.34 | 0.96 ± 0.95 | .28 |
| LV volume z-score | 2.62 ± 1.50 | 1.72 ± 1.20 | .02 |
| LV mass z-score | 1.09 ± 1.60 | 0.96 ± 1.40 | .24 |
| LV mass index, g/ht2.7 | 43.83 ± 7.90 | 40.38 ± 11.40 | .20 |
| LVSF, % | 36 ± 5 | 37 ± 3 | .29 |
| LVEF, % | 60 ± 6 | 62 ± 4 | .32 |
| TR, m/s | 2.30 ± 0.20 | 2.40 ± 0.30 | .53 |
| . | HU <1 y (n = 25) . | HU >1 year (n = 35) . | P . |
|---|---|---|---|
| Age, y | 12 ± 4.2 | 13 ± 3.7 | .42 |
| Hb, g/dL | 9.23 ± 3.50 | 9.54 ± 2.90 | .36 |
| MCV, fL | 87.30 ± 10.80 | 95.50 ± 11.80 | .09 |
| Platelet count, K/µL | 365 ± 122 | 368 ± 144 | .92 |
| HbF, % | 7.80 ± 6.20 | 11 ± 9.40 | .03 |
| LVEDd z-score | 2.24 ± 1.30 | 1.57 ± 1.10 | .04 |
| LVESd z-score | 1.28 ± 1.34 | 0.96 ± 0.95 | .28 |
| LV volume z-score | 2.62 ± 1.50 | 1.72 ± 1.20 | .02 |
| LV mass z-score | 1.09 ± 1.60 | 0.96 ± 1.40 | .24 |
| LV mass index, g/ht2.7 | 43.83 ± 7.90 | 40.38 ± 11.40 | .20 |
| LVSF, % | 36 ± 5 | 37 ± 3 | .29 |
| LVEF, % | 60 ± 6 | 62 ± 4 | .32 |
| TR, m/s | 2.30 ± 0.20 | 2.40 ± 0.30 | .53 |
Data are expressed as mean ± standard deviation unless otherwise indicated. MCV, mean corpuscular volume.
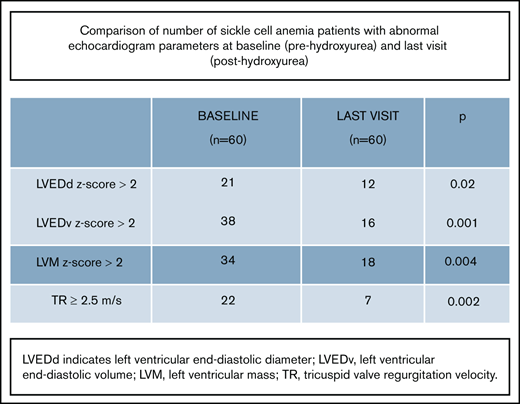
To analyze the effects of HU treatment, a total of 169 serial echocardiograms performed on 60 patients who were on HU treatment were analyzed. Forty (67%) were male. The mean age at the start of HU treatment was 10.3 ± 3.4 years (range, 4-17 years) (Table 4). The mean time between baseline echocardiogram and start date of HU was 448 ± 329 days (range, 3-1047 days). At the last recorded visit, the mean duration of HU treatment was 630 ± 285 days (range, 183-1049 days). Given the nonuniform follow-up, echocardiograms performed up to 3 years before and 3 years after starting HU were included for analysis. Most of the patients (52 of 60) had at least 2 echocardiograms performed after initiating treatment with HU.
Laboratory and echocardiogram findings of patients on HU (N = 60)
| . | Baseline . | Last visit . | P . | ||
|---|---|---|---|---|---|
| Mean ± SD or N . | Range or % . | Mean ± SD or N . | Range or % . | ||
| Age (y) | 10.30 ± 3.40 | 4-17 | 12.70 ± 2.60 | 7-20 | .10 |
| Hb (g/dL) | 8.70 ± 1.10 | 6.90-11.20 | 9.30 ± 0.90 | 7.50-11.10 | .07 |
| MCV (fL) | 76.20 ± 6.80 | 60.30-86.40 | 96.30 ± 14.20 | 66.80-141.10 | .01 |
| Platelet count (K/µL) | 345 ± 87 | 154-544 | 311 ± 95 | 155-490 | .40 |
| HbF (%) | 4.60 ± 6.20 | 1-41 | 14.60 ± 6.90 | 1.60-26 | .002 |
| LVESd z-score | 1.30 ± 0.80 | 0.10-3.30 | 1.10 ± 0.80 | 0.10-2.60 | .45 |
| LVESd z-score ≥2 | 16 | 27 | 7 | 12 | .04 |
| LVEDd z-score | 1.70 ± 1.30 | 0.10-5 | 1.60 ± 1.10 | 0.10-3.60 | .35 |
| LVEDd z-score ≥2 | 21 | 35 | 12 | 20 | .02 |
| LVEDv z-score | 2.20 ± 1.50 | 0.01-6.40 | 1.30 ± 1.10 | 0.10-3.90 | .02 |
| LVEDv z-score ≥2 | 38 | 63 | 16 | 27 | .001 |
| LV mass z-score | 2.50 ± 1.60 | 0.10-5.80 | 1.40 ± 1.20 | 0.10-5.90 | .01 |
| LV mass z-score ≥2 | 34 | 57 | 18 | 30 | .004 |
| LV mass index (g/ht2.7) | 44.80 ± 10.30 | 17.60-71.40 | 42.50 ± 11.30 | 24-69.40 | .47 |
| LVSF (%) | 37 ± 5 | 27-55 | 37 ± 4 | 27-48 | .88 |
| <28% | 3 | 5 | 2 | 3 | .95 |
| LVEF (%) | 60 ± 5 | 47-74 | 61 ± 5 | 48-73 | .73 |
| <55% | 4 | 7 | 3 | 5 | .87 |
| TR (m/s) | 2.30 ± 0.30 | 1.80-3.20 | 2.10 ± 0.20 | 1.70-2.50 | .28 |
| ≥2.5 m/s | 22 | 37 | 7 | 11.5 | .002 |
| . | Baseline . | Last visit . | P . | ||
|---|---|---|---|---|---|
| Mean ± SD or N . | Range or % . | Mean ± SD or N . | Range or % . | ||
| Age (y) | 10.30 ± 3.40 | 4-17 | 12.70 ± 2.60 | 7-20 | .10 |
| Hb (g/dL) | 8.70 ± 1.10 | 6.90-11.20 | 9.30 ± 0.90 | 7.50-11.10 | .07 |
| MCV (fL) | 76.20 ± 6.80 | 60.30-86.40 | 96.30 ± 14.20 | 66.80-141.10 | .01 |
| Platelet count (K/µL) | 345 ± 87 | 154-544 | 311 ± 95 | 155-490 | .40 |
| HbF (%) | 4.60 ± 6.20 | 1-41 | 14.60 ± 6.90 | 1.60-26 | .002 |
| LVESd z-score | 1.30 ± 0.80 | 0.10-3.30 | 1.10 ± 0.80 | 0.10-2.60 | .45 |
| LVESd z-score ≥2 | 16 | 27 | 7 | 12 | .04 |
| LVEDd z-score | 1.70 ± 1.30 | 0.10-5 | 1.60 ± 1.10 | 0.10-3.60 | .35 |
| LVEDd z-score ≥2 | 21 | 35 | 12 | 20 | .02 |
| LVEDv z-score | 2.20 ± 1.50 | 0.01-6.40 | 1.30 ± 1.10 | 0.10-3.90 | .02 |
| LVEDv z-score ≥2 | 38 | 63 | 16 | 27 | .001 |
| LV mass z-score | 2.50 ± 1.60 | 0.10-5.80 | 1.40 ± 1.20 | 0.10-5.90 | .01 |
| LV mass z-score ≥2 | 34 | 57 | 18 | 30 | .004 |
| LV mass index (g/ht2.7) | 44.80 ± 10.30 | 17.60-71.40 | 42.50 ± 11.30 | 24-69.40 | .47 |
| LVSF (%) | 37 ± 5 | 27-55 | 37 ± 4 | 27-48 | .88 |
| <28% | 3 | 5 | 2 | 3 | .95 |
| LVEF (%) | 60 ± 5 | 47-74 | 61 ± 5 | 48-73 | .73 |
| <55% | 4 | 7 | 3 | 5 | .87 |
| TR (m/s) | 2.30 ± 0.30 | 1.80-3.20 | 2.10 ± 0.20 | 1.70-2.50 | .28 |
| ≥2.5 m/s | 22 | 37 | 7 | 11.5 | .002 |
MCV, mean corpuscular volume; SD, standard deviation.
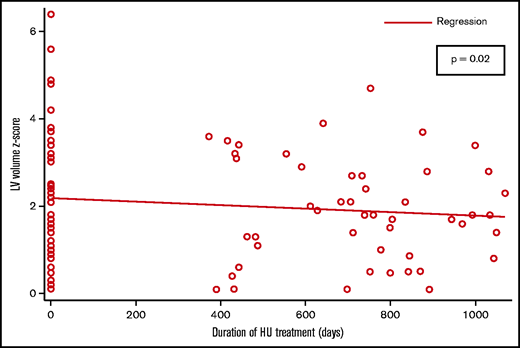
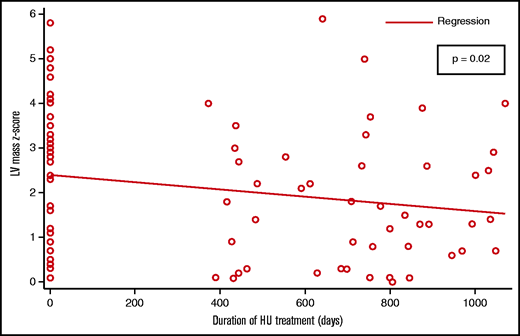
Overall, 43 (72%) patients had an echocardiographic abnormality (abnormal LV size, LV mass, LV systolic function, or TR) compared with 23 (38%) patients with an echocardiographic abnormality on their most recent echocardiogram (P = .01). The measures of LV size and mass were the most commonly noted abnormalities. Patients with LV dilation were more likely to also have LV hypertrophy (31 of 38). A total of 38 children had LV dilation at baseline, and 22 (58%) of them had a normal reading at their most recent visit. The measures of LV size consistently showed a negative association with the duration of HU treatment; the association between LVEDv and duration of HU treatment was statistically significant (P = .02) (Figure 1). There were 34 patients with abnormal LV mass at baseline; 16 (47%) of them had a normal LV mass measurement at their most recent visit. The association between LV mass and duration of HU treatment was statistically significant (P = .02) (Figure 2).
The vast majority of patients on HU had normal LV systolic function; only 3 (5%) had abnormal LVSF, and 4 (7%) had abnormal LVEF. All these patients with abnormal LV systolic function also had LV dilation. The association between LVSF or LVEF and duration of HU treatment was not statistically significant.
Although the association between TR and duration of HU treatment was not statistically significant, the prevalence of elevated TR readings decreased from 37% at baseline to 11% at the most recent visit.
There was no statistically significant correlation between Hb and mean corpuscular volume, and measures of LV size and mass. The HbF was negatively and significantly correlated with LVESd z-score (β = –0.195; P = .001) and LV mass z-score (β = –0.195; P = .001).
Fifty patient echocardiograms (of 169) were interpreted by an additional reader. This was a randomly chosen sample. The tests for interrater agreement analysis (Cohen’s κ and Gwet’s AC1 analyses) revealed an “almost perfect agreement,” with ĸ of 0.9 and AC1 statistic of 0.9, in all echocardiographic measurements by the 2 readers.
Discussion
To compensate for the reduced oxygen-carrying capacity in the face of chronic anemia, patients with SCA develop an increase in plasma volume and maintain increased cardiac output at rest, leading to ventricular dilation.7,17-19 Over time, these patients develop compensatory eccentric hypertrophy in response to the increased wall stress from ventricular dilation.5,6,20-22 Eccentric hypertrophy allows the left ventricle to adapt to chronic volume overload by initially preserving diastolic compliance and maintaining normal filling pressures. However, this hypertrophy is maladaptive and leads to diastolic dysfunction over time. The findings of our study are reflective of this pathophysiological process. In our cohort, the most common echocardiographic abnormalities were LV dilation (50%), LV hypertrophy (24%), and elevated TR (32%). Patients with LV dilation were also more likely to have LV hypertrophy. We assessed LVSF and LVEF as measures of systolic function and found that systolic function was mostly preserved in our cohort. However, these are load-dependent methods, and a decrease in function might become evident when load-independent methods, such as LV end-systolic stress-volume index, are used to assess systolic function, as reported by Poludasu et al.9
In patients with SCA receiving HU therapy, the longer duration of treatment with HU was associated with decreased LV dilation and hypertrophy. We found that older patients at the time of HU initiation were more likely to have LV hypertrophy and elevated TR, and HU use beyond 1 year seemed to reverse these cardiac abnormalities. To our knowledge, this study is the first to analyze longitudinal echocardiographic findings in children and young adults with SCA receiving treatment with HU, and the first to show improvement in LV dilation and hypertrophy with HU therapy.
In our study population, 32% of patients had elevated TR. The reported prevalence of elevated TR in the pediatric age group with SCA varies, with studies including older patient populations reporting a higher prevalence.23,24 For instance, in a study by Onyekwere et al,23 the reported prevalence of elevated TR was 46% in a population with a mean age of 16.2 ± 3.26 years, whereas Caldas et al24 reported a prevalence of 10% in a population with a mean age of 10.1 ± 4.7 years. The prevalence in our cohort is somewhere in-between these 2 reported values, given that the mean age of our study population was 12.0 ± 4.9 years. A recent study by Yates et al25 reported a similar prevalence of elevated TR in their study group with a mean age of 11.0 ± 3.7 years. In our HU treatment group, there was a significant improvement in the number of patients with elevated TR from baseline to last visit (37% of patients with elevated TR at baseline, 11% of patients with elevated TR at last visit). Several studies have reported this improvement in TR. In a study by Rai et al,26 HU therapy was associated with a decrease in TR velocity in children with SCA. The authors postulated that a reduction in hemolysis and improvement in anemia led to this improvement. Another group reported increased levels of HbF, decreased hemolytic parameters, and improvement in TR in 5 adult patients treated with HU.27
Our study included limitations, such as our inability to discern if poor patient medication adherence contributed to the lack of significant difference found in change in certain echocardiographic parameters among patients taking HU. However, our study suggests that the use of sickle cell disease–modifying therapies such as HU may limit the progression of, and potentially improve cardiac abnormalities over time, including dilation, hypertrophy, and elevated TR.
Additional limitations include that the mean time between the baseline echocardiogram and start date of HU was 448 ± 329 days with a wide range (3-1047 days). We assumed the baseline echocardiogram did not change over time before HU treatment. It is possible that the echocardiographic abnormalities might have progressed in the interim, but we did not see an improvement after starting HU because we used a much older baseline echocardiogram for analysis (which was the only available study prior to initiating HU).
In our pediatric SCA population, echocardiographic abnormalities such as LV dilation, LV hypertrophy, and elevated TR were common. In addition, the age at HU initiation was positively associated with LV mass and TR velocity. This study suggests that timely initiation of HU therapy may lead to reverse cardiac remodeling and improvement in these cardiac abnormalities before additional maladaptive changes set in.
Further studies are needed to follow up patients for a longer time on HU, as well as monitoring these patients with SCA into adult life, when their cardiac comorbidities become even more prevalent. Patients with SCA have a unique form of cardiomyopathy with restrictive physiology that is superimposed on hyperdynamic physiology.28 Myocardial strain, an early marker of systolic dysfunction, has also been inconsistently evaluated in this population.29 Therefore, screening echocardiogram should include additional parameters such as measures of LV diastolic function, left atrial volume, and myocardial strain to improve the accuracy of noninvasive screening for cardiac complications in SCA.
Authorship
Contribution: A.D. wrote the first draft; and all authors made substantial contributions to the study conception and design, data acquisition, data analysis, and/or have revised it critically for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arushi Dhar, Department of Pediatric Cardiology, Cohen Children’s Medical Center, 1111 Marcus Ave, Suite M15, New Hyde Park, NY 11042; e-mail: adhar1@northwell.edu.
References
Author notes
Requests for data sharing may be submitted to the corresponding author (Arushi Dhar; e-mail: adhar1@northwell.edu).