Key Points
Gata2 haploinsufficiency promotes HSC proliferation, monocytosis, and decreases CLP generation during aging.
Elderly Gata2 haploinsufficient HSCs are functionally impaired with evidence of myeloid bias.
Abstract
During aging, hematopoietic stem cell (HSC) function wanes with important biological and clinical implications for benign and malignant hematology, and other comorbidities, such as cardiovascular disease. However, the molecular mechanisms regulating HSC aging remain incompletely defined. GATA2 haploinsufficiency driven clinical syndromes initially result in primary immunodeficiencies and routinely evolve into hematologic malignancies on acquisition of further epigenetic mutations in both young and older patients. Using a conditional mouse model of Gata2 haploinsufficiency, we discover that during aging Gata2 promotes HSC proliferation, monocytosis, and loss of the common lymphoid progenitor. Aging of Gata2 haploinsufficient mice also offsets enhanced HSC apoptosis and decreased granulocyte-macrophage progenitor number normally observed in young Gata2 haploinsufficient mice. Transplantation of elderly Gata2 haploinsufficient HSCs impairs HSC function with evidence of myeloid bias. Our data demonstrate that Gata2 regulates HSC aging and suggest the mechanisms by which Gata2 mediated HSC aging has an impact on the evolution of malignancies in GATA2 haploinsufficiency syndromes.
Introduction
Aging of the hematopoietic system is marked by an irrevocable decrease in blood cell function accompanied by fundamental alterations including an increased abundance of hematopoietic stem cells (HSCs), which have diminished function, and imbalanced differentiation potential toward myeloid cell production at the expense of lymphoid cells.1-3 Increasing HSC numbers manifest during aging with a rise in proliferation and a decrease in apoptosis,1,2 yet reduction in their function appears to be associated with upregulation of stress and inflammatory responses and downregulation of genome integrity, including DNA repair, and chromatin modifications.4 Notably, aged HSCs highly express genes associated with myeloid malignancy.3 In the elderly, a reduced number of HSC clones contribute to hematopoiesis, a phenomenon known as clonal hematopoesis of indeterminate potential (CHIP), and this occurs together with frequent mutations in epigenetic regulators that pose an increased risk for both myeloid malignancy and atherosclerosis.5 Thus, overall, aging of HSCs has important biological and clinical implications for hematologic malignancy, anemia, clotting disorders, immunodeficiency, and other comorbidities, such as cardiovascular disease. However, the molecular mechanisms regulating aging of HSCs remain incompletely defined.
Gata2, a zinc-finger transcription factor, has been identified as critical for HSC function in both the embryo and adult.6-9 Deregulation of GATA2 expression leads to hematopoietic aplasia, dysplasia, and neoplasia, underscoring the functional importance of GATA2 in maintaining normal hematopoiesis.10,11 For example, overexpression of GATA2 is observed in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), and gain-of-function mutations in chronic myeloid leukemia correlate with poor prognosis.10,12,13 In contrast, sporadic or germline GATA2 haploinsufficiency mutations in coding or enhancer regions give rise to MDS or AML that is typically preceded by a phase of immunodeficiency.10,11,13,14 Given that up to 20% of GATA2 haploinsufficient patients develop MDS or AML after middle age10,13 and little is known about the impact of Gata2 in this setting, we sought to evaluate Gata2 haploinsufficiency during hematopoietic aging.
Methods
Animals
All mice were housed at the Heath Park Unit, Cardiff University. All animal experiments were executed under PPL 30/3380 in accordance with the 1986 Animals (Scientific Procedures) Act.
Flow cytometry
Stained cells were analyzed on an LSR Fortessa (Becton Dickinson Biosciences) flow cytometer, and fluorescence-activated cell sorter plots were generated using FlowJo 10.6.1 software (Tree Star, Inc).
Transplantation
Transplantation was performed as described previously.15
Statistical analysis
Statistical analysis was performed with GraphPad Prism 8.3.0 software (GraphPad Software, Inc.).
Further details for these methods are provided in supplemental Methods.
Results and discussion
We first assessed Gata2 expression in prospectively isolated hematopoietic cell populations from aged wild-type bone marrow (BM). Consistent with their young BM counterparts,8,9 Gata2 expression was highest in HSCs, attenuated during lympho-myeloid differentiation (hematopoietic progenitor cell 1 [HPC1], hematopoietic progenitor cell 2 [HPC2], lymphoid-primed multipotent progenitor [LMPP], common myeloid progenitor [CMP], granulocyte-macrophage progenitor [GMP], and megakaryocyte–erythroid progenitor [MEP]; and mature myeloid cells), and was notably absent in the common lymphoid progenitor (CLP) and mature lymphoid (T-cell and B-cell) compartments (Figure 1A).
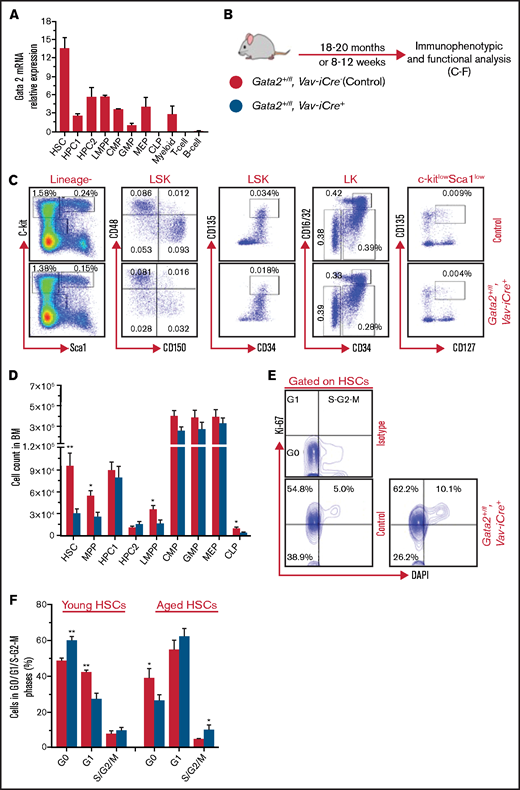
Long-term Gata2 haploinsufficiency enhances HSC proliferation, decreases HSC abundance, and causes a reduction in common lymphoid progenitors. (A) Relative expression of Gata2 mRNA in purified hematopoietic compartments, including HSCs (LSK_CD150+CD48−), HPC1 (LSK_CD150−CD48+), HPC2 (LSK_CD150+CD48+), LMPP (LSK_CD34+CD135hi), CMP (LK_CD34+CD16/32−), GMP (LK_CD34+CD16/32+), MEP (LK_CD34−CD16/32−), CLP (Lin−c-kitloSca1loCD127+CD135+), myeloid cells (Mac1+Gr1+), T cells (CD3ε), and B cells (B220). n = 2 replicates per population. (B) Experimental design for analysis of long-term and short-term Gata2 haploinsufficiency. Gata2+/fl; Vav-iCre− (control) and Gata2+/fl; Vav-iCre+ mice were analyzed at 18 to 20 months and at 8 to 12 weeks old. (C-D) Representative immunophenotypic HSPC analysis and gating scheme for Gata2 +/fl; Vav-iCre− (control) and Gata2+/fl; Vav-iCre+ mice at 18 to 20 months (C) and absolute cell count of primitive and committed hematopoietic populations (D) from control (n = 9) and Gata2+/fl; Vav-iCre+ (n = 7) mice. MPPs are LSK_CD150−CD48−. Percentages represent a frequency of live nucleated BM cells. Data from 3 independent experiments. (E) Representative flow cytometry plots for cell cycle analysis of BM HSCs from Gata2+/fl; Vav-iCre− (control) and Gata2+/fl; Vav-iCre+ mice at 18 to 20 months using Ki-67/4′,6-diamidino-2-phenylindole (DAPI). Bivariate plots showing the frequency of HSCs in G0 (Ki-67−DAPI−), G1 (Ki-67+DAPI−), and S/G2/M (Ki-67+DAPI+) phases from control (n = 9) and Gata2+/fl; Vav-iCre+ (n = 7) mice from 3 independent experiments. (F) The percentage of BM HSCs in G0, G1, and S/G2/M cell cycle phases from aged mice (n = 9 control and 7 Gata2+/fl; Vav-iCre+) and young mice (n = 6 for each genotype) from 3 independent experiments for each condition. Data presented as mean ± standard error of the mean (SEM). Statistical analysis is performed using Mann-Whitney U test. Significant data: *P < .05; **P < .01; ***P < .001.
Long-term Gata2 haploinsufficiency enhances HSC proliferation, decreases HSC abundance, and causes a reduction in common lymphoid progenitors. (A) Relative expression of Gata2 mRNA in purified hematopoietic compartments, including HSCs (LSK_CD150+CD48−), HPC1 (LSK_CD150−CD48+), HPC2 (LSK_CD150+CD48+), LMPP (LSK_CD34+CD135hi), CMP (LK_CD34+CD16/32−), GMP (LK_CD34+CD16/32+), MEP (LK_CD34−CD16/32−), CLP (Lin−c-kitloSca1loCD127+CD135+), myeloid cells (Mac1+Gr1+), T cells (CD3ε), and B cells (B220). n = 2 replicates per population. (B) Experimental design for analysis of long-term and short-term Gata2 haploinsufficiency. Gata2+/fl; Vav-iCre− (control) and Gata2+/fl; Vav-iCre+ mice were analyzed at 18 to 20 months and at 8 to 12 weeks old. (C-D) Representative immunophenotypic HSPC analysis and gating scheme for Gata2 +/fl; Vav-iCre− (control) and Gata2+/fl; Vav-iCre+ mice at 18 to 20 months (C) and absolute cell count of primitive and committed hematopoietic populations (D) from control (n = 9) and Gata2+/fl; Vav-iCre+ (n = 7) mice. MPPs are LSK_CD150−CD48−. Percentages represent a frequency of live nucleated BM cells. Data from 3 independent experiments. (E) Representative flow cytometry plots for cell cycle analysis of BM HSCs from Gata2+/fl; Vav-iCre− (control) and Gata2+/fl; Vav-iCre+ mice at 18 to 20 months using Ki-67/4′,6-diamidino-2-phenylindole (DAPI). Bivariate plots showing the frequency of HSCs in G0 (Ki-67−DAPI−), G1 (Ki-67+DAPI−), and S/G2/M (Ki-67+DAPI+) phases from control (n = 9) and Gata2+/fl; Vav-iCre+ (n = 7) mice from 3 independent experiments. (F) The percentage of BM HSCs in G0, G1, and S/G2/M cell cycle phases from aged mice (n = 9 control and 7 Gata2+/fl; Vav-iCre+) and young mice (n = 6 for each genotype) from 3 independent experiments for each condition. Data presented as mean ± standard error of the mean (SEM). Statistical analysis is performed using Mann-Whitney U test. Significant data: *P < .05; **P < .01; ***P < .001.
To explore the impact of Gata2 haploinsufficiency in hematopoiesis during aging, one allele of the floxed Gata2 gene was deleted in hematopoietic cells using the pan-hematopoietic Vav-iCre promoter.16 Gata2+/+; Vav-iCre+ with Gata2fl/fl; Vav-iCre− were bred to produce Gata2+/fl; Vav-iCre+ (heterozygote) and Gata2+/fl; Vav-iCre− (control) littermates. Genomic DNA polymerase chain reaction genotyping of ear notch biopsies and BM cells confirmed Gata2 haploinsufficiency and control status (supplemental Figure 1A). Gata2+/fl; Vav-iCre+ and control (Gata2+/fl; Vav-iCre−) mice were subsequently aged for 18 to 20 months old, which correlates with humans aged 56 to 69 years (Figure 1B).17 In agreement with previous data,1-3 skewed differentiation capacity toward myeloid lineages and away from lymphoid maturation was observed over time in the peripheral blood (PB) of both aged Gata2+/fl; Vav-iCre+ and control mice (data not shown). At 18 to 20 months, the proportion of myeloid cells (Mac1+Gr1+ and Mac1+Gr1−), B cells (B220+), and T cells (CD4+ and CD8+) in PB was also similar between genotypes (supplemental Figure 1B). Although most GATA2 haploinsufficiency patients present with immunodeficiency, a substantial proportion of patients display normal hematologic profiles before acquiring infections that characterize disease progression.10,13 Thus, unchanged hematologic parameters observed in our mouse model of Gata2 haploinsufficiency may reflect what is seen in this subset of patients. Yet, when comparing the proportion of myeloid and lymphoid cells in PB from aged vs young Gata2+/fl; Vav-iCre+ mice, a significant increase in Mac1+Gr1− myeloid cells was noted in aged Gata2+/fl; Vav-iCre+ mice (supplemental Figure 1C), demonstrating that Gata2 haploinsufficiency causes steady-state accumulation of monocytes during aging.
Age-related changes in cellular composition of BM and extramedullary hematopoietic organs occur during aging.18 However, cellularity and mature myeloid, erythroid, and lymphoid cell proportions were found to be similar in BM, spleen, and thymus of aged Gata2+/fl; Vav-iCre+ and control mice (supplemental Figure 1D-H).
Evaluating the impact of Gata2 haploinsufficiency on the frequencies of hematopoietic stem/progenitor cells (HSPCs) (HSCs, MPPs, HPC1, HPC2) and committed progenitors (CMPs, GMPs, MEPs, CLPs) in BM,19,20 we noted a decrease only in HSC, MPP, LMPP, and CLP populations of aged Gata2+/fl; Vav-iCre+ (Figure 1C-D). Although reduction of HSCs in young Gata2 haploinsufficient mice is well established,6-9 the reduction in HSCs was more pronounced in older Gata2+/fl; Vav-iCre+ vs younger Gata2+/fl; Vav-iCre+ mice (supplemental Figure 1I). Of further note, a decrease in CLP abundance in aged Gata2+/fl; Vav-iCre+ mice contrasted with the lack of impact of Gata2 haploinsufficiency on CLPs from young mice (data not shown) and an overall twofold reduction in CLPs compared with their young counterparts (supplemental Figure 1I). GMP abundance was reduced in the context of young Gata2 haploinsufficient mice21 (and data not shown). Unexpectedly, GMP abundance was comparable between aged Gata2+/fl; Vav-iCre+ and control mice, demonstrating that aging of Gata2 haploinsufficient mice normalizes numbers of GMPs (Figure 1C-D). Therefore, during aging, haploinsufficiency of Gata2 selectively drives attrition of both HSC and CLP compartments and rescues the number of GMPs.
To discern the cellular mechanisms leading to reduced HSCs during aging of Gata2+/fl; Vav-iCre+ mice, we evaluated their apoptosis and cell cycle status. Differing from young Gata2 haploinsufficient mice, where HSCs display increased apoptosis (supplemental Figure 1K),7 no significant difference was observed in early- and late-stage apoptosis in HSCs from aged Gata2+/fl; Vav-iCre+ mice compared with their control counterparts, as judged by the Annexin V assay (supplemental Figure 1J-K). Similarly, a significant increase in late-stage apoptosis in MPPs of young Gata2 haploinsufficient mice was lost during aging (supplemental Figure 1J-K). In stark contrast to enhanced quiescence observed in young Gata2 haploinsufficient mice (Figure 1F),7 Ki-67 analysis revealed a significant decrease in the proportion of G0 HSCs together with an increase in the frequency of HSCs in S/G2/M phases in aged Gata2+/fl; Vav-iCre+ mice (Figure 1E-F), indicating Gata2 haploinsufficiency promotes HSC proliferation during aging.
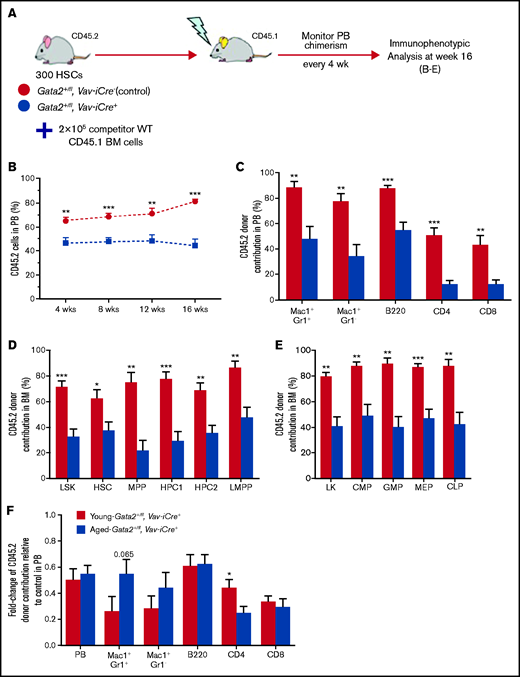
To assess the impact of enhanced HSC proliferation on the function of aged Gata2+/fl; Vav-iCre+ HSCs, we performed competitive transplantation experiments. To do this, 300 CD45.2 purified aged HSCs from control or Gata2+/fl; Vav-iCre+ mice were cotransplanted with 2 × 105 CD45.1 competitor BM cells into lethally irradiated CD45.1 recipients (Figure 2A). Engraftment analysis was conducted every 4 weeks and revealed that the frequency of PB donor contribution was significantly lower in recipients of aged Gata2+/fl; Vav-iCre+ derived cells compared with control derived cells during the entire 16-week posttransplant period (Figure 2B), which was reflected in lower donor contribution to all mature myeloid and lymphoid lineages in BM, PB, and spleen (Figure 2C; supplemental Figure 2) and lower HSPC and committed progenitor engraftment in BM at week 16 (Figure 2D-E). Notably, engraftment of the T-cell compartment was more affected than myeloid or B-cell compartments (Figure 2C), suggesting myeloid bias after transplantation of elderly Gata2 haploinsufficient HSCs. This contention is supported by data showing a twofold expansion in myeloid cells and an approximately twofold decrease in CD4+ T cells in the PB of recipients of aged Gata2+/fl; Vav-iCre+ HSCs compared with recipients of HSCs from young Gata2+/fl; Vav-iCre+ mice (Figure 2F). Thus, aged Gata2 haploinsufficient HSCs fail to reconstitute multilineage hematopoiesis and functionally impart myeloid bias on aged HSCs in vivo.
Elderly Gata2 haploinsufficient HSCs have a functional defect in reconstitution of multilineage hematopoietic compartments with a myeloid bias. (A) Schematic representation of competitive HSC transplantation experiment. Three hundred HSCs from aged control or Gata2+/fl; Vav-iCre+ mice (CD45.2+) together with 2 × 105 unfractionated BM competitor cells (CD45.1+) were transplanted into lethally irradiated (9.5 Gy) recipient mice (CD45.1+). Four independent biological replicates were used for each genotype. Donor chimerism in PB was tested every 4 weeks until week 16 after transplant. (B) Proportion of CD45.2 donor-derived cells in PB after transplantation of donor cells from control (n = 8 recipients) or Gata2+/fl; Vav-iCre+ mice (n = 8 recipients). (C-E) Percentages of CD45.2 donor-derived cells contribution to mature cells in PB (C) BM HSPCs (D) and BM committed myeloid/lymphoid progenitors (E) at week 16 after transplantation of control or Gata2+/fl; Vav-iCre+ donor cells. n = 8 recipients for each genotype from 2 independent experiments. (F) Fold change ratios of CD45.2 donor-derived cell contribution to mature PB cells in aged mice (n = 8 control and 8 Gata2+/fl; Vav-iCre+) in relation to young mice (n = 8 control and 8 Gata2+/fl; Vav-iCre+) donor cells after normalizing to their control counterparts. 2 to 3 independent experiments were performed for each condition. Data is presented as mean ± SEM. Statistical analysis is performed using the Mann-Whitney U test. Significant data: *P < .05; **P < .01; ***P < .001.
Elderly Gata2 haploinsufficient HSCs have a functional defect in reconstitution of multilineage hematopoietic compartments with a myeloid bias. (A) Schematic representation of competitive HSC transplantation experiment. Three hundred HSCs from aged control or Gata2+/fl; Vav-iCre+ mice (CD45.2+) together with 2 × 105 unfractionated BM competitor cells (CD45.1+) were transplanted into lethally irradiated (9.5 Gy) recipient mice (CD45.1+). Four independent biological replicates were used for each genotype. Donor chimerism in PB was tested every 4 weeks until week 16 after transplant. (B) Proportion of CD45.2 donor-derived cells in PB after transplantation of donor cells from control (n = 8 recipients) or Gata2+/fl; Vav-iCre+ mice (n = 8 recipients). (C-E) Percentages of CD45.2 donor-derived cells contribution to mature cells in PB (C) BM HSPCs (D) and BM committed myeloid/lymphoid progenitors (E) at week 16 after transplantation of control or Gata2+/fl; Vav-iCre+ donor cells. n = 8 recipients for each genotype from 2 independent experiments. (F) Fold change ratios of CD45.2 donor-derived cell contribution to mature PB cells in aged mice (n = 8 control and 8 Gata2+/fl; Vav-iCre+) in relation to young mice (n = 8 control and 8 Gata2+/fl; Vav-iCre+) donor cells after normalizing to their control counterparts. 2 to 3 independent experiments were performed for each condition. Data is presented as mean ± SEM. Statistical analysis is performed using the Mann-Whitney U test. Significant data: *P < .05; **P < .01; ***P < .001.
In this report, we identify Gata2 as a critical determinant of HSC function during aging. Although HSCs continue to decline in prevalence and function in Gata2 haploinsufficient mice during aging, we demonstrate significant alterations in HSC and progenitor cell behavior that selectively occur in aged Gata2 haploinsufficient mice rather than in their young counterparts, including (1) augmented HSC proliferation (contrasting with enhanced quiescence in young Gata2 haploinsufficient HSCs), (2) normalization of HSC apoptosis (whereas HSC apoptosis was enhanced in young Gata2 haploinsufficient HSCs), (3) normalization of GMP abundance (compared with the decrease in GMPs observed in young Gata2 haploinsufficient mice), (4) monocytosis, and (5) decreased lymphoid progenitor capacity exemplified by loss of CLPs. These data offer insights into the specific HSC fates that are mediated by Gata2 during aging, setting the stage for studies that further decipher the transcriptional underpinnings of Gata2 regulation in the aging hematopoietic system.
Our data also have implications for GATA2 haploinsufficiency-driven clinical syndromes. Aged Gata2+/fl; Vav-iCre+ mice fail to fully recapitulate the immunodeficiency and progression to MDS/AML observed clinically, which may be ascribed to a number of factors. First, our hematopoietic-specific conditional Gata2 mouse model does not assess niche-driven effects of Gata2 haploinsufficiency on the hematopoietic system. Second, in striking contrast to mice, GATA2 haploinsufficient patients are routinely exposed to infectious agents that push the hematopoietic system to exhaustion where they either succumb or clonally adapt to this inflammatory environment, acquire secondary mutations, and develop MDS/AML. Further work will be needed to assess whether infectious or inflammatory stimuli will induce disease progression in Gata2+/fl; Vav-iCre+ mice. These caveats notwithstanding, atypical sequela includes monocytosis and B-cell loss in GATA2-related chronic myelomonocytic leukemia and MDS,11,22 which mirrors monocytosis observed here in aged Gata2 haploinsufficient mice. Where monocytosis occurs in young patients, this may be caused by premature aging of HSCs, diminishing their function and promoting myeloid bias. Such myeloid bias may explain the presence of lymphoid progenitor deficiency in GATA2 haploinsufficiency despite the absence of Gata2 expression in lymphoid progenitors and mature lymphoid cells.22-24 During aging, Gata2 haploinsufficient HSCs were driven out of quiescence and into proliferation, and they relinquish their apoptotic advantage; if such a mechanism operates in any GATA2 haploinsufficient patient, it may cause a gradual accrual of DNA damage and a proliferative environment conducive for acquiring secondary epigenetic mutations with clonal dominance in developing MDS/AML. Finally, paralleling recent findings that inflammatory processes driven by atherosclerosis enhance both HSC proliferation and CHIP,25 it will be of interest to investigate whether monocytosis and increased HSC proliferation observed in Gata2 haploinsufficient mice during aging are directly linked and clinically relevant to malignant progression of GATA2 haploinsufficiency syndromes.
Acknowledgments
A. Abdelfattah was supported by The Hashemite University, Zarqa, Jordan. N.P.R. laboratory is supported by the Saudi Arabian Cultural Bureau, Leukemia Cancer Society and Leukemia Research Appeal for Wales.
Authorship
Contribution: A. Abdelfattah designed and performed experiments, analyzed and interpreted data, prepared figures, and contributed to writing the manuscript; A.H.-D., L.C., A. Almotiri, and B.A. performed experiments and contributed to data analysis; J.B.M.-G. performed experiments and contributed to data analysis and interpretation; A.T. contributed to experimental design, data analysis, and interpretation; N.P.R. conceived and supervised the project, designed experiments, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neil P. Rodrigues, European Cancer Stem Cell Research Institute, School of Biosciences, Cardiff University, Hadyn Ellis Building, Maindy Road, Cardiff, CF24 4HQ, United Kingdom; e-mail: rodriguesn@cardiff.ac.uk.
References
Author notes
Requests for data sharing should be made to the corresponding author: rodriguesn@cardiff.ac.uk.
The full-text version of this article contains a data supplement.