Key Points
Treatment with PE, rituximab, IVIg, and donor buffy coat is effective in promoting engraftment in patients with DSA <20 000 MFI.
Patients with persistent positive C1q at transplant have a higher risk of engraftment failure and poor survival.
Abstract
Donor-specific anti-HLA antibodies (DSAs) are a major cause of engraftment failure in patients receiving haploidentical stem cell transplantation (HaploSCT). Effective treatments are needed for these patients, who often have no other donor options and/or are in need to proceed urgently to transplantation. We studied a multimodality treatment with alternate-day plasma exchange (PE), rituximab, intravenous γ globulin (IVIg) and an irradiated donor buffy coat for patients with DSAs at 2 institutions. Thirty-seven patients with a median age of 51 years were treated with this desensitization protocol. Treatment outcomes were compared with a control group of HaploSCT patients without DSAs (n = 345). The majority of patients in the DSA group were female (83.8% vs 37.1% in controls, P < .001) and received stem cells from a child as the donor (67.6% vs 44.1%, P = .002). Mean DSA level before and after desensitization was 10 198 and 5937 mean fluorescence intensity (MFI), respectively, with mean differences of 4030 MFI. Fourteen of 30 tested patients (46.7%) had C1q positivity, while 8 of 29 tested patients (27.6%) remained positive after desensitization. In multivariable analysis, patients with initial DSA > 20 000 MFI and persistent positive C1q after desensitization had a significantly lower engraftment rate, which resulted in significantly higher non-relapse mortality and worse overall survival (OS) than controls, whereas graft outcome and survival of patients with initial DSA < 20 000 MFI and those with negative C1q after treatment were comparable with controls. In conclusion, treatment with PE, rituximab, IVIg, and donor buffy coat is effective in promoting engraftment in patients with DSAs ≤20 000 MFI.
Introduction
With the development of several innovative methods to control alloreactivity between the donor and recipient, haploidentical stem cell transplantation (HaploSCT) has significantly expanded donor availability and extended allogeneic hematopoietic stem cell transplantation (AHSCT) to almost all patients in need, with similar outcomes with HLA-matched donor transplants.1-5
Despite this significant success, some obstacles still need to be overcome. One of the main limitations is the occurrence of anti-HLA antibodies against donor HLA antigens (donor-specific anti-HLA antibodies [DSAs]) on the mismatched haplotype, which have been shown to be a major cause of primary graft failure (PGF) and poor survival posttransplant.6-10 Moreover, we have previously identified a high correlation between DSA levels >5000 mean fluorescence intensity (MFI) and complement fixation, assessed by the C1q assay, which is currently believed to be the main mechanism of DSA-induced engraftment failure in recipient of AHSCT.8,11,12 Based on accumulated experience to date, the European Society for Blood and Marrow Transplantation recently published recommendations for testing and treating patients with DSA13 and its use for donor selection in HaploSCT.14
While selecting a donor without corresponding HLA to recipient’s anti-HLA antibodies is an ideal option, it might not always be possible to avoid such donors due to the limited donor availability and/or urgent need to proceed to transplant. To reduce the risk of PGF, several desensitization methods have been proposed.6-8,15,16 Our group initially developed a multimodality desensitization method targeting antibody removal, inhibition of antibody production, antibody neutralization, and inhibition of complement cascade.6,8
In this study, we report the experience with this desensitization treatment of HaploSCT patients with DSA as studied at 2 major institutions in the United States using the same treatment protocol.
Methods
Patients and transplant procedures
Data of consecutive hematologic malignancy patients, ≥18 years of age, with DSA who received desensitization prior to HaploSCT from November 2010 to January 2019 at the University of Texas MD Anderson Cancer Center (UTMDACC) (Houston, TX) and City of Hope National Medical Center (COH) (Duarte, CA) were included in the study group (DSA group). Transplant outcomes of patients in the DSA group were compared with a control group of patients without DSA who received a HaploSCT at UTMDACC during the same period of time. All patients in the DSA and control groups received unmanipulated HaploSCT with high-dose posttransplant cyclophosphamide, tacrolimus, and mycophenolate mofetil for graft-versus-host prophylaxis as previously described.17
The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki. All patients provided written informed consent for treatment and transplantation. A retrospective data review protocol and waiver of informed consent approved by UTMDACC and COH institutional review boards were used to analyze the results.
DSA AND C1q testing
Pretransplant sera of all patients were tested prospectively for anti-HLA class I and II antibodies using multiplex bead assays performed on the Luminex platform, including LABScreen PRA and LABScreen Mixed methods for screening. A semiquantitative measurement of DSA level was performed by the LABScreen single antigen bead assay (One Lambda, part of Thermo Fisher Scientific; Canoga Park, CA) according to the manufacturer’s instructions, and results were expressed as MFI. The initial screening test was performed at the time of HLA typing. All patients with positive antibody screen were retested within 30 days of admission for transplant (predesensitization) and postdesensitization. Individual DSAs against all HLA antigens were recorded and, for the purpose of the analysis, the maximum MFI levels were considered.
DSA desensitization prior to haploidentical transplantation
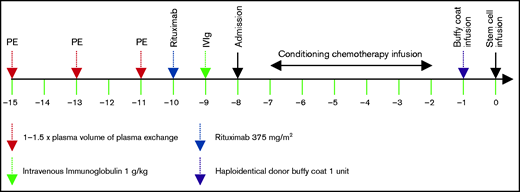
The decision on desensitization for patients with DSA was based on DSA levels, C1q positivity, and physician’s discretion. Both UTMDACC and COH DSA desensitization protocols included 3 sessions of alternate-day plasma exchange (PE) with 1× to 1.5× plasma volume (replaced with either fresh frozen plasma or 4% to 5% human plasma albumin), starting 1 week prior to admission for transplantation, followed by a single dose of rituximab (Rituxan 375 mg/m2) the next day after completion of PE, followed 1 day later by 1 dose of intravenous γ globulin (IVIg) (1 g/kg), as previously described.8 In March 2011, the desensitization protocol was amended by adding an irradiated buffy coat prepared from 1 U whole blood of the same haploidentical donor and infused on transplant day −1 to neutralize the remaining DSA prior to administration of hematopoietic stem cells on day 0, as summarized in Figure 1.
Statistical analysis and outcome definitions
Primary outcome was the cumulative incidence of neutrophil engraftment, while cumulative incidence of platelet engraftment, non-relapse mortality (NRM), relapse, overall survival (OS), and progression-free survival (PFS) were analyzed as secondary outcomes. All outcomes were computed from date of stem cell infusion to date of the event or censored at last follow-up. Neutrophil engraftment was defined as the first date of absolute neutrophil count recovery ≥0.5 × 109 /L for 3 consecutive days. Platelet engraftment was defined as the first date of platelet count >20 × 109/L and sustained for 7 consecutive days independent of transfusion. Death from any cause was considered an event for OS where death or relapse for PFS. The Kaplan-Meier method was used to estimate all survival measures. The cumulative incidences of engraftment, relapse, and NRM were evaluated by the competing risks method, where death was the competing risk for relapse and relapse was the competing risk for NRM. Death, relapse, or second transplant without engraftment were considered competing events of engraftment.
The impact of desensitization treatment in patients with different MFI ranges (<10 000, 10 000-20 000, and >20 000 MFI) and C1q status on survival outcomes was determined using univariable and multivariable Cox proportional hazards regression models, while a proportional subdistribution hazards regression model was used for cumulative incidence outcomes with competing risks. Covariates used for adjustment in the multivariable regression models were age, sex, hematopoietic cell transplant comorbidity index (HCT-CI), ABO mismatch, donor-recipient relation, the refined disease risk index (DRI-R), conditioning regimen intensity, and stem cell source. All variables included in the regression models were tested for the proportional hazard assumption and interaction terms. All statistical calculations were carried out using STATA 13.1 (Stata, College Station, TX). P values < .05 were considered significant, and all tests were 2 sided.
Results
Patients and transplant characteristics
A total of 37 HaploSCT patients with DSAs were included in the study, including 27 patients from UTMDACC and 10 from COH. The control group was composed of 345 consecutive patients without DSA receiving HaploSCT from UTMDACC. The majority of patients in the DSA group were female (83.8%), compared with only 37.1% in control group (P < .001), and received stem cells from a child donor (67.6% vs 44.1%, P = .002). Child donor to mother recipient transplantation accounted for 64.9% in DSA group compared with 18.6% in controls (P < .001).
Patient and transplant characteristics are summarized in Table 1.
Characteristics of patients in the DSA (receiving desensitization) and control groups
| . | DSA group (n = 37), (%,range or SD) . | Control group (n = 345), (%, range or SD) . | P value . |
|---|---|---|---|
| Age (y), median (range) | 51 (19-66) | 47 (18-72) | .374 |
| Sex: female | 31 (83.8) | 128 (37.1) | <.001 |
| Sex mismatch | 17 (46.0) | 154 (44.6) | .507 |
| Diagnosis | .887 | ||
| AML/MDS | 21 (56.8) | 200 (58.0) | |
| Others | 16 (43.2) | 145 (42.03) | |
| ABO mismatch | .368 | ||
| Minor | 5 (14.7) | 57 (15.6) | |
| Major | 3 (8.8) | 55 (16.0) | |
| Bidirectional | 1 (2.9) | 3 (0.9) | |
| Donor-recipient relation | .002 | ||
| Child | 25 (67.6) | 44 (44.1) | |
| Sibling | 7 (18.9) | 150 (43.5) | |
| Parent | 3 (8.1) | 40 (11.6) | |
| Other | 2 (5.4) | 3 (0.9) | |
| Child donor to mother recipient | 24 (64.9) | 64 (18.6) | <.001 |
| DRI-R (n = 309) | .170 | ||
| Low | 3 (9.7) | 32 (12.1) | |
| Intermediate | 14 (45.2) | 114 (43.2) | |
| High | 7 (22.6) | 92 (34.9) | |
| Very high | 7 (22.6) | 26 (9.9) | |
| Prior autologous transplant | 2 (6.1) | 29 (8.4) | 1.000 |
| CR 1/2 | 11 (34.4) | 128 (37.1) | .460 |
| Median HCT-CI (range) | 2 (0-7) | 2 (0-9) | .226 |
| Conditioning regimen intensity | .056 | ||
| MAC | 27 (73.0) | 195 (56.5) | |
| NMA/RIC | 10 (27.0) | 150 (43.5) | |
| Conditioning regimen | |||
| Flu-Mel (±TBI, thiotepa) | 26 (70.3) | 292 (84.6) | .003 |
| Flu-Bu (±TBI, thiotepa) | 5 (13.5) | 43 (12.5) | |
| Others (Flu-TBI, Flu-Cy) | 6 (16.2) | 10 (2.9) | |
| Stem source: marrow | 21 (56.8) | 282 (81.7) | .001 |
| Buffy coat infusion | 27 (77.1) | NA | |
| Antibody specificity | |||
| HLA class I | 14 (37.8) | NA | |
| HLA class II | 12 (32.4) | NA | |
| HLA class I and II | 11 (29.7) | NA | |
| Predesensitization MFI level, mean (SD) | 10 198.2 (8618.6) | NA | |
| Postdesensitization MFI level, mean (SD) | 5937.2 (8336) | NA | |
| Initial C1q positivity (n = 30) | 14 (46.7) | ||
| Pretransplant C1q positivity (n = 29) | 8 (27.6) | NA |
| . | DSA group (n = 37), (%,range or SD) . | Control group (n = 345), (%, range or SD) . | P value . |
|---|---|---|---|
| Age (y), median (range) | 51 (19-66) | 47 (18-72) | .374 |
| Sex: female | 31 (83.8) | 128 (37.1) | <.001 |
| Sex mismatch | 17 (46.0) | 154 (44.6) | .507 |
| Diagnosis | .887 | ||
| AML/MDS | 21 (56.8) | 200 (58.0) | |
| Others | 16 (43.2) | 145 (42.03) | |
| ABO mismatch | .368 | ||
| Minor | 5 (14.7) | 57 (15.6) | |
| Major | 3 (8.8) | 55 (16.0) | |
| Bidirectional | 1 (2.9) | 3 (0.9) | |
| Donor-recipient relation | .002 | ||
| Child | 25 (67.6) | 44 (44.1) | |
| Sibling | 7 (18.9) | 150 (43.5) | |
| Parent | 3 (8.1) | 40 (11.6) | |
| Other | 2 (5.4) | 3 (0.9) | |
| Child donor to mother recipient | 24 (64.9) | 64 (18.6) | <.001 |
| DRI-R (n = 309) | .170 | ||
| Low | 3 (9.7) | 32 (12.1) | |
| Intermediate | 14 (45.2) | 114 (43.2) | |
| High | 7 (22.6) | 92 (34.9) | |
| Very high | 7 (22.6) | 26 (9.9) | |
| Prior autologous transplant | 2 (6.1) | 29 (8.4) | 1.000 |
| CR 1/2 | 11 (34.4) | 128 (37.1) | .460 |
| Median HCT-CI (range) | 2 (0-7) | 2 (0-9) | .226 |
| Conditioning regimen intensity | .056 | ||
| MAC | 27 (73.0) | 195 (56.5) | |
| NMA/RIC | 10 (27.0) | 150 (43.5) | |
| Conditioning regimen | |||
| Flu-Mel (±TBI, thiotepa) | 26 (70.3) | 292 (84.6) | .003 |
| Flu-Bu (±TBI, thiotepa) | 5 (13.5) | 43 (12.5) | |
| Others (Flu-TBI, Flu-Cy) | 6 (16.2) | 10 (2.9) | |
| Stem source: marrow | 21 (56.8) | 282 (81.7) | .001 |
| Buffy coat infusion | 27 (77.1) | NA | |
| Antibody specificity | |||
| HLA class I | 14 (37.8) | NA | |
| HLA class II | 12 (32.4) | NA | |
| HLA class I and II | 11 (29.7) | NA | |
| Predesensitization MFI level, mean (SD) | 10 198.2 (8618.6) | NA | |
| Postdesensitization MFI level, mean (SD) | 5937.2 (8336) | NA | |
| Initial C1q positivity (n = 30) | 14 (46.7) | ||
| Pretransplant C1q positivity (n = 29) | 8 (27.6) | NA |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; Bu, busulfan; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CR1/2, first or second complete remission; Cy, cyclophosphamide; Flu, fludarabine; MA, myeloablative conditioning; MDS, myelodysplastic syndrome; Mel, melphalan; MPN, myeloproliferative neoplasm; NA, not available; NMA, non-myeloablative intensity conditioning; RIC, reduced intensity conditioning; TBI, total body irradiation.
DSA levels and complement fixation ability before and after desensitization
Among 37 patients with DSA who received desensitization, 27 patients (77.1%) also received a donor buffy coat infusion before transplant. Fourteen (38.7%), 12 (32.4%), and 11 patients (29.7%) had antibodies against donor HLA class I, II, and both classes, respectively. The mean baseline DSA levels before desensitization were 10 198 MFI (standard deviation [SD], 8618). Twenty-one (56.8%), 10 (27%), and 6 patients (16.2%) with DSA levels <10 000, 10 000 to 20 000, and >20 000 MFI, respectively, while 14 of 30 tested patients (46.7%) also had C1q positivity. Significantly higher DSA levels were seen in patients with positive C1q than in those with negative C1q (mean 19 490.7 MFI [SD 5482.2] vs 3701 MFI [SD 2484.1], respectively; P < .001). All 6 patients with DSA >20 000 MFI had antibodies against both class I and II HLA antigens.
The mean DSA level after desensitization was 5937 MFI (SD, 8336), which was significantly lower compared with predesensitization levels (P = .026). Eight of 29 tested patients (27.6%) remained C1q positive after treatment.
Impact of DSA desensitization on engraftment
The cumulative incidences of neutrophil engraftment at day 28 were 75.7% and 83.8% at 60 days posttransplant compared with 90.7% and 94.2% in controls, with unadjusted subdistribution hazard ratio (SHR) of 0.77 (95% confidence interval [CI], 0.50-1.17; P = .216). Stratified by DSA levels, the cumulative incidences of neutrophil engraftment at 60 days for patients with DSA <10 000, 10 000 to 20 000, and >20 000 MFI were 95.2% (SHR, 0.94; 95% CI, 0.59-1.52; P = .813), 75% (SHR, 0.67; 95% CI, 0.24-1.87; P = .144) and 50% (SHR 0.32, 95% CI, 0.18-1.12, P = .052), respectively. Among 6 patients with DSA > 20 000 MFI, 2 patients had PGF and 1 patient died of bleeding at 25 days posttransplant without engraftment. Based on C1q status posttreatment, patients with persistent C1q positivity after desensitization had a 50% engraftment rate at day 60 posttransplant (SHR, 0.34; 95% CI, 0.09-1.15; P = .054), whereas 85.7% of C1q-negative patients engrafted within 60 days posttransplant (SHR, 0.87; 95% CI, 0.38-1.96; P = .744) (Table 2).
Transplant outcomes and univariable analysis of impact of DSA desensitization at different MFI cutoffs and C1q on transplant outcomes
| . | At day 28, % . | At day 60, % . | Unadjusted SHR (95% CI) . | P value . |
|---|---|---|---|---|
| Neutrophil engraftment | ||||
| Control | 90.7 | 94.2 | Ref | Ref |
| DSA (all cases) | 75.7 | 83.8 | 0.77 (0.50-1.17) | .216 |
| DSA <10 000 MFI | 80.9 | 95.2 | 0.94 (0.59-1.52) | .813 |
| DSA 10 000-20 000 MFI | 75.0 | 75.0 | 0.67 (0.24-1.87) | .144 |
| DSA >20 000 MFI | 50.0 | 50.0 | 0.32 (0.18-1.12) | .052 |
| C1q positive | 64.3 | 88.9 | 0.50 (0.22-1.13) | .102 |
| C1q persistently positive after treatment | 50.0 | 50.0 | 0.34 (0.09-1.15) | .054 |
| C1q positive to negative after treatment | 85.7 | 85.7 | 0.87 (0.38-1.96) | .744 |
| Platelet engraftment | ||||
| Control | 45.5 | 77.2 | Ref | Ref |
| DSA | 36.0 | 58.9 | 0.65 (0.41-1.03) | .068 |
| DSA <10 000 MFI | 47.7 | 64.8 | 0.79 (0.43-1.47) | .462 |
| DSA 10 000-20000 MFI | 12.5 | 50.0 | 0.46 (0.19-1.16) | .099 |
| DSA >20 000 MFI | 16.7 | 33.3 | 0.30 (0.08-0.97) | .044 |
| C1q positive | 14.3 | 42.9 | 0.39 (0.18-0.84) | .017 |
| C1q persistently positive after treatment | 12.5 | 37.5 | 0.32 (0.11-0.97) | .045 |
| C1q positive to negative after treatment | 28.6 | 57.1 | 0.63 (0.25-1.58) | .325 |
| . | At day 28, % . | At day 60, % . | Unadjusted SHR (95% CI) . | P value . |
|---|---|---|---|---|
| Neutrophil engraftment | ||||
| Control | 90.7 | 94.2 | Ref | Ref |
| DSA (all cases) | 75.7 | 83.8 | 0.77 (0.50-1.17) | .216 |
| DSA <10 000 MFI | 80.9 | 95.2 | 0.94 (0.59-1.52) | .813 |
| DSA 10 000-20 000 MFI | 75.0 | 75.0 | 0.67 (0.24-1.87) | .144 |
| DSA >20 000 MFI | 50.0 | 50.0 | 0.32 (0.18-1.12) | .052 |
| C1q positive | 64.3 | 88.9 | 0.50 (0.22-1.13) | .102 |
| C1q persistently positive after treatment | 50.0 | 50.0 | 0.34 (0.09-1.15) | .054 |
| C1q positive to negative after treatment | 85.7 | 85.7 | 0.87 (0.38-1.96) | .744 |
| Platelet engraftment | ||||
| Control | 45.5 | 77.2 | Ref | Ref |
| DSA | 36.0 | 58.9 | 0.65 (0.41-1.03) | .068 |
| DSA <10 000 MFI | 47.7 | 64.8 | 0.79 (0.43-1.47) | .462 |
| DSA 10 000-20000 MFI | 12.5 | 50.0 | 0.46 (0.19-1.16) | .099 |
| DSA >20 000 MFI | 16.7 | 33.3 | 0.30 (0.08-0.97) | .044 |
| C1q positive | 14.3 | 42.9 | 0.39 (0.18-0.84) | .017 |
| C1q persistently positive after treatment | 12.5 | 37.5 | 0.32 (0.11-0.97) | .045 |
| C1q positive to negative after treatment | 28.6 | 57.1 | 0.63 (0.25-1.58) | .325 |
| . | At 1 y, % . | At 2 y, % . | . | . |
|---|---|---|---|---|
| NRM | ||||
| Control | 28.8 | 31.7 | Ref | Ref |
| DSA | 17.2 | 25.5 | 0.67 (0.31-1.47) | .323 |
| DSA <10 000 MFI | 15.6 | 15.6 | 0.49 (0.15-1.57) | .232 |
| DSA 10 000-20 000 MFI | 12.5 | 12.5 | 0.46 (0.06-3.81) | .475 |
| DSA >20 000 MFI | 37.5 | 58.3 | 2.18 (1.08-6.98) | .034 |
| C1q positive | 22.7 | 38.2 | 1.13 (0.40-3.20) | .815 |
| C1q persistently positive after treatment | 50 | 87.5 | 3.42 (1.30-8.90) | .013 |
| C1q positive to negative after treatment | 0 | 0 | NA | NA |
| Relapse | ||||
| Control | 22.1 | 25.8 | Ref | Ref |
| DSA | 27.8 | 41.6 | 1.41 (0.74-2.69) | .296 |
| DSA <10 000 MFI | 24.8 | 36.7 | 1.20 (0.50-2.90) | .676 |
| DSA 10 000-20 000 MFI | 38.9 | 38.9 | 1.45 (0.36-5.85) | .599 |
| DSA >20 000 MFI | 20.8 | 41.7 | 1.63 (0.42-6.40) | .479 |
| C1q positive | 29.4 | 45.6 | 1.85 (0.75-4.53) | .175 |
| C1q persistently positive after treatment | 12.5 | 12.5 | 2.68 (0.99-7.27) | .063 |
| C1q positive to negative after treatment | 40.0 | 70.0 | 0.56 (0.07-4.41) | .581 |
| . | At 1 y, % . | At 2 y, % . | . | . |
|---|---|---|---|---|
| NRM | ||||
| Control | 28.8 | 31.7 | Ref | Ref |
| DSA | 17.2 | 25.5 | 0.67 (0.31-1.47) | .323 |
| DSA <10 000 MFI | 15.6 | 15.6 | 0.49 (0.15-1.57) | .232 |
| DSA 10 000-20 000 MFI | 12.5 | 12.5 | 0.46 (0.06-3.81) | .475 |
| DSA >20 000 MFI | 37.5 | 58.3 | 2.18 (1.08-6.98) | .034 |
| C1q positive | 22.7 | 38.2 | 1.13 (0.40-3.20) | .815 |
| C1q persistently positive after treatment | 50 | 87.5 | 3.42 (1.30-8.90) | .013 |
| C1q positive to negative after treatment | 0 | 0 | NA | NA |
| Relapse | ||||
| Control | 22.1 | 25.8 | Ref | Ref |
| DSA | 27.8 | 41.6 | 1.41 (0.74-2.69) | .296 |
| DSA <10 000 MFI | 24.8 | 36.7 | 1.20 (0.50-2.90) | .676 |
| DSA 10 000-20 000 MFI | 38.9 | 38.9 | 1.45 (0.36-5.85) | .599 |
| DSA >20 000 MFI | 20.8 | 41.7 | 1.63 (0.42-6.40) | .479 |
| C1q positive | 29.4 | 45.6 | 1.85 (0.75-4.53) | .175 |
| C1q persistently positive after treatment | 12.5 | 12.5 | 2.68 (0.99-7.27) | .063 |
| C1q positive to negative after treatment | 40.0 | 70.0 | 0.56 (0.07-4.41) | .581 |
| . | At 1 y, % (95% CI) . | At 2 y, % (95% CI) . | Unadjusted HR (95% CI) . | P value . |
|---|---|---|---|---|
| OS | ||||
| Control | 57.7 (52.1-62.8) | 50.2 (44.5-55.6) | Ref | Ref |
| DSA | 68.1 (48.1-81.7) | 52.4 (26.7-72.9) | 0.89 (0.50-1.56) | .680 |
| DSA <10 000 MFI | 70.2 (41.5-86.7) | 60.1 (29.7-80.8) | 0.67 (0.30-1.53) | .352 |
| DSA 10 000-20 000 MFI | 70.0 (22.5-91.8) | 70.0 (22.5-91.8) | 0.72 (0.17-2.89) | .640 |
| DSA >20 000 MFI | 41.7 (15.6-76.7) | 20.8 (8.7-59.5) | 2.74 (1.13-4.68) | .026 |
| C1q positive | 58.4 (26.2-80.6) | 39.0 (17.8-50.5) | 1.52 (0.71-3.23) | .280 |
| C1q persistently positive after treatment | 33.3 (5.6-65.7) | 0 | 3.56 (1.57-8.09) | .002 |
| C1q positive to negative after treatment | 80.8 (30.3-96.9) | 80.8 (30.3-96.9) | 0.67 (0.17-2.72) | .578 |
| PFS | ||||
| Control | 49.1 (43.6-54.4) | 42.5 (37.0-49.0) | Ref | Ref |
| DSA | 54.9 (35.1-70.9) | 32.9 (12.7-55.0) | 1.01 (0.62-1.67) | .944 |
| DSA <10 000 MFI | 59.7 (32.2-79.3) | 47.7 (19.1-71.9) | 0.78 (0.38-1.58) | .491 |
| DSA 10 000-20 000 MFI | 48.6 (17.7-81.6) | 48.6 (17.7-81.6) | 0.89 (0.28-2.77) | .836 |
| DSA >20 000 MFI | 41.7 (15.6-56.7) | 0 | 2.49 (1.02-4.05) | .044 |
| C1q positive | 48.5 (17.8-53.8) | 16.2 (1.9-39.2) | 1.48 (0.73-3.00) | .276 |
| C1q persistently positive after treatment | 37.5 (12.8-67.4) | 0 | 2.89 (1.28-6.54) | .011 |
| C1q positive to negative after treatment | 60.0 (22.6-88.2) | 30.0 (11.2-51.9) | 0.88 (0.28-2.78) | .838 |
| . | At 1 y, % (95% CI) . | At 2 y, % (95% CI) . | Unadjusted HR (95% CI) . | P value . |
|---|---|---|---|---|
| OS | ||||
| Control | 57.7 (52.1-62.8) | 50.2 (44.5-55.6) | Ref | Ref |
| DSA | 68.1 (48.1-81.7) | 52.4 (26.7-72.9) | 0.89 (0.50-1.56) | .680 |
| DSA <10 000 MFI | 70.2 (41.5-86.7) | 60.1 (29.7-80.8) | 0.67 (0.30-1.53) | .352 |
| DSA 10 000-20 000 MFI | 70.0 (22.5-91.8) | 70.0 (22.5-91.8) | 0.72 (0.17-2.89) | .640 |
| DSA >20 000 MFI | 41.7 (15.6-76.7) | 20.8 (8.7-59.5) | 2.74 (1.13-4.68) | .026 |
| C1q positive | 58.4 (26.2-80.6) | 39.0 (17.8-50.5) | 1.52 (0.71-3.23) | .280 |
| C1q persistently positive after treatment | 33.3 (5.6-65.7) | 0 | 3.56 (1.57-8.09) | .002 |
| C1q positive to negative after treatment | 80.8 (30.3-96.9) | 80.8 (30.3-96.9) | 0.67 (0.17-2.72) | .578 |
| PFS | ||||
| Control | 49.1 (43.6-54.4) | 42.5 (37.0-49.0) | Ref | Ref |
| DSA | 54.9 (35.1-70.9) | 32.9 (12.7-55.0) | 1.01 (0.62-1.67) | .944 |
| DSA <10 000 MFI | 59.7 (32.2-79.3) | 47.7 (19.1-71.9) | 0.78 (0.38-1.58) | .491 |
| DSA 10 000-20 000 MFI | 48.6 (17.7-81.6) | 48.6 (17.7-81.6) | 0.89 (0.28-2.77) | .836 |
| DSA >20 000 MFI | 41.7 (15.6-56.7) | 0 | 2.49 (1.02-4.05) | .044 |
| C1q positive | 48.5 (17.8-53.8) | 16.2 (1.9-39.2) | 1.48 (0.73-3.00) | .276 |
| C1q persistently positive after treatment | 37.5 (12.8-67.4) | 0 | 2.89 (1.28-6.54) | .011 |
| C1q positive to negative after treatment | 60.0 (22.6-88.2) | 30.0 (11.2-51.9) | 0.88 (0.28-2.78) | .838 |
HR, hazard ratio; Ref, reference.
There was no significant difference in neutrophil engraftment rate for patients who had DSAs against HLA class I (85.7% at 60 days; SHR, 1.10; 95% CI, 0.55-2.19; P = .784) or II (83.3% at 60 days; SHR, 1.04; 95% CI, 0.47-2.32; P = .916) antigens in comparison with control patients without DSA. On the contrary, patients who developed antibodies against donor HLA antigens targeting both class I and II (54.5% at 60 days; SHR, 0.35; 95% CI, 0.15-0.81; P = .014) had a significantly lower neutrophil engraftment rate compared with controls. However, since antibody specification and MFI levels were highly correlated, all patients with high DSA levels (>20 000 MFI) also had antibodies against both HLA class I and II; therefore, we analyzed the impact of treatment on engraftment only in patients with DSA ≤ 20 000 MFI, which showed similar neutrophil engraftment rate between patients with DSA targeting HLA class I, II, or both classes and controls.
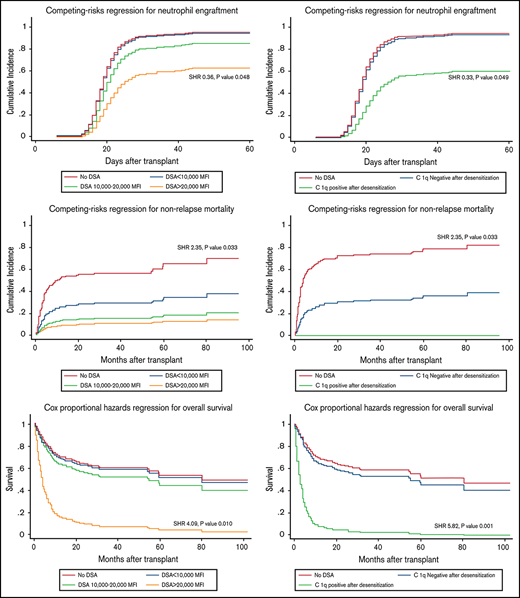
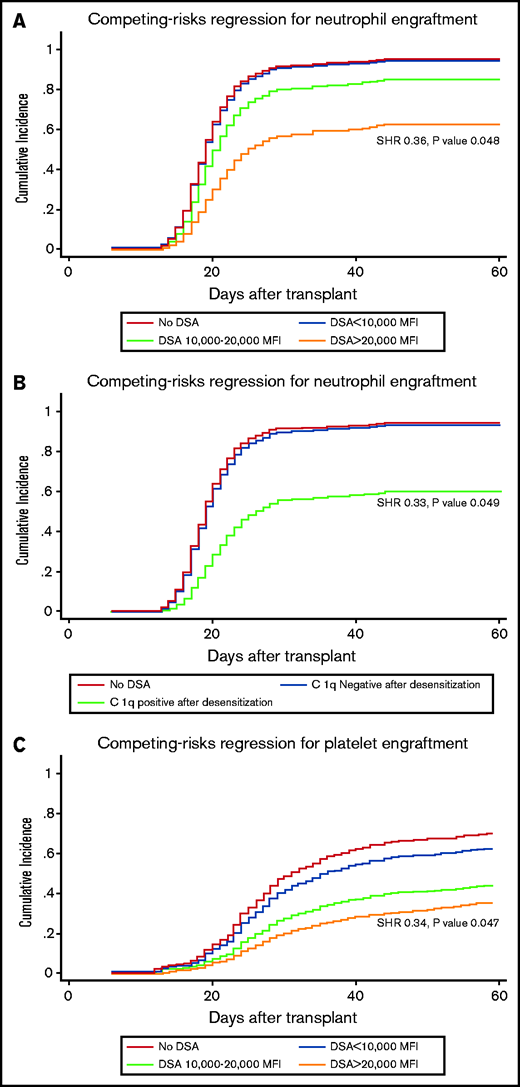
Having baseline DSA levels > 20 000 MFI and persistent C1q positivity after desensitization were associated with a significantly lower neutrophil engraftment rate after adjusting for other potential risk factors in multivariable regression analysis, with SHRs of 0.36 (95% CI, 0.11-0.99; P = .048) (Figure 2A) and 0.33 (95% CI, 0.09-0.99; P = .049) (Figure 2B), respectively, while patients with DSA range < 20 000 MFI and those with negative C1q after desensitization had rates of neutrophil engraftment comparable with controls (Table 3).
Impact of initial DSA level and C1q status after desensitization on transplant outcomes. Impact of initial DSA level and C1q status after desensitization on neutrophil engraftment (A,B), platelet engraftment (C,D), non-relapse mortality (E,F) and overall survival (G,H).
Impact of initial DSA level and C1q status after desensitization on transplant outcomes. Impact of initial DSA level and C1q status after desensitization on neutrophil engraftment (A,B), platelet engraftment (C,D), non-relapse mortality (E,F) and overall survival (G,H).
Multivariable analysis
| . | Adjusted SHR . | 95% CI . | P value . |
|---|---|---|---|
| Neutrophil engraftment* | |||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 0.97 | 0.59-1.61 | .905 |
| DSA 10 000-20 000 MFI | 0.68 | 0.24-1.93 | .468 |
| DSA >20 000 MFI | 0.36 | 0.11-0.99 | .048 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 0.92 | 0.37-2.25 | .859 |
| Persistent C1q positive | 0.33 | 0.09-0.99 | .049 |
| Platelet engraftment* | |||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 0.81 | 0.44-1.46 | .478 |
| DSA 10 000-20 000 MFI | 0.48 | 0.20-1.16 | .103 |
| DSA >20 000 MFI | 0.34 | 0.13-0.98 | .047 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 0.63 | 0.27-1.46 | .284 |
| Persistent C1q positive | 0.36 | 0.22-0.97 | .042 |
| Non-relapse mortality* | |||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 0.33 | 0.10-2.66 | .299 |
| DSA 10 000-20 000 MFI | 0.48 | 0.12-4.31 | .516 |
| DSA >20 000 MFI | 2.35 | 1.54-6.05 | .033 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | NA | NA | NA |
| Persistent C1q positive | 4.00 | 1.14-11.01 | .030 |
| Relapse | |||
| Model 1: impact of DSA level* | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 2.48 | 0.82-7.54 | .109 |
| DSA 10 000-20 000 MFI | 1.81 | 0.38-8.58 | .455 |
| DSA >20 000 MFI | 2.61 | 0.59-9.53 | .204 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 2.11 | 0.58-6.73 | .367 |
| Persistent C1q positive | 0.48 | 0.15-4.95 | .538 |
| Overall survival† | Adjusted HR | ||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 0.81 | 0.24-2.69 | .741 |
| DSA 10 000-20 000 MFI | 0.76 | 0.18-3.24 | .718 |
| DSA >20 000 MFI | 4.09 | 1.45-8.90 | .010 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 0.84 | 0.20-3.53 | .812 |
| Persistent C1q positive | 5.82 | 2.15-11.69 | .001 |
| Progression-free survival† | |||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 1.06 | 0.38-2.96 | .917 |
| DSA 10 000-20 000 MFI | 1.13 | 0.34-3.73 | .835 |
| DSA >20 000 MFI | 4.58 | 1.58-11.28 | .005 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 1.30 | 0.40-4.25 | .657 |
| Persistent C1q positive | 4.56 | 1.71-11.20 | .002 |
| . | Adjusted SHR . | 95% CI . | P value . |
|---|---|---|---|
| Neutrophil engraftment* | |||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 0.97 | 0.59-1.61 | .905 |
| DSA 10 000-20 000 MFI | 0.68 | 0.24-1.93 | .468 |
| DSA >20 000 MFI | 0.36 | 0.11-0.99 | .048 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 0.92 | 0.37-2.25 | .859 |
| Persistent C1q positive | 0.33 | 0.09-0.99 | .049 |
| Platelet engraftment* | |||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 0.81 | 0.44-1.46 | .478 |
| DSA 10 000-20 000 MFI | 0.48 | 0.20-1.16 | .103 |
| DSA >20 000 MFI | 0.34 | 0.13-0.98 | .047 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 0.63 | 0.27-1.46 | .284 |
| Persistent C1q positive | 0.36 | 0.22-0.97 | .042 |
| Non-relapse mortality* | |||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 0.33 | 0.10-2.66 | .299 |
| DSA 10 000-20 000 MFI | 0.48 | 0.12-4.31 | .516 |
| DSA >20 000 MFI | 2.35 | 1.54-6.05 | .033 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | NA | NA | NA |
| Persistent C1q positive | 4.00 | 1.14-11.01 | .030 |
| Relapse | |||
| Model 1: impact of DSA level* | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 2.48 | 0.82-7.54 | .109 |
| DSA 10 000-20 000 MFI | 1.81 | 0.38-8.58 | .455 |
| DSA >20 000 MFI | 2.61 | 0.59-9.53 | .204 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 2.11 | 0.58-6.73 | .367 |
| Persistent C1q positive | 0.48 | 0.15-4.95 | .538 |
| Overall survival† | Adjusted HR | ||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 0.81 | 0.24-2.69 | .741 |
| DSA 10 000-20 000 MFI | 0.76 | 0.18-3.24 | .718 |
| DSA >20 000 MFI | 4.09 | 1.45-8.90 | .010 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 0.84 | 0.20-3.53 | .812 |
| Persistent C1q positive | 5.82 | 2.15-11.69 | .001 |
| Progression-free survival† | |||
| Model 1: impact of DSA level | |||
| No DSA | Ref | ||
| DSA <10 000 MFI | 1.06 | 0.38-2.96 | .917 |
| DSA 10 000-20 000 MFI | 1.13 | 0.34-3.73 | .835 |
| DSA >20 000 MFI | 4.58 | 1.58-11.28 | .005 |
| Model 2: impact of C1q status after desensitization | |||
| No DSA | Ref | ||
| C1q positive to negative | 1.30 | 0.40-4.25 | .657 |
| Persistent C1q positive | 4.56 | 1.71-11.20 | .002 |
Impacts of covariates on outcomes of interest are not presented.
Abbreviations are explained in Table 2.
Subdistribution hazards regression model adjusted for age (continuous), sex, ABO mismatch, donor-recipient relation, DRI-R, conditioning regimen intensity, stem cell source, and HCT-CI.
Cox proportional hazards regression model adjusted for age (continuous), sex, ABO mismatch, donor-recipient relation, DRI-R, conditioning regimen intensity, stem cell source, and HCT-CI.
Cumulative incidences of platelet engraftment at day 60 for patients with DSA <10 000, 10 000 to 20 000, and >20 000 MFI were 64.8%, 50% and 33.3%, respectively, compared with 77.2% in the control group with unadjusted SHRs of 0.79 (95% CI, 0.43-1.47; P = .462), 0.46 (95% CI, 0.19-1.16; P = .099), and 0.30 (95% CI, 0.08-0.97; P = .044), respectively.
Patients with baseline C1q positivity and those with persistent C1q positivity after desensitization had 60-day cumulative incidences of platelet engraftment of 42.9% and 37.5%, respectively, with unadjusted SHRs of 0.39 (95% CI, 0.18-0.84; P = .017) and 0.32 (95% CI, 0.11-0.97; P = .045), respectively (Table 2).
Multivariable analyses showed no significant differences in platelet engraftment between patients with DSA <20 000 and controls as well as patients with C1q negativity after desensitization vs controls (Table 3). However, DSA level >20 000 MFI and persistent C1q positivity independently predicted poor platelet engraftment, with adjusted SHRs of 0.34 (95% CI, 0.13-0.98; P = .047) (Figure 2C) and 0.36 (95% CI, 0.22-0.97; P = .042) (Figure 2D), respectively.
Impact of DSA desensitization on NRM, relapse, and survival posttransplant
Median follow-up duration of survivors was 25 months (range, 1.3-104.5). NRM at 1 year for all patients with DSA was 17.2% vs 28.8% in control group (SHR 0.67, 95% CI, 0.31-1.47, P = .323). No significant differences of NRM were seen in subgroup with DSA <10 000 MFI (15.6%) and 10 000 to 20 000 MFI (12.5%) when compared with controls. Also, none of the C1q-negative patients with after desensitization died of NRM. However, patients with initial DSA >20 000 MFI and with persistently positive C1q had a significantly increased risk of NRM, 37.5% (SHR, 2.18; 95% CI, 1.08-6.98; P = .034) and 50% (SHR, 3.42; 95% CI, 1.30-8.90; P = .013) at 1 year, respectively. The significance remained after adjusting for other potential confounders in multivariable analyses, with SHRs of 2.35 (95% CI, 1.54-6.05; P = .033) (Figure 2E) and 4.00 (95% CI, 1.14-11.01; P = .030) (Figure 2F), respectively.
The higher NRM for patients with DSA >20 000 MFI and persistently positive C1q resulted in lower OS (20.8% and 0% at 2 years, respectively) and PFS (0% at 2 years) compared with the control group (50.2% OS and 42.5% PFS at 2 years, respectively). While OS and PFS between desensitized patients at DSA levels <20 000 MFI as well as those who C1q became negative after desensitization were comparable with control group (Table 2). Multivariable analyses confirmed a significant association of high DSA levels >20 000 MFI and persistent C1q positivity on both OS and PFS as summarized in Table 3. After adjusting for other potential risk factors, patients with DSA >20 000 MFI had an HR for risk of death of 4.09 (95% CI, 1.45-8.90; P = .010) (Figure 2G), while the HR was 5.82 (95% CI, 2.15-11.69, P = .001) for persistent C1q positivity after desensitization (Figure 2H) compared with patients without DSA.
Neither C1q positivity nor DSA levels influenced risk of relapse posttransplant (Table 2).
Impact of buffy coat infusion on graft outcomes
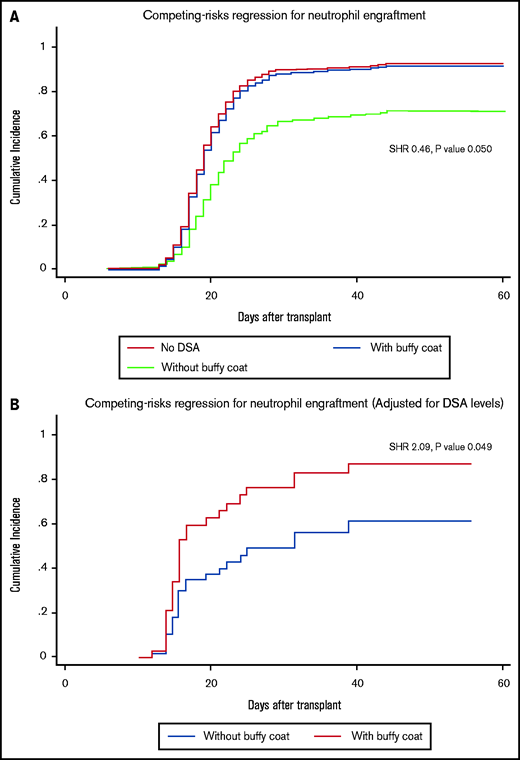
Twenty patients received desensitization treatment with buffy coat. Compared with patients without DSA, engraftment rate of patients receiving buffy coat was comparable (SHR, 0.89; 95% CI, 0.53-1.50; P = .661), whereas there was a strong trend toward a reduced rate of engraftment in DSA patients who received desensitization without buffy infusion (SHR, 0.46; 95% CI, 0.21-1.00; P = .050) (Figure 3A). In addition, including the buffy coat infusion into the desensitization regimen doubled the chance of neutrophil engraftment when compared with desensitization without buffy coat after adjusting for DSA levels (SHR, 2.09; 95% CI, 1.02-4.40; P = .049) (Figure 3B).
Impact of buffy coat infusion on engraftment. Impact of desensitization with and without buffy coat infusion on neutrophil engraftment before (A) and after adjustment for DSA levels (B).
Impact of buffy coat infusion on engraftment. Impact of desensitization with and without buffy coat infusion on neutrophil engraftment before (A) and after adjustment for DSA levels (B).
Discussion
The presence of DSA has been recognized as a critical cause of primary graft rejection both in solid organ transplant and in HLA-mismatched AHSCT.6-9,19-26 Similar to a previous study by our group,8 in the current study, we found that the majority of patients with DSA were multiparous females (84%) who had DSA against their child donor (65%), in a significantly higher proportion than those without DSA. These findings emphasize, again, the importance of DSA testing in all recipients of HaploSCT, particularly in multiparous female patients receiving a HaploSCT from a child donor, as pregnancy appears to be the major risk factor for allosensitization in these patients.
To reduce the risk of PGF, we and others have reported beneficial effects of a combined modality desensitization to decrease DSA levels.7,8,16,27 In this study, we report the largest experience to date of desensitization therapy for HaploSCT patients with DSA using a single protocol developed at UTMDACC and adopted at COH, confirming its efficacy in these patients. Recognizing that some patients had extremely high DSA levels (>20 000 MFI), in addition to antibody removal and decreased antibody production and antibody neutralization using PE, rituximab, and IVIg, we added (early on) an infusion of an irradiated buffy coat on day −1 of transplant, prepared from 1 U donor peripheral blood, to block the remaining circulating antibodies. As compared with random platelet infusions, which have only HLA class I antigens on their surface,15,28,29 the buffy coat has both class I and II HLA antigens and is likely more effective at adsorbing the remaining DSA and sparing the hematopoietic stem cells infused the next day, more so because DSAs against multiple donor HLA antigens, and not infrequently against the entire mismatched haplotype, are often present.30
The buffy coat infusion appears to have a significant impact on the likelihood of engraftment after adjusting for DSA levels, as patients with highest levels were more likely to receive a buffy coat.
In addition to providing the largest experience with desensitization treatment to date as well as treatment of patients with the highest DSA levels ever reported, we demonstrate that this desensitization protocol is effective in reducing DSA levels and complement binding activity as well as decreasing the risk of PGF and NRM, and these results were confirmed in 2 major US transplant centers. Our results showed a significant reduction of DSA levels after desensitization, which resulted in comparable graft outcome and survival for patients with DSAs against donor HLA class I and II antigens with levels up to 20 000 MFI who received desensitization, in comparison with control patients without DSA, suggesting that this is an effective treatment strategy for most patients. However, patients with very high pretreatment DSA levels (>20 000 MFI), in addition to having lower neutrophil engraftment, had a significantly lower incidence of platelet engraftment, higher NRM, and lower survival than patients without DSAs, despite desensitization.9 Proceeding with HaploSCT in the presence of such high levels of DSAs is not indicated at present, unless more effective desensitization methods are developed.
Interestingly, when stratified by DSA levels, we found similar graft outcomes in patients with HLA antibodies against class I, II, or both HLA antigens and controls, suggesting that this treatment could successfully neutralize antibodies targeting both class I and II HLA antigens.
In addition, results from the current study demonstrate that persistent C1q positivity at transplantation (after desensitization) was associated with lower engraftment, higher NRM, and poor survival compared with patients without DSAs. Most patients with C1q positivity who became negative after desensitization and engrafted successfully had outcomes comparable with those of patients without DSAs. However, half of the patients who remained C1q positive after desensitization and before transplant rejected the graft, suggesting that more effective desensitization strategies might be needed in these cases and that the primary aim of treatment should be to obtain C1q negativity before transplant.
In a solid-phase antibody assay, the MFI value from a single antigen bead merely reflects the amount of antibody bound to the bead and not the amount of circulating antibody in the serum.31 As previously summarized by a Food and Drug Administration antibody-mediated rejection workshop, the level of DSAs measured by a solid-phase assay may be insufficient in determining a successful desensitization.32 In contrast, a recent study in kidney transplantation demonstrated that posttreatment, achieving negative C1q DSAs was associated with a significantly better graft survival compared with >50% reduction in levels of MFI of DSAs, suggesting that the C1q assay is more reliable than immunoglobulin G MFI in monitoring intervention efficacy.33 Additionally, the C1q assay provides a yes or no answer for complement binding and may be more time and cost efficient than other titration assays using serial dilutions.34 We therefore chose the C1q assay as the surrogate marker of responsiveness to the desensitization treatment in the current study. Prospective studies to address this issue are ongoing.
In conclusion, a multimodality treatment with PE, rituximab, and IVIg and infusion of an irradiated donor buffy coat on day −1 of transplantation is an effective strategy to desensitize patients with DSA before haploidentical transplantation. Patients with very high DSA levels or and/or those who remain C1q positive at transplantation have a very high risk of engraftment failure and should not proceed to transplantation or other/additional therapeutic approaches should be investigated.
Authorship
Contribution: S.O.C. designed the study, treated patients, and participated in data collection, interpretation of results, and manuscript writing; P.K. contributed to statistical design, data analysis, interpretation of results, and manuscript writing; J.Z. contributed to antibody testing and results interpretation and reviewed and approved the manuscript; F.M.A. contributed to antibody testing and results interpretation and reviewed and approved the manuscript; G.R. and J.C. contributed to data collection and reviewed and approved the manuscript; M.T., S.O., and A.N. contributed to antibody testing and data collection and reviewed and approved the manuscript; S.J.F. and R.C. treated patients and reviewed and approved the manuscript; M.M.A.M. contributed to treatment of patients and data collection and reviewed and approved the manuscript; and K.G. and K.C. contributed to data collection, antibody testing, and interpretation of results and reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan O. Ciurea, Department of Stem Cell Transplantation and Cellular Therapy, Unit 423, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: sciurea@uci.edu.
References
Author notes
For data sharing, e-mail the corresponding author: sciurea@uci.edu.
S.O.C. and M.M.A.M. are joint first authors.
K.G. and K.C. contributed equally to this study.