Key Points
Hyper-CVAD-R is an effective regimen for BL that prevents CNS relapse.
Hyper-CVAD-R therapy is effective especially among patients with high-risk features such as BM and CNS involvement.
Abstract
Burkitt leukemia/lymphoma (BL) and high-grade B-cell lymphoma (HGBL) have a high incidence of central nervous system (CNS) involvement, which is associated with poor prognosis. The hyper-cyclophosphamide, vincristine, Adriamycin, and dexamethasone plus rituximab (CVAD-R) regimen includes systemic and intrathecal CNS-directed therapy to treat and prevent CNS disease. We report here the long‐term safety and efficacy of the hyper-CVAD-R regimen in adults with BL and HGBL, focusing on its efficacy to prevent CNS relapse. Among 79 adults (54 BL, 25 HGBL), the median age was 44 years (25% ≥60 years old), 73% had bone marrow (BM) involvement, and 28% had CNS involvement. The complete response rate was 91% (BL 96%; HGBCL 79%; P = .16). The 5-year relapse-free survival (RFS) and overall survival (OS) rates were 58% and 52%, respectively. The cumulative incidence of relapse (CIR) was 21% (BL 14%; HGBCL 37%, P = .06) and was associated with baseline BM (27% vs 0%; P = .02) and CNS (42% vs 12%; P < .01) involvement. In multivariate analyses, age and CNS involvement were independent predictors for OS and RFS. The 5-year CNS CIR was 6% (BL 4%; HGBL 11%; P = .31); 16% with baseline CNS involvement (P = .03). Our data support the use of hyper-CVAD-R in preventing CNS relapse, especially among high-risk patients with BM or CNS involvement.
Introduction
Burkitt leukemia/lymphoma (BL) and high-grade B-cell lymphoma (HGBL, previously “atypical Burkitt lymphoma”) are aggressive lymphomas that share similar histologic and biologic features with high risk of central nervous system (CNS) involvement.1-4 The standard of care for BL is dose-intense chemoimmunotherapy regimens with rituximab and CNS-directed therapy such as high-dose methotrexate (MTX) and cytarabine (Ara-C).5-8 The optimal management of HGBL remains uncertain.9 The dose-adjusted (DA)-etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) regimen was developed to increase efficacy in proliferative lymphoid neoplasms while limiting toxicity associated with BL-like chemoimmunotherapy regimens. It showed favorable efficacy and tolerability in patients with aggressive lymphomas, including HGBL and BL, without improvement of outcomes in patients with diffuse large B-cell lymphoma when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone.10-12 The DA-EPOCH-rituximab (EPOCH-R) regimen does not include systemic CNS-directed therapy and therefore may not be suitable for patients at higher risk of CNS relapse, such as those with baseline bone marrow (BM) or CNS involvement.6,9,13,14 In contrast, the hyper-cyclophosphamide, vincristine, Adriamycin, and dexamethasone plus rituximab (CVAD-R) regimen includes 4 cycles of high-dose MTX and Ara-C and a total of 16 intrathecal (IT) chemotherapy. Here, we report the long‐term safety and efficacy of the hyper-CVAD-R in patients with BL and HGBL focusing on patterns of relapse.
Methods
Between January 2000 and January 2018, 102 patients with newly diagnosed BL or HGBL treated with the hyper-CVAD-R regimen were retrospectively reviewed. Patients provided informed consent according to MD Anderson Cancer Center guidelines after approval by the institutional review board. Eligibility criteria included age ≥15 years and Eastern Cooperative Oncology Group (ECOG) performance status ≤3 with no restrictions for older age, organ dysfunction, or presence of HIV. Because the diagnostic criteria of HGBL have undergone recent changes, all cases were reviewed retrospectively (J.D.K. and S.E.H.) to confirm that they meet current diagnostic criteria according to the 2017 World Health Organization classification.15 Among 102 cases, 54 were confirmed BL, 25 were confirmed HGBL, and 23 cases were excluded because they did not have sufficient information to be confirmed as BL or HGBL.
The doses and schedule of hyper-CVAD-R were previously described.8,16,17 CNS prophylaxis consisted of 16 IT alternating doses of MTX/Ara-C. For patients with baseline CNS involvement, IT therapy was administered twice weekly until clearance, then weekly for 4 doses, after which prophylaxis was resumed. No routine cranial irradiation was administered.
We evaluated complete response (CR) rates, relapse‐free survival (RFS), overall survival (OS), and cumulative incidence of relapse (CIR).
Results
The patient characteristics are shown in Table 1. No differences were observed between BL and HGBL. The median age was 44 years; 25% were older than 60 years. Eleven patients (13%) were HIV-positive. Fifty-four patients (73%) had BM involvement and 22 (28%) had CNS disease (21 leptomeningeal and 1 parenchymal). Three patients received cranial radiation (1 for leptomeningeal disease and 2 for cranial nerve palsies). Among the 25 patients with HGBL, 7 (28%) had double-hit lymphoma (all with BCL2 rearrangement).
Characteristics and outcomes of patients with BL and HGBL treated with hyper-CVAD-R
| Characteristic N (%) or median [range] . | Total (n = 79) . | BL (n = 54) . | HGBL (n = 25) . | P value . |
|---|---|---|---|---|
| Age at Dx, y | 44 [18-77] | 42 [18-77] | 47 [20-75] | .24 |
| Age ≥60 y | 20 (25) | 12 (22) | 8 (32) | .51 |
| Sex (male) | 51 (65) | 36 (67) | 15 (60) | .75 |
| ECOG PS 0-1 | 52/75 (69) | 35/52 (67) | 17/23 (74) | .76 |
| Stage 4 | 70 (89) | 49 (91) | 21 (84) | .67 |
| BM disease | 58 (73) | 42 (78) | 16 (64) | .31 |
| Extramedullary disease | 36 (46) | 24 (44) | 12 (48) | .96 |
| CNS disease at Dx | 22 (28) | 15 (28) | 7 (28) | 1.00 |
| Positive CSF | 16 (20) | 11 (20) | 5 (20) | |
| Parenchymal disease | 1 (2) | 0 | 1 (4) | |
| Clinical manifestations | 5 (6) | 4 (8) | 1 (4) | |
| LDH ≥ 3 ULN, n (%) | 50 (63) | 38 (70) | 12 (48) | .10 |
| HIV-related | 11 (14) | 9 (17) | 2 (8) | .49 |
| DHL | NA | NA | 7 (28) | |
| IPI | NA | NA | 3.5 [2-5] | |
| CNS-IPI | NA | NA | 3 [1-4] | |
| Treatment outcome, n (%) | ||||
| CR rate | 68/75 (91) | 49/51 (96) | 19/24 (79) | .16 |
| Early death <30 d | 2 (3) | 2 (4) | 0 | .84 |
| Early death <60 d | 5 (6) | 4 (7) | 1 (4) | .93 |
| Relapse | 15/68 (22) | 8/49 (16) | 7/19 (37) | .13 |
| CNS relapse | 4 (5) | 2 (4) | 2 (8) | .43 |
| Isolated CNS relapse | 2 (50) | 1 (50) | 1 (50) | |
| Death | 46 (58) | 31 (57) | 15 (60) | 1.00 |
| Death in CR | 21 (27) | 18 (33) | 3 (12) | |
| Overall survival | .86 | |||
| Median, mo (95% CI) | 71 (18-NR) | 125 (13-NR) | 25 (17-NR) | |
| 5-y, % (95% CI) | 52 (42-64) | 55 (44-70) | 44 (28-68) | |
| Relapse-free survival | .62 | |||
| Median, mo (95% CI) | 127 (54-NR) | 126 (50-NR) | NR (10-NR) | |
| 5-y, % (95% CI) | 58 (48-72) | 59 (47-75) | 57 (39-85) | |
| Cumulative incidence of relapse | ||||
| 5-y, % (95% CI) | 21 (11-30) | 14 (4-24) | 37 (14-59) | .06 |
| Cumulative incidence of CNS relapse | ||||
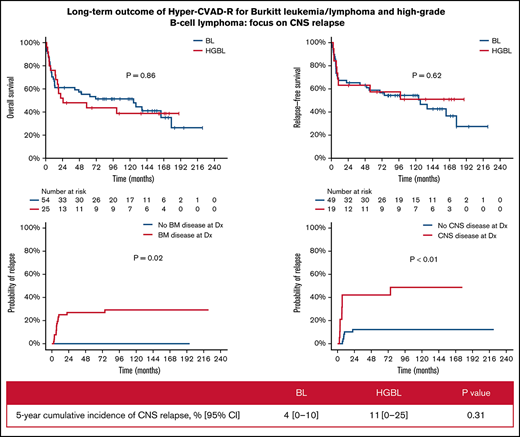
| 5-y, % (95% CI) | 6 (0 − 12) | 4 (0-10) | 11 (0-25) | .31 |
| Characteristic N (%) or median [range] . | Total (n = 79) . | BL (n = 54) . | HGBL (n = 25) . | P value . |
|---|---|---|---|---|
| Age at Dx, y | 44 [18-77] | 42 [18-77] | 47 [20-75] | .24 |
| Age ≥60 y | 20 (25) | 12 (22) | 8 (32) | .51 |
| Sex (male) | 51 (65) | 36 (67) | 15 (60) | .75 |
| ECOG PS 0-1 | 52/75 (69) | 35/52 (67) | 17/23 (74) | .76 |
| Stage 4 | 70 (89) | 49 (91) | 21 (84) | .67 |
| BM disease | 58 (73) | 42 (78) | 16 (64) | .31 |
| Extramedullary disease | 36 (46) | 24 (44) | 12 (48) | .96 |
| CNS disease at Dx | 22 (28) | 15 (28) | 7 (28) | 1.00 |
| Positive CSF | 16 (20) | 11 (20) | 5 (20) | |
| Parenchymal disease | 1 (2) | 0 | 1 (4) | |
| Clinical manifestations | 5 (6) | 4 (8) | 1 (4) | |
| LDH ≥ 3 ULN, n (%) | 50 (63) | 38 (70) | 12 (48) | .10 |
| HIV-related | 11 (14) | 9 (17) | 2 (8) | .49 |
| DHL | NA | NA | 7 (28) | |
| IPI | NA | NA | 3.5 [2-5] | |
| CNS-IPI | NA | NA | 3 [1-4] | |
| Treatment outcome, n (%) | ||||
| CR rate | 68/75 (91) | 49/51 (96) | 19/24 (79) | .16 |
| Early death <30 d | 2 (3) | 2 (4) | 0 | .84 |
| Early death <60 d | 5 (6) | 4 (7) | 1 (4) | .93 |
| Relapse | 15/68 (22) | 8/49 (16) | 7/19 (37) | .13 |
| CNS relapse | 4 (5) | 2 (4) | 2 (8) | .43 |
| Isolated CNS relapse | 2 (50) | 1 (50) | 1 (50) | |
| Death | 46 (58) | 31 (57) | 15 (60) | 1.00 |
| Death in CR | 21 (27) | 18 (33) | 3 (12) | |
| Overall survival | .86 | |||
| Median, mo (95% CI) | 71 (18-NR) | 125 (13-NR) | 25 (17-NR) | |
| 5-y, % (95% CI) | 52 (42-64) | 55 (44-70) | 44 (28-68) | |
| Relapse-free survival | .62 | |||
| Median, mo (95% CI) | 127 (54-NR) | 126 (50-NR) | NR (10-NR) | |
| 5-y, % (95% CI) | 58 (48-72) | 59 (47-75) | 57 (39-85) | |
| Cumulative incidence of relapse | ||||
| 5-y, % (95% CI) | 21 (11-30) | 14 (4-24) | 37 (14-59) | .06 |
| Cumulative incidence of CNS relapse | ||||
| 5-y, % (95% CI) | 6 (0 − 12) | 4 (0-10) | 11 (0-25) | .31 |
Abbreviations: BM, bone marrow; CI, confidence interval; CNS, central nervous system; CR, complete response; CSF, cerebrospinal fluid; DHL, double-hit lymphoma; Dx, diagnosis; IPI, international prognostic index; LDH, lactate dehydrogenase; mo, months; NA, not available; NR, not reported; PS, performance status; ULN, upper limit normal; y, year.
Among 75 evaluable patients, the CR rate was 91% (BL 96%; HGBCL 79%; P = .16) and the 30-day mortality was 3%. Death in CR occurred in 21 patients (28%, median age 56 [range, 22-77]). Most deaths in CR (76%) were treatment-related, primarily from infections (48%) and secondary myeloid malignancies (19%) (supplemental Table 1).
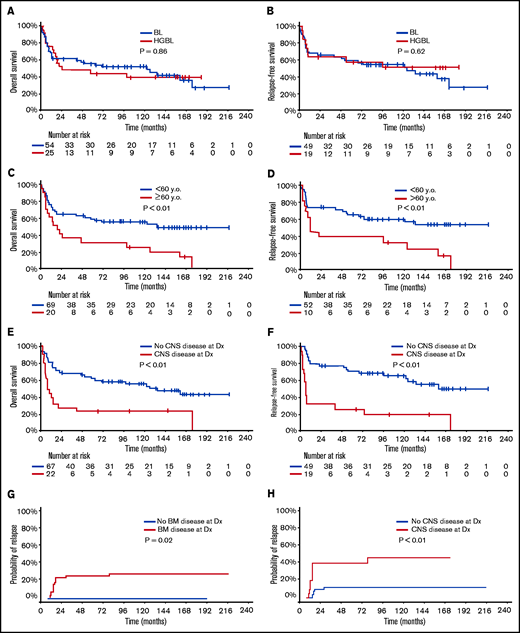
With a median follow-up of 50 months, 33 patients (42%) were alive in CR (23 BL, 10 HGBL). Only 1 patient with relapsed disease (first CR [CR1] duration 6 year) remains alive in second CR (CR2) for 5+ years after salvage with DA-EPOCH-R. The 5-year OS and RFS rates were 52% (BL 55%; HGBL 44%; P = .86) and 58% (BL 59%; HGBL 57%; P = .62), respectively (Figure 1A-B). The 5-year OS and RFS rates were inferior in patients ≥60 years (OS 30% vs 59%, P < .01; RFS 38% vs 65%, P < .01, Figure 1C-D) and those with baseline CNS involvement (OS 23% vs 63%, P < .01; RFS 26% vs 71%, P < .01, Figure 1E-F). In patients aged <60 years with no CNS or BM involvement (n = 19; 10 BL and 9 HGBL), the 5-year OS and RFS rates were 68% and 86%, respectively. Patients with higher BL International Prognostic Index had worse outcome: 5-year OS 83% vs 67% vs 42%; 5-year RFS 91% vs 80% vs 45% in patients with BL International Prognostic Index low vs intermediate vs high, respectively (supplementary Figure 1).
Outcome of patients with Burkitt leukemia/lymphoma and high-grade B-cell lymphoma treated with hyper-CVAD plus rituximab. Overall survival (A) and relapse-free (B) survival according to disease type. Overall survival (C) and relapse-free survival (D) according to age. Overall survival (E) and relapse-free survival (F) according to the status of baseline CNS disease. Cumulative incidence of relapse according to bone marrow involvement (G) and CNS involvement (H). y.o., years old.
Outcome of patients with Burkitt leukemia/lymphoma and high-grade B-cell lymphoma treated with hyper-CVAD plus rituximab. Overall survival (A) and relapse-free (B) survival according to disease type. Overall survival (C) and relapse-free survival (D) according to age. Overall survival (E) and relapse-free survival (F) according to the status of baseline CNS disease. Cumulative incidence of relapse according to bone marrow involvement (G) and CNS involvement (H). y.o., years old.
Multivariate analysis adjusted for age, ECOG performance status, lactate dehydrogenase, CNS disease, and HIV status showed that only age and CNS disease at baseline were independently associated with worse OS (P < .01 for both) and RFS (P = .02 and P < .01, respectively). ECOG performance status was predictive of OS when the multivariate analysis was restricted to patients with CNS disease. The CIR was 21% (BL 14%; HGBCL 37%, P = .06) and was higher in patients with baseline BM (27% vs 0%; P = .02) or CNS (42% vs 12%; P < .01) involvements (Figure 1G-H). The median time to relapse was 75 months (range, 0.5-228.1 months).
Four patients had CNS relapses (2 isolated parenchymal relapses, 2 leptomeningeal with systemic relapses) at a median of 5.6 months from diagnosis (range, 7.1-11.3). Three of them had baseline CNS disease. All 4 died of their disease at median of 2.5 months after CNS relapse. The 5-year CNS CIR disease was 6% (BL 4%; HGBL 11%; P = .31). Baseline CNS involvement was the main factor associated with CNS relapse (P = .03). Of the 22 patients with CNS involvement at baseline, 3 had CNS relapse (1 isolated parenchymal, 2 leptomeningeal with systemic relapse). Eighteen of the 22 patients died, and the cause of death was disease (N = 10), infections (N = 5, all but 1 were in CR), toxicity (N = 1), and unknown (N = 2). Among the 22 patients with CNS involvement, multivariate analysis adjusting for age, ECOG performance status, lactate dehydrogenase, and HIV status showed that ECOG performance status was predictive of OS.
Discussion
This long-term follow-up of the hyper-CVAD-R regimen in BL and HGBL supports the efficacy of dose-intensive regimens in preventing CNS relapses, especially among high-risk patients. Patients <60 years without CNS or BM disease had the best outcome (5-year OS, RFS and CIR rates of 68%, 86%, and 0%, respectively). When stratified by age and disease risk, our findings seem comparable to other previously reported regimens.6,7,14
Importantly, our cohort includes a population with high frequency of baseline CNS (28%) and BM (73%) involvement. In a recent report evaluating the DA-EPOCH-R regimen18 in 113 patients with BL, the median age was 38 years and the frequency of CNS and BM involvement at diagnosis was 10% and 28%, respectively, representing a lower risk population. In their study, the 4-year OS was 87% and the 4-year event-free survival was worse for patients with baseline CNS (46%) and BM (67%) involvement. Among 11 patients with baseline CNS disease, 6 either progressed (n = 3 including 1 with systemic and brain parenchyma) or died (n = 3). Two of 81 high-risk patients (2%) without baseline CNS involvement relapsed with parenchymal CNS disease despite IT chemotherapy (contrasting with no relapses in our BL cohort).
The low incidence of CNS relapses in our cohort is consistent with a recent study among 557 adults in newly diagnosed BL, where treatment with DA-EPOCH was associated with an increased risk of CNS relapse (CIR 13% vs 2% to 4% with dose-intense regimens).19 In another retrospective study of 64 adults with BL (n = 38) or HGBCL (n = 26), the 1-year incidence of CNS relapse was 36% in patients treated with DA-EPOCH-R versus 0% in patients treated with dose-intensive regimens.20 These studies and our data support the use of high-dose MTX and Ara-C for patients with BL or HGBL at high risk of systemic and CNS relapse. A major limitation is the high risk of treatment-related toxicity and deaths in CR especially in patients older than 40 or 60 years. For these patients, future modification of induction therapy with hyper-CVAD-R in combination with blinatumomab and inotuzumab followed possibly by chimeric antigen receptor T-cell therapy are expected to further improve the outcome of patients with baseline or at higher risk for CNS disease. DA-EPOCH-R should possibly be reserved for patients with low-risk disease and those who may not tolerate more intensive regimens.
Treating and preventing CNS disease in patients with BL or HGBL remains a challenge. Worse outcomes among elderly patients may be attributable to the empiric dose reduction to minimize the excess toxicity associated with systemic chemotherapy. Incorporation of novel compounds such as antibody-drug conjugates, immunotherapy, and inhibitors of the mTOR/phosphoinositide-3-kinase pathway will hopefully increase our ability to prevent and treat CNS disease while minimizing toxicity.21-24
In conclusion, the hyper-CVAD-R regimen is effective in patients with BL and HGBCL and is associated with a low incidence of CNS relapse, even in high-risk patients. Regimens containing high-dose MTX and Ara-C are preferable for patients with higher risk of CNS relapse. CNS relapse is associated with dismal prognosis, indicating a major unmet need.
Authorship
Contribution: B.S. and K.M. analyzed the data and wrote the manuscript; E.J. designed the study and wrote the manuscript; J.D.K. and S.E.H. performed pathological analysis; G.R.-C. analyzed the data; F.R., N.J.S., P.T., N.J., and H.K. collected data and treated patients; E.J., J.K., and H.K. provided leadership and managed the study team; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: E.J. has research grants with Amgen, AbbVie, Spectrum, BMS, Takeda Oncology, Pfizer, and Adaptive. F.R. has had honoraria and has been a member of advisory boards with BMS, Novartis, AbbVie, and Amgen. H.K. reports grants from AbbVie, Agios, Amgen, Ariad, Astex, BMS, Cyclacel, Daiichi-Sankyo, Immunogen, Jazz Pharma, Novartis, and Pfizer. N.J.S. has served as consultant for Takeda Oncology and AstraZeneca, reports receiving commercial research grants from Takeda Oncology and Astellas Pharma Inc, and has received speakers’ bureau honoraria from Amgen.
Data sharing statement: For data sharing, contact the corresponding author: ejabbour@mdanderson.org.
Correspondence: Elias Jabbour, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ejabbour@mdanderson.org.
References
Author notes
B.S. and K.M. contributed equally to this study.
The full-text version of this article contains a data supplement.