Key Points
A pediatric chemotherapy regimen was tolerable and effective for AYAs up to age 40 years with acute lymphoblastic leukemia.
Toxicities were similar when the same treatment was given by pediatric or adult hematologist/oncologists.
Visual Abstract
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography ©2020. All Rights Reserved.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography ©2020. All Rights Reserved.
Abstract
Adolescents and young adults (AYAs) with acute lymphoblastic leukemia have improved outcomes when treated with pediatric-inspired regimens. CALGB 10403 was the largest prospective study to evaluate the feasibility of using a pediatric regimen in AYAs with acute lymphoblastic leukemia up to 40 years of age. This article presents the toxicity events observed in the CALGB 10403 study and compares these toxicities vs those observed among AYAs treated on the same arm of the companion Children’s Oncology Group (COG) AALL0232 study. Toxicities in CALGB 10403 were similar to those observed in COG AALL0232. Some grade 3 to 4 adverse events were more often reported in CALGB 10403 compared with COG AALL0232 (hyperglycemia, hyperbilirubinemia, transaminase elevation, and febrile neutropenia). Adverse events correlated with body mass index ≥30 kg/m2 and some with increasing age. The mortality rate in CALGB 10403 was low (4%) and similar to that in the COG AALL0232 trial. A caveat to this analysis is that only 39% of CALGB 10403 patients completed all planned protocol treatment. In COG AALL0232, although 74% of patients aged <18 years completed treatment, only 57% of patients aged ≥18 years completed treatment. This scenario suggests that issues associated with age and treating physician may be a factor. Due to its improved survival rates compared with historical controls, the CALGB 10403 regimen is now a standard of care. The hope is that the rate of protocol completion will increase as more familiarity is gained with this regimen. These trials were registered at www.clinicaltrials.gov as #NCT00558519 (CALGB 10403) and #NCT00075725 (COG AALL0232).
Introduction
Several retrospective studies reported a superior outcome when young adults with acute lymphoblastic leukemia (ALL) were treated with pediatric-inspired regimens compared with conventional adult regimens.1-4 One concern was the potential toxicity of using a childhood ALL regimen in an “older” young adult population. Therefore, a prospective trial (CALGB 10403) was conducted to determine the feasibility of adult hematologist/oncologists treating adolescents and young adults (AYAs; 16-39 years of age) using one arm of a randomized trial performed by the Children’s Oncology Group (COG), AALL0232.4,5 One objective of the CALGB 10403 study was to identify age-related increases in specific treatment-related toxicities that may limit the applicability of pediatric-inspired regimens in AYAs with ALL.
We describe here the adverse event profiles according to age cohort for patients enrolled on CALGB 10403 and compare them vs adverse events reported from the same treatment arm of the COG AALL0232 trial in patients ≥16 years of age. In the very large COG study, these AYAs comprised 20% of all enrolled patients, and 92% were between the ages of 16 and 21 years; 8% were aged >21 years.
Methods
From November 2007 through September 2012, a total of 318 AYAs (age 18-39 years) with newly diagnosed B- or T-precursor ALL (World Health Organization criteria) were enrolled on CALGB 10403 from 3 US cooperative groups (Alliance [Alliance for Clinical Trials in Oncology], SWOG [Southwest Oncology Group], and Eastern Cooperative Oncology Group [ECOG]). CALGB is now part of Alliance.
Research was approved by the relevant institutional review boards, and participants provided written informed consent. The eligibility criteria, treatment schema, and outcomes for CALGB 10403 have been previously published, and the regimen is listed in the supplemental Appendix.5 The date of data lock for CALGB 10403 was July 31, 2018. The comparison group from COG AALL0232 included 158 patients (146 patients aged 16-21 years and 12 patients aged 22-30 years) with precursor B-cell ALL randomized to the “PC” arm (prednisone during induction and Capizzi methotrexate/pegaspargase during interim maintenance).4 However, in COG AALL0232, slow responders received additional treatment consisting of a second interim maintenance (IM2) and delayed intensification (DI2) phase, entailing 4 more months of intensive therapy, plus 12 Gy cranial irradiation, compared with CALGB 10403 patients. These patients were still included in this analysis, but toxicities during IM2 and DI2 were not included.
For COG AALL0232 and CALGB 10403, data were collected regarding all grade 3 or higher nonhematologic toxicities. Descriptive statistics were used to summarize toxicities. For the current report, we focused on grade 3 to 5 events with at least a possible relationship to treatment. In CALGB 10403, toxicities were graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. COG AALL0232 initially used CTCAE version 3.0 for toxicity reporting; this was changed to CTCAE version 4.0 for the final year of accrual with toxicities mapped to CTCAE version 4.0 using National Cancer Institute–defined algorithms for both trials in this analysis. Proportions were compared by using the χ2 or Fisher’s exact test. P values ≤.05 were considered significant.
Results
Of the 318 patients registered to CALGB 10403, a total of 289 were evaluable and analyzed for adverse events. Most of the ineligible subjects were found to have Philadelphia chromosome–positive ALL (n = 20) and were excluded. Sixty-one percent were male; 75% were White, 10% were African American, and 15% were Hispanic. The median age was 24 years for CALGB 10403 patients and 17 years for the COG AALL0232 AYA patients. In CALGB 10403, 33% of patients were aged 16 to 21 years, 45% were aged 22 to 30 years, and 22% were aged 31 to 39 years vs 92%, 8%, and 0% of the COG AALL0232 AYA patients (P < .001), respectively.
Remission induction toxicities
Induction toxicities are summarized in Table 1. The rates of grade 3 to 4 hyperglycemia, hyperbilirubinemia, transaminase elevation, and febrile neutropenia during induction were higher in the CALGB 10403 trial than in AYAs treated in COG AALL0232. Importantly, induction mortality rates for CALGB 10403 and COG AALL0232 were both low: 3.1% and 1.3%, respectively (P = .34). The causes of death in individual patients in both studies during induction included: COG AALL0232, acute kidney injury (n = 1), infection (n = 1); CALGB 10403, hepatic failure (n = 2), sepsis (n = 2), ventricular tachycardia (n = 1), unknown (n = 1), blood and lymphatic disorders (n = 1), multiorgan failure (n = 1), and nervous system disorders (n = 1).
Comparison of grade 3 to 4 adverse events during the induction course
| Adverse event . | CALGB 10403 (N = 289) . | COG AALL0232 (N = 158) . | P* . |
|---|---|---|---|
| Hyperglycemia | 31.1% (90) | 22.8% (36) | .06 |
| Aspartate aminotransferase | 12.8% (37) | 5.7% (9) | .02 |
| Alanine aminotransferase | 28.7% (83) | 17.7% (28) | .01 |
| Hyperbilirubinemia | 19.0% (55) | 7.0% (11) | <.001 |
| Anaphylaxis | 1.4% (4) | 0.6% (1) | .66 |
| Pancreatitis | 2.8% (8) | 1.3% (2) | .51 |
| Thrombosis | 5.2% (15) | 1.9% (3) | .13 |
| Febrile neutropenia* | 23.9% (69) | 5.7% (9) | <.001 |
| Infection | 24.6% (71) | 22.8% (36) | .67 |
| Adverse event . | CALGB 10403 (N = 289) . | COG AALL0232 (N = 158) . | P* . |
|---|---|---|---|
| Hyperglycemia | 31.1% (90) | 22.8% (36) | .06 |
| Aspartate aminotransferase | 12.8% (37) | 5.7% (9) | .02 |
| Alanine aminotransferase | 28.7% (83) | 17.7% (28) | .01 |
| Hyperbilirubinemia | 19.0% (55) | 7.0% (11) | <.001 |
| Anaphylaxis | 1.4% (4) | 0.6% (1) | .66 |
| Pancreatitis | 2.8% (8) | 1.3% (2) | .51 |
| Thrombosis | 5.2% (15) | 1.9% (3) | .13 |
| Febrile neutropenia* | 23.9% (69) | 5.7% (9) | <.001 |
| Infection | 24.6% (71) | 22.8% (36) | .67 |
Number of patients is given in parentheses.
χ2 or Fisher’s exact test.
Postremission therapy toxicities
Postremission mortality rates in CALGB 10403 and COG AALL0232 were both low: 1.3% and 0.8%, respectively (P = .64). Grade 3 to 4 adverse events during postremission treatment are listed in Table 2. Although the rates of infection during induction were similar between COG AALL0232 and CALGB 10403, the rates of infection were significantly higher postinduction in COG AALL0232 (55.0%), compared with CALGB 10403 (38.7%) (P = .002). After induction, the incidence of febrile neutropenia was not different. During interim maintenance with methotrexate and pegaspargase, 9.1% of patients in CALGB 10403 and 16.4% in COG AALL0232 developed grade 3 to 4 mucositis (P = .037). Grade 3 to 4 hypersensitivity reactions declined from 10% to 4% after a CALGB 10403 protocol amendment to require premedication for pegaspargase with corticosteroids, acetaminophen, and diphenhydramine. In comparison, AYAs in COG AALL0232, who did not receive routine premedication before pegaspargase, had higher rates of hypersensitivity reactions requiring discontinuation of pegaspargase (14.6% grade 3/4). Other differences included an increased incidence of hepatic toxicity, pancreatitis, elevated bilirubin, decreased fibrinogen, and increased thrombosis in CALGB 10403. In CALGB 10403, specific grade 3 and higher thrombotic events included: central nervous system embolic infarcts (n = 1), cavernous sinus thrombosis (n = 6), pulmonary embolus (n = 7), extensive lower extremity deep vein thrombosis (n = 2), and extensive right internal jugular/subclavian deep vein thrombosis (n = 1). Of note, detailed information on thrombotic events was only present in 15 of the 33 patients. Conversely, there was an increased incidence of encephalopathy (5.1% vs 0.7%) and motor neuropathy (15.8% vs 6.6%) in COG ALL0232 patients compared with CALGB 10403 patients. The incidence of avascular necrosis of bone (2.5%-2.8%) and fracture (0.6%-0.7%) was similar between the 2 studies.
Comparison of grade 3 to 4 adverse events during postremission therapy
| Adverse event . | CALGB 10403 (N = 238) . | COG AALL0232 (N = 149) . | P* . |
|---|---|---|---|
| Allergic reaction | 0 | 2.0 | .056 |
| Anaphylaxis | 12.6 | 17.5 | .19 |
| Coagulation abnormalities | |||
| Decreased fibrinogen | 12.2 | 1.3 | <.001 |
| Prothrombin time | 0.4 | 0.7 | 1 |
| Partial thromboplastin time | 5.0 | 0.7 | .02 |
| Disseminated intravascular coagulation | 0 | 1.3 | .15 |
| Central nervous system hemorrhage | 0.8 | 0 | .53 |
| Thrombosis | 10.1 | 2.0 | .002 |
| Hepatic | |||
| Liver failure | 0 | 0 | 1 |
| Aspartate aminotransferase | 34.0 | 17.5 | .0004 |
| Alanine aminotransferase | 55.5 | 35.6 | .0001 |
| Bilirubin | 15.6 | 16.1 | .88 |
| Pancreatitis | 8.0 | 2.7 | .045 |
| Bone | |||
| Fracture | 0.8 | 0.7 | 1 |
| Osteonecrosis | 3.4 | 2.7 | .77 |
| Hyperglycemia | 19.3 | 10.7 | .025 |
| Neurologic | |||
| Central nervous system ischemia | 0 | 0 | NA |
| Confusion | 1.7 | 0.7 | .65 |
| Encephalopathy | 0 | 4.7 | .001 |
| Motor neuropathy | 6.3 | 14.8 | .006 |
| Sensory neuropathy | 18.5 | 10.1 | .025 |
| Seizure | 3.4 | 0 | .026 |
| Dysphasia | 2.9 | 4.0 | .56 |
| Somnolence | 1.3 | 1.3 | 1 |
| Febrile neutropenia | 49.2 | 40.9 | .11 |
| Infection | 38.7 | 55.0 | .002 |
| Adverse event . | CALGB 10403 (N = 238) . | COG AALL0232 (N = 149) . | P* . |
|---|---|---|---|
| Allergic reaction | 0 | 2.0 | .056 |
| Anaphylaxis | 12.6 | 17.5 | .19 |
| Coagulation abnormalities | |||
| Decreased fibrinogen | 12.2 | 1.3 | <.001 |
| Prothrombin time | 0.4 | 0.7 | 1 |
| Partial thromboplastin time | 5.0 | 0.7 | .02 |
| Disseminated intravascular coagulation | 0 | 1.3 | .15 |
| Central nervous system hemorrhage | 0.8 | 0 | .53 |
| Thrombosis | 10.1 | 2.0 | .002 |
| Hepatic | |||
| Liver failure | 0 | 0 | 1 |
| Aspartate aminotransferase | 34.0 | 17.5 | .0004 |
| Alanine aminotransferase | 55.5 | 35.6 | .0001 |
| Bilirubin | 15.6 | 16.1 | .88 |
| Pancreatitis | 8.0 | 2.7 | .045 |
| Bone | |||
| Fracture | 0.8 | 0.7 | 1 |
| Osteonecrosis | 3.4 | 2.7 | .77 |
| Hyperglycemia | 19.3 | 10.7 | .025 |
| Neurologic | |||
| Central nervous system ischemia | 0 | 0 | NA |
| Confusion | 1.7 | 0.7 | .65 |
| Encephalopathy | 0 | 4.7 | .001 |
| Motor neuropathy | 6.3 | 14.8 | .006 |
| Sensory neuropathy | 18.5 | 10.1 | .025 |
| Seizure | 3.4 | 0 | .026 |
| Dysphasia | 2.9 | 4.0 | .56 |
| Somnolence | 1.3 | 1.3 | 1 |
| Febrile neutropenia | 49.2 | 40.9 | .11 |
| Infection | 38.7 | 55.0 | .002 |
Data are presented as percentages.
NA, not applicable.
*χ2 or Fisher’s exact test.
Sixty-one percent of the CALGB 10403 patients completed the planned intensive chemotherapy and began the prolonged maintenance treatment, but only 39% completed all protocol-designated treatment, which continued for 2 to 3 years from diagnosis. Much of the dropout was related to physicians switching patients to “nonprotocol” treatment. The reasons for this dropout are outlined in the original paper.5 Of note, in COG AALL0232, in the “PC” arm, 57.1% of patients aged ≥18 years completed all protocol treatment, compared with 74% of patients <18 years of age (P = .017).
Toxicities according to age
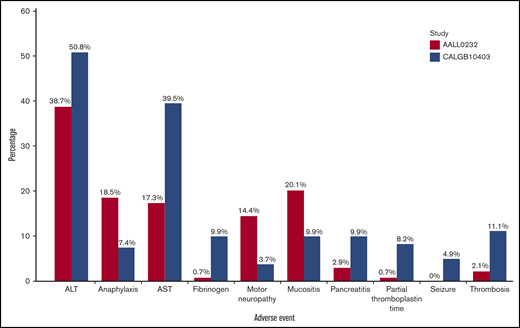
To determine whether there were any differences in toxicities between age cohorts, toxicities were analyzed according to age group (16-21 years, 22-30 years, and >31 years of age) in CALGB 10403 and COG AALL0232. In CALGB 10403, the median ages for the 16- to 21-year-old and 22- to 30-year-old groups were 20 and 25 years, respectively. In COG AALL0232, the median ages for these two groups were 17 and 24.5 years, but only 7.6% of patients were >22 years old. These induction toxicities did not increase in frequency or severity with increasing age cohorts. In fact, there was a trend toward higher toxicities reported in the CALGB 10403 patients aged 16 to 21 years. Among these younger CALGB 10403 patients, there was more hyperglycemia, hyperbilirubinemia, transaminase elevation, pancreatitis, and febrile neutropenia compared with the same age group (aged 16-21 years) treated in COG AALL0232 during induction (Figure 1). Some grade 3 to 4 adverse events were more common among CALGB 10403 patients than COG AALL0232 patients treated during the postremission treatment period (Figure 2). However, when analyzed as a continuous variable, increased age correlated with a decreased fibrinogen level during induction and postremission therapy (odds ratios [ORs] of 1.103 [P < .0001] and 1.111 [P = .0002], respectively) and elevated alanine aminotransferase levels during induction and postremission therapy (ORs of 1.037 [P = .039] and 1.045 [P = .011]).
Comparison of select adverse events (grade 3-4) during induction (age group 16-21 years). ALT, alanine aminotransferase.
Comparison of select adverse events (grade 3-4) during induction (age group 16-21 years). ALT, alanine aminotransferase.
Comparison of select adverse events (grade 3-4) during postremission therapy (age group 16-21 years). ALT, alanine aminotransferase; AST, aspartate aminotransferase. *Decreased fibrinogen level.
Comparison of select adverse events (grade 3-4) during postremission therapy (age group 16-21 years). ALT, alanine aminotransferase; AST, aspartate aminotransferase. *Decreased fibrinogen level.
Toxicities according to body mass index
Body mass index (BMI) was an independent determinant of outcome in the CALGB 10403 study. To determine whether BMI contributed to differences in toxicities, BMI was also analyzed according to age group and study (Table 3). There was a statistically significant difference in BMI by age group in COG AALL0232 (P = .037) but not in CALGB 10403. The median BMI of patients overall was higher in CALGB 10403 compared with COG AALL0232 (P = .056). COG AALL0232 had a higher percentage of patients with BMI <30 kg/m2; conversely, CALGB 10403 had a higher percentage of patients with BMI ≥30 kg/m2. In both COG AALL0232 and CALGB 10403, patients with a lower BMI (<30 kg/m2) had a lower frequency of grade 3 to 5 toxicities (P = .002 for COG AALL0232). The morbidly obese group (BMI ≥40 kg/m2) in the CALGB 10403 study had the highest number of grade 3 to 5 toxicities. During induction, patients with a higher BMI had a higher incidence of grade 3 to 4 toxicities in both trials (CALGB 10403 and COG AALL0232) (Table 4).
BMI according to age in CALGB 10403 and COG AALL0232
| Age group . | BMI <30 kg/m2 . | BMI 30-40 kg/m2 . | BMI ≥40 kg/m2 . | Total N . | P . |
|---|---|---|---|---|---|
| CALGB 10403 | 197 | 71 | 21 | 289 | |
| 16-21 y | 70.2% (66) | 22.3% (21) | 7.5% (7) | 94 | .23 |
| 22-30 y | 72.5% (95) | 21.4% (28) | 6.1% (8) | 131 | |
| ≥31 y | 56.3% (36) | 34.4% (22) | 9.4% (6) | 64 | |
| COG AALL0232 | 124 | 25 | 9 | 158 | |
| 16-21 y | 80.8% (118) | 14.4% (21) | 4.8% (7) | 146 | .04 |
| 22-30 y | 50.0% (6) | 33.3% (4) | 16.7% (2) | 12 |
| Age group . | BMI <30 kg/m2 . | BMI 30-40 kg/m2 . | BMI ≥40 kg/m2 . | Total N . | P . |
|---|---|---|---|---|---|
| CALGB 10403 | 197 | 71 | 21 | 289 | |
| 16-21 y | 70.2% (66) | 22.3% (21) | 7.5% (7) | 94 | .23 |
| 22-30 y | 72.5% (95) | 21.4% (28) | 6.1% (8) | 131 | |
| ≥31 y | 56.3% (36) | 34.4% (22) | 9.4% (6) | 64 | |
| COG AALL0232 | 124 | 25 | 9 | 158 | |
| 16-21 y | 80.8% (118) | 14.4% (21) | 4.8% (7) | 146 | .04 |
| 22-30 y | 50.0% (6) | 33.3% (4) | 16.7% (2) | 12 |
Number of patients is given in parentheses.
Selected grade 3 to 4 toxicity comparison according to BMI group during induction
| Adverse event . | BMI <30 kg/m2 (n = 197) . | BMI 30-40 kg/m2 (n = 71) . | BMI ≥40 kg/m2 (n = 21) . | P . |
|---|---|---|---|---|
| CALGB 10403 (N = 289) | ||||
| Nonhematologic toxicity | 77.2% (152) | 80.3% (57) | 85.7% (18) | .685 |
| Hepatic toxicity | 31.0% (61) | 52.1% (37) | 61.9% (13) | .001 |
| Infection | 21.8% (43) | 26.8% (19) | 42.9% (9) | .092 |
| Alanine aminotransferase | 23.9% (47) | 35.2% (25) | 52.4% (11) | .009 |
| Aspartate aminotransferase | 7.1% (14) | 23.9% (17) | 28.6% (6) | <.0001 |
| Hyperbilirubinemia | 11.7% (23) | 31.0% (22) | 47.6% (10) | <.0001 |
| Pancreatitis | 2.0% (4) | 2.8% (2) | 9.5% (2) | .123 |
| Hyperglycemia | 26.4% (52) | 39.4% (28) | 47.6% (10) | .030 |
| Adverse event . | BMI <30 kg/m2 (n = 197) . | BMI 30-40 kg/m2 (n = 71) . | BMI ≥40 kg/m2 (n = 21) . | P . |
|---|---|---|---|---|
| CALGB 10403 (N = 289) | ||||
| Nonhematologic toxicity | 77.2% (152) | 80.3% (57) | 85.7% (18) | .685 |
| Hepatic toxicity | 31.0% (61) | 52.1% (37) | 61.9% (13) | .001 |
| Infection | 21.8% (43) | 26.8% (19) | 42.9% (9) | .092 |
| Alanine aminotransferase | 23.9% (47) | 35.2% (25) | 52.4% (11) | .009 |
| Aspartate aminotransferase | 7.1% (14) | 23.9% (17) | 28.6% (6) | <.0001 |
| Hyperbilirubinemia | 11.7% (23) | 31.0% (22) | 47.6% (10) | <.0001 |
| Pancreatitis | 2.0% (4) | 2.8% (2) | 9.5% (2) | .123 |
| Hyperglycemia | 26.4% (52) | 39.4% (28) | 47.6% (10) | .030 |
| . | BMI <30 kg/m2 (n = 124) . | BMI 30-40 kg/m2 (n = 25) . | BMI ≥40 kg/m2 (n = 9) . | P . |
|---|---|---|---|---|
| COG AALL0232 (N = 158) | ||||
| Nonhematologic toxicity | 62.1% (77) | 84.0% (21) | 55.6% (5) | .077 |
| Hepatic toxicity | 17.7% (22) | 40.0% (10) | 11.1% (1) | .042 |
| Infection | 18.6% (23) | 32.0% (8) | 55.6% (5) | .018 |
| Alanine aminotransferase | 15.3% (19) | 32.0% (8) | 11.1% (1) | 0.140 |
| Aspartate aminotransferase | 4.8% (6) | 8.0% (2) | 11.1% (1) | 0.394 |
| Hyperbilirubinemia | 4.0% (5) | 20.0% (5) | 11.1% (1) | .018 |
| Pancreatitis | 0% (0) | 8.0% (2) | 0% (0) | .046 |
| Hyperglycemia | 20.2 (25) | 32.0% (8) | 33.3% (3) | .303 |
| . | BMI <30 kg/m2 (n = 124) . | BMI 30-40 kg/m2 (n = 25) . | BMI ≥40 kg/m2 (n = 9) . | P . |
|---|---|---|---|---|
| COG AALL0232 (N = 158) | ||||
| Nonhematologic toxicity | 62.1% (77) | 84.0% (21) | 55.6% (5) | .077 |
| Hepatic toxicity | 17.7% (22) | 40.0% (10) | 11.1% (1) | .042 |
| Infection | 18.6% (23) | 32.0% (8) | 55.6% (5) | .018 |
| Alanine aminotransferase | 15.3% (19) | 32.0% (8) | 11.1% (1) | 0.140 |
| Aspartate aminotransferase | 4.8% (6) | 8.0% (2) | 11.1% (1) | 0.394 |
| Hyperbilirubinemia | 4.0% (5) | 20.0% (5) | 11.1% (1) | .018 |
| Pancreatitis | 0% (0) | 8.0% (2) | 0% (0) | .046 |
| Hyperglycemia | 20.2 (25) | 32.0% (8) | 33.3% (3) | .303 |
Number of patients is given in parentheses.
Although there were some differences in incidence between the trials, many of the toxicities were overlapping (hepatic toxicity, hyperbilirubinemia). When analyzed as a continuous variable, BMI was associated with an increased incidence of pancreatitis (OR, 1.078; P = .048), increased aspartate aminotransferase (OR, 1.072; P = .001), increased alanine aminotransferase (OR, 1.052; P = .001), and increased bilirubin (OR, 1.109; P < .0001) during induction. In addition, increased BMI was associated with an increased rate of aspartate aminotransferase (OR, 1.046; P = .006) and bilirubin (OR, 1.044; P = .025) during postremission therapy.
Delay in treatment related to toxicity
One hundred seventy-seven CALGB 10403 patients (61%) and 125 COG AALL0232 patients (79%) completed all planned protocol therapy up to starting the prolonged maintenance phase. We evaluated whether grade 3 to 4 toxicities in liver function tests or pancreatitis had led to delays in treatment. Toxicities occurring during DI2 and IM2 (for COG AALL0232) were excluded for this analysis. Treatment delay was calculated as the sum of the length of the overall treatment time (induction, consolidation, interim maintenance, and delayed intensification courses) minus 184 days (the ideal number of days per protocol if all therapy was given on time) for patients who completed all protocol treatment before starting maintenance. Overall, there was a trend (P = .051) toward more delays in treatment (time from starting induction to beginning of maintenance) in CALGB 10403 (median, 64 days) compared with COG AALL0232 (59 days). Evaluating treatment delay by selected toxicity on CALGB 10403 or COG AALL0232 (pancreatitis, bilirubin, transaminase, or patients with at least one toxicity), the average delay time for patients who had grade 3 to 4 transaminase elevation, hyperbilirubinemia, or pancreatitis was slightly longer (median, 5 days) but was not statistically significant.
Completion of treatment on CALGB 10403
Only 39% of patients in CALGB 10403 completed treatment, compared with 57% of the AYA patients in COG AALL0232. In contrast, 74% of the patients <18 years of age in COG AALL0232 completed all therapy (P = .017). To analyze potential reasons for the higher dropout rate of the CALGB 10403 patients, we analyzed serial recorded performance status in CALGB 10403 patients who completed treatment vs patients who did not complete treatment. Patients who did not complete treatment had a worse ECOG performance status during interim maintenance, intensification, and maintenance (Table 5). To further assess the reason for dropout, we also analyzed differences in toxicities (grade 3-4) between patients who stayed on treatment vs those who went off treatment for reasons other than progression or transplant at the beginning of each treatment course. There was no correlation between increased rate of serious (grade 3-4) toxicities and not completing treatment.
Serial record of performance status during treatment on CALGB 10403
| . | N . | . | ECOG performance status . | P . | ||
|---|---|---|---|---|---|---|
| Phase of therapy . | Completion of therapy . | 0 . | 1 . | ≥2 . | ||
| Induction | 236 | Completed course | 33.9% (80) | 56.8% (134) | 9.3% (22) | .61 |
| 48 | Did not complete therapy | 41.7% (20) | 50% (24) | 8.3% (4) | ||
| Consolidation | 206 | Completed course | 30.6% (63) | 59.2% (122) | 10.2% (21) | .77 |
| 29 | Did not complete therapy | 27.6% (8) | 58.6% (17) | 13.8% (4) | ||
| Interim maintenance | 191 | Completed course | 37.7% (72) | 54.5% (104) | 7.9% (15) | .40 |
| 16 | Did not complete therapy | 25% (4) | 62.5% (10) | 12.5% (2) | ||
| Intensification | 171 | Completed course | 40.4% (69) | 57.9% (99) | 1.8% (3) | .31 |
| 18 | Did not complete therapy | 22.2% (4) | 77.8% (14) | 0 | ||
| Maintenance | 111 | Completed all treatment | 79.3% (88) | 18.0% (20) | 2.7% (2) | .001 |
| 65 | Did not complete therapy | 53.9% (35) | 41.5% (27) | 4.6% (3) | ||
| . | N . | . | ECOG performance status . | P . | ||
|---|---|---|---|---|---|---|
| Phase of therapy . | Completion of therapy . | 0 . | 1 . | ≥2 . | ||
| Induction | 236 | Completed course | 33.9% (80) | 56.8% (134) | 9.3% (22) | .61 |
| 48 | Did not complete therapy | 41.7% (20) | 50% (24) | 8.3% (4) | ||
| Consolidation | 206 | Completed course | 30.6% (63) | 59.2% (122) | 10.2% (21) | .77 |
| 29 | Did not complete therapy | 27.6% (8) | 58.6% (17) | 13.8% (4) | ||
| Interim maintenance | 191 | Completed course | 37.7% (72) | 54.5% (104) | 7.9% (15) | .40 |
| 16 | Did not complete therapy | 25% (4) | 62.5% (10) | 12.5% (2) | ||
| Intensification | 171 | Completed course | 40.4% (69) | 57.9% (99) | 1.8% (3) | .31 |
| 18 | Did not complete therapy | 22.2% (4) | 77.8% (14) | 0 | ||
| Maintenance | 111 | Completed all treatment | 79.3% (88) | 18.0% (20) | 2.7% (2) | .001 |
| 65 | Did not complete therapy | 53.9% (35) | 41.5% (27) | 4.6% (3) | ||
Number of patients is given in parentheses.
Discussion
These data indicate that when adult hematologists/oncologists used a pediatric ALL regimen (CALGB 10403) in an AYA population up to 40 years of age, there was an increase in some toxicities but they were not treatment limiting. Most of the differences in serious toxicities between the 2 populations occurred during induction therapy. Importantly, in both study populations, the treatment-related mortality was low and not different. The toxicities were not unexpected and generally resolved quickly; importantly, selected grade 3 to 4 toxicities did not lead to a statistically significant delay in treatment. A caveat to this analysis is that only 39% of patients on CALGB 10403 completed all 2 to 3 years of the planned protocol treatment. However, notably in COG AALL0232, although 74% of patients <18 years of age completed all protocol treatment, this percentage was significantly lower (57.1%) in patients aged ≥18 years, suggesting that factors associated with age and treating physician may play a role. The reasons for this in CALGB 10403 are shown in supplemental Figure 2. There is a suggestion that patients who did not complete treatment had a worse ECOG performance status later during interim postremission therapy; however, the numbers of patients are small, and the differences were only statistically significant during maintenance treatment. The most common reason for lack of treatment completion was not necessarily due to unresolved toxicity but due to CALGB 10403 physicians switching patients to nonprotocol treatment, possibly as a result of lack of familiarity with an intensive pediatric regimen. With improving familiarity and comfort with this intensive outpatient regimen, we anticipate that fewer patients will be counseled to abandon this therapy for alternative treatment approaches.
There is also the interesting observation that factors other than physician choice and serious treatment toxicities may play a role in treatment discontinuation. Even in the COG AALL0232 trial, with the same physicians familiar with these regimens caring for all patients, there was a significantly lower rate of protocol completion for the AYA patients aged ≥18 years. Over the years, much discussion about the AYA psyche and the challenges associated with young adulthood (eg, independent living away from “parents,” education, job and relationship challenges) have been proposed as potential reasons for lower adherence to clinical trials and may be particularly relevant for the long arduous treatment programs for ALL.6 Although our data cannot confirm the role of these more difficult-to-quantify issues, these factors are undoubtedly important and require more careful study in future AYA trials of ALL.
The major toxicities more frequently observed in the AYA population of CALGB 10403 compared with the COG AALL0232 patients were hyperglycemia, febrile neutropenia, thromboembolism, pancreatitis, and increased bilirubin. These differences were noted primarily during induction and are similar to those observed in other pediatric-inspired trials for AYA ALL.7-11 After induction therapy, there were no significant differences in toxicities between our 2 populations; there was also no significant increase in toxicity in the older AYA patients in CALGB 10403. The incidence of toxicities in NOPHO ALL2008 was also very similar to those seen in CALGB 10403, except for a lower incidence of liver toxicities in induction in the NOPHO patients.12
Because obesity rates also increase with age, it is perhaps not surprising that obesity may have contributed significantly to the increased incidence of hepatic, hyperglycemic, thrombotic, and septic complications noted, particularly during induction therapy in CALGB 10403 patients. Nearly one-third (30%) of these patients were considered obese (BMI >30 kg/m2) and had significantly worse survival compared with the COG AALL0232 trial, in which 19% of patients were considered obese.5 Denton et al8 found that both obesity and age ≥10 years were predictors of hepatotoxicity and pancreatitis in a retrospective study of children and adolescents receiving treatment of ALL; similar to this report, the highest incidence occurred during induction. Higher BMI in both trials (CALGB 10403 and COG ALL0232) was, in fact, associated with a significantly increased risk of grade 3 to 4 induction toxicities. Obesity has also been associated with nonalcoholic steatohepatitis. The increased hepatic complications (transaminitis and hyperbilirubinemia) observed in CALGB 10403 may be related to exposure to pegaspargase and 28 days of prednisone during the remission induction phase. Prospective evaluation is needed to evaluate the association of obesity with the degree of asparagine depletion (using asparaginase levels as a surrogate marker),13 and its impact on inflammation and disseminated intravascular coagulation often noted at diagnosis, and subsequent rates of thrombosis. We do not have data on patient alcohol use from this study; however, it is possible that alcohol use was more common in the CALGB 10403 population and may have contributed to an increased incidence of hepatic complications.
Lowering or capping the dose of pegaspargase may be a way to minimize hepatic toxicity.14 Similarly, using pulses of glucocorticoid therapy rather than 4 weeks of continuous exposure may decrease toxicity in the AYA population. This approach has been pursued by the German Multicenter ALL group when treating older adults with ALL, with success.15 In the NOPHO ALL2008 study, pegaspargase was not used during induction; instead, more frequent dosing was performed later but at a dose of 1000 IU/ m2.12 The risk of thrombosis was lower (5%-6%) and the rate of liver dysfunction was very low (2.4%-4.2%) in the 10- to 45-year-old age group. This could be related to the timing of the pegaspargase as well as lower BMI in this population compared with our North American patients. In a randomized trial, the NOPHO group reported similar clinical outcomes but a decreased incidence of pancreatitis and thromboembolic complications when intermittent rather than continuous pegaspargase was used after the first 10 weeks of therapy.16
Dutch investigators have recently published their study of targeted asparaginase dosing, reporting good levels with lower doses.14,17 Various “chemopreventive” strategies have been investigated with pegaspargase such as milk thistle,18 levocarnitine,19 and antithrombin (AT) supplementation or prophylactic anticoagulation to decrease the risk of thrombosis. In the PARKAA (Prophylactic Antithrombin Replacement in Kids With Acute Lymphoblastic Leukemia Treated With L-Asparaginase) study, children were randomized to receive AT infusions or no AT treatment.20 The incidence of thrombosis in patients treated with AT was 28%, compared with 37% in the nontreated arm, suggesting a trend for efficacy. A subsequent trial, THROMBOTECT, randomized children and adolescents with ALL to receive thromboprophylaxis with low-molecular-weight heparin, AT, or unfractionated heparin during induction.21 Patients assigned to heparin had a high risk of thromboembolism (8.0%) compared with those randomized to receive enoxaparin (3.5%; P = .011) or AT (1.9%; P < .001). These data show a benefit for the prophylactic use of AT or enoxaparin to reduce the risk of thromboembolism. Evaluating genetic polymorphisms may also help to determine if there are etiologies other than age-related changes explaining the increased incidence of toxicity with respect to these agents.
In summary, treatment with a pediatric regimen in AYAs aged up to 40 years was feasible, and mortality was low. Certain toxicities (hyperglycemia, hepatic toxicity, and febrile neutropenia) were more common on the adult trial, but these were mainly limited to induction therapy. Room remains for improvement given the high rates of dropout from the trial and the correlation of poor outcomes with high BMI and possible underlying nonalcoholic steatohepatitis. Strategies to mitigate these toxicities could include reducing or delaying pegaspargase during induction and using pulse glucocorticoid therapy rather than 28 continuous days of prednisone. Familiarity with these regimens on the part of adult hematologists/oncologists who treat AYAs with ALL and training may be just as critical to improved outcomes.
Importantly, as we move forward with the next generation of trials for AYA ALL, it may be possible that further dose adjustments of these established chemotherapeutic approaches will be possible as the addition of immune targeting agents such as blinatumomab or inotuzumab ozogamicin into frontline therapy may both allow for treatment modifications to reduce toxicity while further improving outcomes for AYAs with ALL.
Data sets and protocols are available to other investigators by contacting the corresponding author (Anjali S. Advani; e-mail: advania@ccf.org).
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to Alliance), U10CA180886, U24CA196173, and U10CA180899 (to COG), UG1CA233180, UG1CA233253, UG1CA233290, UG1CA233302, UG1CA233328, UG1CA233327, UG1CA233373, U10CA180820 (ECOG-ACRIN), U10CA180888 (SWOG), and by St. Baldrick’s Foundation. M.L.L. is the UCSF Benioff Chair of Children’s Health and Deborah and Arthur Ablin Endowed Chair in Pediatric Molecular Oncology. S.P.H. is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at the Children’s Hospital of Philadelphia. The study was also supported in part by Servier (CALGB 10403; https://acknowledgements.alliancefound.org).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: W.S. and R.A.L. designed the study; A.S.A. and W.S. accrued patients to this trial, analyzed the data, and wrote the primary manuscript; J.Y., K.L., M.D., Z.C., and K.C. helped with the data collection and statistical analysis; A.S,A., S.M.L., and W.S. analyzed the data with the help of the statisticians J.Y., K.L., M.D., and Z.C.; S.M.L., E.L., M.L., M.C.F., and D.C. accrued patients to the trial and reviewed the final version of the manuscript; M.S.T., F.R.A., H.E., R.M.S., J.L.M., M.L.L., N.W., W.C., and R.A.L. reviewed the final version of the manuscript; and S.P.H., E.R., N.W., R.A.L.,W.S. made significant additions to the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: D.C. has received research support for clinical studies conducted by the following organizations: Daiichi Sankyo Co. and Astellas Pharma, Incyte Corporation, and Cyclacel Pharmaceuticals, Inc. A.S.A. has received honoraria from Jazz Pharmaceuticals, Sigma Tau, Pfizer, Amgen, Seattle Genetics, and Kite Pharmaceuticals. S.P.H. owns stock in Amgen; and has received consulting fees from Novartis and honoraria from Amgen. R.A.L. has received consulting fees and/or research support for clinical trials to his institution from Amgen, Ariad/Takeda, Cellectis, Celgene, CVS Caremark, Epizyme, Forty Seven, Novartis, and Rafael Pharmaceuticals. M.C.F. reports research funding from Bellicum and Celgene/BMS; research funding and consulting from MacroGenics; and consulting from Agios. R.M.S. reports grant/personal fees from AbbVie, Agios, and Novartis; grants from AROG; and personal fees from Actinium, Argenx, Astellas, AstraZeneca, BioLineRx, Celgene, Daiichi Sankyo, Elevate, Gemoab, Janssen, Jazz, MacroGenics, Otsuka, Pfizer, Hoffmann-La Roche, Stemline, Syndax, Syntrix, Syros, Takeda, and Trovagene. S.M.L. reports honoraria from Daiichi Sankyo, Jazz, Bristol Myers Squibb, Acceleron, and Agios; research funding from Biosight, Celgene, Hoffmann-La Roche, Kura, Onconova, and Ariad. M.L.L. reports advisory board membership for MediSix Therapeutics, Inc. M.S.T. reports research funding from AbbVie, Cellerant, Orsenix, ADC Therapeutics, Biosight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; advisory board membership for AbbVie, BioLineRx, Daiichi Sankyo, Orsenix, KAHR, Rigel, Nohla, Delta-Fly Pharma, Tetraphase, Oncolyze, Jazz Pharma, Roche, Biosight, and Novartis; and royalties from UpToDate. W.S. reports advisory board memberships for Agios, Amgen, Astellas, Kite, Jazz, MorphoSys, and Servier; speaking honoraria from AbbVie and Pfizer; and other from UpToDate and Research to Practice. The remaining authors declare no competing financial interests.
Correspondence: Anjali S. Advani, Desk CA60, 10201 Carnegie Ave, Cleveland, OH 44195; e-mail: advania@ccf.org.
References
Author notes
The full-text version of this article contains a data supplement.