Key Points
African inheritance provides some protection against bone toxicities caused by ALL treatment in Black and Hispanic patients.
Abstract
Hispanic children have a higher incidence of acute lymphoblastic leukemia (ALL) and inferior treatment outcomes relative to non-Hispanic White children. We previously reported that Hispanic children with ALL had lower risk of fracture and osteonecrosis. To unravel the genetic root of such ethnic differences, we genotyped 449 patients from the DFCI 05-001 cohort and analyzed their ancestry. Patients with discordant clinical and genetic ancestral groups were reclassified, and those with unknown ancestry were reassigned on the basis of genetic estimates. Both clinical and genetic ancestries were analyzed in relation to risk of bone toxicities and survival outcomes. Consistent with clinically reported race/ethnicity, genetically defined Hispanic and Black patients had significantly lower risk of fracture (Hispanic: subdistribution hazard ratio [SHR], 0.42; 95% confidence interval [CI], 0.22-0.81; P = .01; Black: SHR, 0.28; 95% CI, 0.10-0.75; P = .01), and osteonecrosis (Hispanic: SHR, 0.12; 95% CI, 0.02-0.93; P = .04; Black: SHR, 0.24; 95% CI, 0.08-0.78; P = .02). The lower risk was driven by African but not Native American or Asian ancestry. In addition, patients with a higher percentage of Native American ancestry had significantly poorer overall survival and event-free survival. Our study revealed that the lower risk of bone toxicities among Black and Hispanic children treated for ALL was attributed, in part, to the percentage of African ancestry in their genetic admixture. The findings provide suggestive evidence for the protective effects of genetic factors associated with African decent against bone damage caused by ALL treatment and clues for future studies to identify underlying biological mechanisms.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy among children and young adolescents.1 Because overall survival (OS) in patients with this disease has improved over the last several decades, efforts to reduce toxicities while maintaining excellent outcomes are imperative.2 Another area in childhood ALL garnering research attention is the impact of race/ethnicity and health disparities on patient outcomes.3 For instance, Hispanic children have a higher incidence rate of ALL and inferior treatment outcomes compared with children of European ancestry.4 Because race/ethnicity can be defined in multiple ways, including geography, ancestral descent, language, religion, and sociocultural identity, drivers of racial/ethnic disparities should be carefully considered. This may be particularly true for patients of Hispanic ethnicity, which is a highly heterogeneous group with contributions to their admixed genetic inheritance from European, African, and Native American ancestries.5
A multisite clinical trial (Dana-Farber Cancer Institute [DFCI] ALL Consortium Protocol 05-001; clinicaltrials.gov #NCT00400946) had a total of 794 eligible patients with ALL age 1 to 18 years.6 We previously reported that Hispanic children in that trial had lower rates of therapy-related bone toxicities, including osteonecrosis and fracture, but significantly higher risk of relapse and inferior event-free survival (EFS) than non-Hispanic children.7 By using widely accessible high-throughput genotyping technologies, we can now objectively estimate the composition of ancestral admixture in each individual, which is an accurate and efficient approach to unraveling genetic causes of underlying health disparities.8-10 Previous studies have identified the proportion of Native American ancestry that explains, in part, the inferior treatment outcomes among Hispanic children with ALL,8-10 but no studies have examined genetic ancestry in relation to ALL therapy-related bone toxicities.
Our previous report from the DFCI 05-001 trial used clinically reported race/ethnicity; in this analysis, we aimed to reclassify race/ethnicity on the basis of the patients’ genetic background and to investigate the associations of the estimates of global ancestry with risk of bone toxicities. Considering the importance of Native American ancestry in driving the ethnic differences in ALL outcomes between Hispanic and non-Hispanic patients, we hypothesized that Native American ancestry might be also associated with lower risk of ALL treatment-related bone toxicities in Hispanic children and adolescents.Methods
Patient population
Samples and clinical data were derived from the Dana-Farber Cancer Institute ALL Consortium Protocol 05-001, a phase 3 open-label, randomized multisite trial with the primary end point of comparing the overall occurrence of asparaginase-induced toxicities between IV PEG-asparaginase vs intramuscular native Escherichia coli L-asparaginase. The trial has been described in detail in previous publications.6,7,11 Between 2005 and 2011, 794 pediatric patients age 1 to 18 years with newly diagnosed ALL were enrolled at 11 consortium sites in Canada and the United States (including Puerto Rico). After achieving complete remission (CR), patients received 2 years of risk-adapted multiagent chemotherapy, including 30 weeks of L-asparaginase and dexamethasone as corticosteroid preparation during post-induction treatment phases. This study has been approved by the Institutional Review Board at Roswell Park Comprehensive Cancer Center.
Bone toxicity assessment
Treatment-related toxicities, including symptomatic osteonecrosis (grade 2 or worse) and fractures (all grades), were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 and were prospectively collected for each patient over the course of therapy. The study protocol did not require imaging monitoring for osteonecrosis. All cases of osteonecrosis and fractures were confirmed by radiographic imaging.
Genotyping and data quality control
For this analysis, we used DNA samples extracted from bone marrow or peripheral blood samples collected after achieving CR. Samples were processed by the Data Bank and Biorepository laboratories at Roswell Park Comprehensive Cancer Center. Adequate DNA samples from 484 patients, plus 2% blind duplicates, were acquired for genotyping, which was performed by the Genomic Shared Resource using the Illumina OmniExpress Beadchip array. After a quality control check, 19 samples with missing rates of >5% and another 16 samples with cryptic relatedness were removed, leaving 449 ALL children in the final analysis.
Estimation of ancestry and genetic reclassification of race/ethnicity
Clinically reported race/ethnicity for children on the DFCI 05-001 trial was documented by a clinical research associate at the time of enrollment and was based on patient/parent report and/or country of origin. After genotyping, global genetic ancestry for each patient was derived from the STRUCTURE program,12 with reference data for European, African, Asian, and Native American ancestral populations obtained from the 1000 Genome Project.13 Ancestry estimates were expressed as a numeric value between 0 and 1 corresponding to the percentage of each of the 4 constitutional ancestries. The estimates of European, African, Asian, and Native American ancestry added up to a unit of 1 for each individual.
Children with discordance between reported and genetic racial/ethnic classification were reclassified according to their genetic ancestry, which was defined as follows: White patients had at least 90% European ancestry, Asian patients had at least 15% Asian ancestry, Black patients had at least 15% African ancestry and less than 5% Native American ancestry, and Hispanic patients had at least 5% Native American ancestry. Approximately 10% of the children (n = 46) were reported as other or unknown race/ethnicity, all but 5 of whom were subsequently assigned to 1 of the 4 groups according to genetic ancestry.
Statistical analysis
Regression models for the subdistribution hazard of the cumulative incidence function were used to relate reported race/ethnicity, genetically reclassified race/ethnicity, and genetic ancestry to risk of fracture and osteonecrosis, with death and recurrence as competing risk factors, with and without adjustment for covariates, including age, sex, and final risk group. For survival outcomes, OS was defined as the time from registration to death from any cause; EFS was defined as the time from registration to the first event of relapse, death, or second malignancy. Induction events, including death and/or failure to achieve CR, were considered as events at time 0. Cox proportional hazards models were used to model OS and EFS with genetic ancestry and covariates, including age, sex, and final risk group. The analyses were performed in R 3.6.1 and a 2-sided P value < .05 was considered statistically significant.
Results
A total of 449 patients were included in the final analysis. A CONSORT diagram is provided in supplemental Figure 1 with information on the inclusion and exclusion of patients for this study. The descriptive characteristics of the genotyped subcohort compared with those of the full DFCI 05-001 cohort are shown in Table 1. The average age of patients in the genotyped subcohort was 6.7 years with 26% age 10 years or older; 44% were female. Based on reported race/ethnicity, 66% were White, 17% were Hispanic, 5% were Black, 3% were Asian, and 10% were other/unknown. The demographic and clinical characteristics of the genotyped subcohort were largely similar to those of the full cohort, although the proportion of Hispanic patients was slightly lower (17% vs 21%; P = .10). The rate of fracture (25% vs 18%) and osteonecrosis (10% vs 8%) was moderately higher in the genotyped subcohort than the full cohort, although it was not statistically significant. The rate of osteonecrosis was higher in non-Canadian study centers than in Canadian centers (7% vs 16%; P = .002), yet the rate of fracture was similar (24% vs 26%; P = .54).
Patient characteristics of the genotyped subcohort and the full cohort of the DFCI 05-001 trial
| Characteristic . | Full cohort (N = 794) . | Genotyped subcohort (n = 449) . | P . |
|---|---|---|---|
| Age, y | .83 | ||
| <10 | 593 (75) | 332 (74) | |
| ≥10 | 201 (25) | 117 (26) | |
| Sex | .89 | ||
| Male | 441 (56) | 252 (56) | |
| Female | 353 (44) | 197 (44) | |
| Hispanic ethnicity, clinical* | .10 | ||
| Hispanic | 150 (21) | 74 (17) | |
| Non-Hispanic | 582 (79) | 375 (83) | |
| Race, clinical | .84 | ||
| White | 617 (78) | 343 (76) | |
| Black | 40 (5) | 20 (5) | |
| Asian | 23 (3) | 15 (3) | |
| Other/unknown | 114 (14) | 71 (16) | |
| Initial risk group | .25 | ||
| Standard | 462 (58) | 277 (62) | |
| High | 332 (42) | 172 (38) | |
| Random assignment | .93 | ||
| Directly assigned IM E coli | 281 (38) | 175 (39) | |
| IV PEG-asparaginase | 232 (32) | 131 (29) | |
| IM E coli | 231 (30) | 139 (31) | |
| Not assigned because of induction toxicity | 5 (1) | 3 (1) | |
| Final risk group† | .84 | ||
| Standard | 407 (54) | 248 (55) | |
| High | 260 (35) | 156 (35) | |
| Very high | 66 (9) | 45 (10) |
| Characteristic . | Full cohort (N = 794) . | Genotyped subcohort (n = 449) . | P . |
|---|---|---|---|
| Age, y | .83 | ||
| <10 | 593 (75) | 332 (74) | |
| ≥10 | 201 (25) | 117 (26) | |
| Sex | .89 | ||
| Male | 441 (56) | 252 (56) | |
| Female | 353 (44) | 197 (44) | |
| Hispanic ethnicity, clinical* | .10 | ||
| Hispanic | 150 (21) | 74 (17) | |
| Non-Hispanic | 582 (79) | 375 (83) | |
| Race, clinical | .84 | ||
| White | 617 (78) | 343 (76) | |
| Black | 40 (5) | 20 (5) | |
| Asian | 23 (3) | 15 (3) | |
| Other/unknown | 114 (14) | 71 (16) | |
| Initial risk group | .25 | ||
| Standard | 462 (58) | 277 (62) | |
| High | 332 (42) | 172 (38) | |
| Random assignment | .93 | ||
| Directly assigned IM E coli | 281 (38) | 175 (39) | |
| IV PEG-asparaginase | 232 (32) | 131 (29) | |
| IM E coli | 231 (30) | 139 (31) | |
| Not assigned because of induction toxicity | 5 (1) | 3 (1) | |
| Final risk group† | .84 | ||
| Standard | 407 (54) | 248 (55) | |
| High | 260 (35) | 156 (35) | |
| Very high | 66 (9) | 45 (10) |
Data are No. (%).
IM, intramuscular.
Data on Hispanic ethnicity unknown for 62 patients.
Data on final risk group are missing for 61 patients.
As shown in Table 2 and supplemental Figure 2, genetic analysis revealed a median of 96% European ancestry among White patients, 76% African ancestry among Black patients, and 58% Asian ancestry among Asian patients. The genetic makeup in Hispanic patients was admixed with a median of 23% Native American ancestry, 17% African ancestry, and 58% European ancestry. On the basis of the ancestry estimates, a small proportion of children with discordant reported and genetic race/ethnicity were reclassified, and most individuals with other or unknown race/ethnicity were assigned to 1 of the 4 ancestral groups (supplemental Table 1; supplemental Figure 3).
Estimates of genetic ancestry by clinical race/ethnicity in the genotyped subcohort of the DFCI 05-001 trial
| . | Ancestry . | |||
|---|---|---|---|---|
| European . | African . | Asian . | Native American . | |
| White | 0.96 (0.01-1) | 0.01 (0-0.93) | 0.02 (0-0.98) | 0.01 (0-0.25) |
| Black | 0.21 (0-0.63) | 0.76 (0.36-0.97) | 0.02 (0-0.07) | 0.02 (0-0.22) |
| Asian | 0.37 (0-0.71) | 0.02 (0-0.07) | 0.58 (0.2-0.99) | 0.02 (0-0.1) |
| Hispanic | 0.58 (0.13-1) | 0.17 (0-0.87) | 0.02 (0-0.08) | 0.23 (0-0.79) |
| Other/unknown | 0.83 (0.03-1) | 0.09 (0-0.96) | 0.03 (0-0.44) | 0.05 (0-0.64) |
| . | Ancestry . | |||
|---|---|---|---|---|
| European . | African . | Asian . | Native American . | |
| White | 0.96 (0.01-1) | 0.01 (0-0.93) | 0.02 (0-0.98) | 0.01 (0-0.25) |
| Black | 0.21 (0-0.63) | 0.76 (0.36-0.97) | 0.02 (0-0.07) | 0.02 (0-0.22) |
| Asian | 0.37 (0-0.71) | 0.02 (0-0.07) | 0.58 (0.2-0.99) | 0.02 (0-0.1) |
| Hispanic | 0.58 (0.13-1) | 0.17 (0-0.87) | 0.02 (0-0.08) | 0.23 (0-0.79) |
| Other/unknown | 0.83 (0.03-1) | 0.09 (0-0.96) | 0.03 (0-0.44) | 0.05 (0-0.64) |
The numbers presented in the table indicate the proportion of each genetic ancestry an individual carries that ranges between 0 and 1.
Children with reported Hispanic ethnicity had significantly lower risk of fracture (Table 3) (subdistribution hazard ratio [SHR], 0.39; 95% confidence interval [CI], 0.21-0.73; P = .003) and osteonecrosis (SHR, 0.20; 95% CI, 0.06-0.65; P = .008) compared with non-Hispanic White children (Table 3). When genetically defined racial/ethnic groups were considered, similar results were observed among Hispanic children for both fracture (SHR, 0.42; 95% CI, 0.22-0.81; P = .01) and osteonecrosis (SHR, 0.24; 95% CI, 0.08-0.78; P = .02) as well as among Black children (fracture: SHR, 0.28; 95% CI, 0.10-0.75; P = .01; osteonecrosis: SHR, 0.12; 95% CI, 0.02-0.93; P = .04).
Risk of fracture and osteonecrosis by clinical and genetic reclassified race/ethnicity in the genotyped subcohort of the DFCI 05-001 trial
| Race/ethnicity . | No. of bone toxicities (%) . | Unadjusted model . | Adjusted model* . | |||
|---|---|---|---|---|---|---|
| Yes . | No . | SHR (95% CI) . | P . | SHR (95% CI) . | P . | |
| Fractures | ||||||
| Clinical | ||||||
| White | 88 (79.3) | 206 (60.9) | 1.00 | 1.00 | ||
| Hispanic | 10 (9.0) | 64 (18.9) | 0.42 (0.22-0.81) | .009 | 0.39 (0.21-0.73) | .003 |
| Black | 1 (0.9) | 19 (5.6) | 0.15 (0.02-1.09) | .06 | 0.15 (0.02-1.06) | .06 |
| Asian | 2 (1.8) | 13 (3.8) | 0.43 (0.10-1.78) | .24 | 0.45 (0.11-1.95) | .29 |
| Other | 10 (9.0) | 36 (10.7) | 0.68 (0.36-1.29) | .24 | 0.79 (0.42-1.50) | .47 |
| Genetically reclassified | ||||||
| White | 92 (82.9) | 213 (63.0) | 1.00 | 1.00 | ||
| Hispanic | 10 (9.0) | 64 (18.9) | 0.43 (0.22-0.84) | .01 | 0.42 (0.22-0.81) | .01 |
| Black | 4 (3.6) | 35 (10.4) | 0.32 (0.12-0.86) | .02 | 0.28 (0.10-0.75) | .01 |
| Asian | 4 (3.6) | 22 (6.5) | 0.50 (0.18-1.36) | .17 | 0.47 (0.16-1.33) | .15 |
| Other | 1 (0.9) | 4 (1.2) | 0.59 (0.10-3.74) | .59 | 0.81 (0.13-5.17) | .82 |
| Osteonecrosis | ||||||
| Clinical | ||||||
| White | 38 (84.4) | 256 (63.4) | 1.00 | 1.00 | ||
| Hispanic | 3 (6.7) | 71 (17.6) | 0.30 (0.09-0.96) | .04 | 0.20 (0.06-0.65) | .008 |
| Black | 0 | 20 (5.0) | NA | NA | NA | NA |
| Asian | 0 | 15 (3.7) | NA | NA | NA | NA |
| Other | 4 (8.9) | 42 (10.4) | 0.66 (0.23-1.85) | .43 | 0.90 (0.34-2.37) | .83 |
| Genetically reclassified | ||||||
| White | 40 (88.9) | 265 (65.6) | 1.00 | 1.00 | ||
| Hispanic | 3 (6.7) | 71 (17.6) | 0.30 (0.09-0.96) | .04 | 0.24 (0.08-0.78) | .02 |
| Black | 1 (2.2) | 38 (9.4) | 0.18 (0.03-1.32) | .09 | 0.12 (0.02-0.93) | .04 |
| Asian | 1 (2.2) | 25 (6.2) | 0.28 (0.04-2.02) | .21 | 0.12 (0.03-1.47) | .12 |
| Other | 0 | 5 (1.2) | NA | NA | NA | NA |
| Race/ethnicity . | No. of bone toxicities (%) . | Unadjusted model . | Adjusted model* . | |||
|---|---|---|---|---|---|---|
| Yes . | No . | SHR (95% CI) . | P . | SHR (95% CI) . | P . | |
| Fractures | ||||||
| Clinical | ||||||
| White | 88 (79.3) | 206 (60.9) | 1.00 | 1.00 | ||
| Hispanic | 10 (9.0) | 64 (18.9) | 0.42 (0.22-0.81) | .009 | 0.39 (0.21-0.73) | .003 |
| Black | 1 (0.9) | 19 (5.6) | 0.15 (0.02-1.09) | .06 | 0.15 (0.02-1.06) | .06 |
| Asian | 2 (1.8) | 13 (3.8) | 0.43 (0.10-1.78) | .24 | 0.45 (0.11-1.95) | .29 |
| Other | 10 (9.0) | 36 (10.7) | 0.68 (0.36-1.29) | .24 | 0.79 (0.42-1.50) | .47 |
| Genetically reclassified | ||||||
| White | 92 (82.9) | 213 (63.0) | 1.00 | 1.00 | ||
| Hispanic | 10 (9.0) | 64 (18.9) | 0.43 (0.22-0.84) | .01 | 0.42 (0.22-0.81) | .01 |
| Black | 4 (3.6) | 35 (10.4) | 0.32 (0.12-0.86) | .02 | 0.28 (0.10-0.75) | .01 |
| Asian | 4 (3.6) | 22 (6.5) | 0.50 (0.18-1.36) | .17 | 0.47 (0.16-1.33) | .15 |
| Other | 1 (0.9) | 4 (1.2) | 0.59 (0.10-3.74) | .59 | 0.81 (0.13-5.17) | .82 |
| Osteonecrosis | ||||||
| Clinical | ||||||
| White | 38 (84.4) | 256 (63.4) | 1.00 | 1.00 | ||
| Hispanic | 3 (6.7) | 71 (17.6) | 0.30 (0.09-0.96) | .04 | 0.20 (0.06-0.65) | .008 |
| Black | 0 | 20 (5.0) | NA | NA | NA | NA |
| Asian | 0 | 15 (3.7) | NA | NA | NA | NA |
| Other | 4 (8.9) | 42 (10.4) | 0.66 (0.23-1.85) | .43 | 0.90 (0.34-2.37) | .83 |
| Genetically reclassified | ||||||
| White | 40 (88.9) | 265 (65.6) | 1.00 | 1.00 | ||
| Hispanic | 3 (6.7) | 71 (17.6) | 0.30 (0.09-0.96) | .04 | 0.24 (0.08-0.78) | .02 |
| Black | 1 (2.2) | 38 (9.4) | 0.18 (0.03-1.32) | .09 | 0.12 (0.02-0.93) | .04 |
| Asian | 1 (2.2) | 25 (6.2) | 0.28 (0.04-2.02) | .21 | 0.12 (0.03-1.47) | .12 |
| Other | 0 | 5 (1.2) | NA | NA | NA | NA |
NA, not available; SHR, subdistribution hazard ratio.
Death and recurrence were considered competing risks. Adjusted covariates included age, sex, and final risk group.
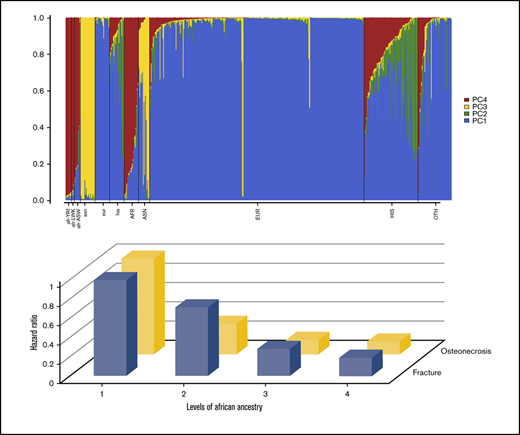
When the 4 genetic ancestries were tested together, the proportion of African ancestry (P = .002), but not Asian (P = .26) or Native American (P = .30) ancestry, was significantly associated with fracture risk and osteonecrosis risk (African ancestry, P = .04; Asian ancestry, P = .08; Native American ancestry (P = .07). When the percentage of African ancestry was categorized into 4 levels based on data distribution (P < .024, P = .024-.066, P = .067-.272, P > .272), an inverse dose-response relationship was observed (Table 4). Those with higher proportions of African ancestry tended to have lower risk of fracture (level 4 vs level 1: SHR, 0.19; 95% CI, 0.06-0.58; P = .003) and osteonecrosis (level 4 vs level 1: SHR, 0.14; 95% CI, 0.02-0.92; P = .04).
Risk of bone toxicities by the levels of African ancestry in the genotyped subcohort of the DFCI 05-001 trial
| . | No. of toxicities (%) . | Unadjusted model . | Adjusted model* . | |||
|---|---|---|---|---|---|---|
| Yes . | No . | SHR (95% CI) . | P . | SHR (95% CI) . | P . | |
| Fracture | ||||||
| Level 1 (<0.024) | 94 (84.7) | 221 (65.4) | 1.00 | 1.00 | ||
| Level 2 (0.024-0.066) | 10 (9.0) | 35 (10.4) | 0.71 (0.38-1.34) | .29 | 0.72 (0.39-1.35) | .31 |
| Level 3 (0.067-0.272) | 4 (3.6) | 41 (12.1) | 0.28 (0.10-0.77) | .01 | 0.29 (0.10-0.81) | .02 |
| Level 4 (>0.272) | 3 (2.7) | 41 (12.1) | 0.20 (0.07-0.63) | .006 | 0.19 (0.06-0.58) | .003 |
| Osteonecrosis | ||||||
| Level 1 (<0.024) | 41 (91.1) | 274 (67.8) | 1.00 | 1.00 | ||
| Level 2 (0.024-0.066) | 2 (4.4) | 43 (10.6) | 0.33 (0.08-1.36) | .12 | 0.32 (0.08-1.25) | .10 |
| Level 3 (0.067-0.272) | 1 (2.2) | 44 (10.9) | 0.16 (0.02-1.16) | .07 | 0.15 (0.02-1.14) | .07 |
| Level 4 (>0.272) | 1 (2.2) | 43 (10.6) | 0.16 (0.02-1.16) | .07 | 0.14 (0.02-0.92) | .04 |
| . | No. of toxicities (%) . | Unadjusted model . | Adjusted model* . | |||
|---|---|---|---|---|---|---|
| Yes . | No . | SHR (95% CI) . | P . | SHR (95% CI) . | P . | |
| Fracture | ||||||
| Level 1 (<0.024) | 94 (84.7) | 221 (65.4) | 1.00 | 1.00 | ||
| Level 2 (0.024-0.066) | 10 (9.0) | 35 (10.4) | 0.71 (0.38-1.34) | .29 | 0.72 (0.39-1.35) | .31 |
| Level 3 (0.067-0.272) | 4 (3.6) | 41 (12.1) | 0.28 (0.10-0.77) | .01 | 0.29 (0.10-0.81) | .02 |
| Level 4 (>0.272) | 3 (2.7) | 41 (12.1) | 0.20 (0.07-0.63) | .006 | 0.19 (0.06-0.58) | .003 |
| Osteonecrosis | ||||||
| Level 1 (<0.024) | 41 (91.1) | 274 (67.8) | 1.00 | 1.00 | ||
| Level 2 (0.024-0.066) | 2 (4.4) | 43 (10.6) | 0.33 (0.08-1.36) | .12 | 0.32 (0.08-1.25) | .10 |
| Level 3 (0.067-0.272) | 1 (2.2) | 44 (10.9) | 0.16 (0.02-1.16) | .07 | 0.15 (0.02-1.14) | .07 |
| Level 4 (>0.272) | 1 (2.2) | 43 (10.6) | 0.16 (0.02-1.16) | .07 | 0.14 (0.02-0.92) | .04 |
The 4 levels of African ancestry were determined on the basis of the distribution of the data, most of which was close to zero and was categorized as level 1; the rest were categorized into 3 levels with approximately equal numbers of patients. The numbers in the parentheses indicate the cutoff points of the proportion of African ancestry an individual carries that ranges between 0 and 1.
Death and recurrence were considered competing risks. Adjusted covariates included age, sex, and final risk group.
Finally, we examined genetic ancestry in relation to OS and EFS in the genotyped subcohort. Among the 4 genetic ancestries, Native American ancestry was significantly associated with OS (P = .03), but not with EFS (P = .08). When the percentage of Native American ancestry was categorized into high and low levels based on the median (as shown in Table 5), those with high levels had significantly poorer OS (adjusted HR, 4.00; 95% CI, 1.45-11.02; P = .007) and EFS (adjusted HR, 2.07; 95% CI, 1.13-3.79; P = .02).
OS and EFS by the levels of Native American ancestry in the genotyped subcohort of the DFCI 05-001 trial
| Native American ancestry . | No. of events/total . | Unadjusted model . | Adjusted model* . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| OS | |||||
| Below median (<0.006) | 5/236 | 1.00 | 1.00 | ||
| Above median (≥0.006) | 15/213 | 3.62 (1.32-9.96) | .01 | 4.00 (1.45-11.02) | .007 |
| EFS | |||||
| Below median (<0.006) | 17/236 | 1.00 | 1.00 | ||
| Above median (≥0.006) | 28/213 | 1.96 (1.07-3.59) | .03 | 2.07 (1.13-3.79) | .02 |
| Native American ancestry . | No. of events/total . | Unadjusted model . | Adjusted model* . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| OS | |||||
| Below median (<0.006) | 5/236 | 1.00 | 1.00 | ||
| Above median (≥0.006) | 15/213 | 3.62 (1.32-9.96) | .01 | 4.00 (1.45-11.02) | .007 |
| EFS | |||||
| Below median (<0.006) | 17/236 | 1.00 | 1.00 | ||
| Above median (≥0.006) | 28/213 | 1.96 (1.07-3.59) | .03 | 2.07 (1.13-3.79) | .02 |
The numbers in the parentheses in the left column indicate the cutoff points of the proportion of Native American ancestry an individual carries that ranges between 0 and 1.
Multivariable Cox models were adjusted for age, sex, and final risk group.
Discussion
In children enrolled on the DFCI ALL Consortium Protocol 05-001, we observed lower risk of fracture and osteonecrosis among Hispanic patients. This risk reduction was associated with the proportion of African ancestry in their genetic admixture, but not Native American ancestry, as we had hypothesized. After reclassification based on genetic ancestry, Black children had an even lower risk of bone toxicities. Our findings suggest that African ancestry may protect against the bone toxicities associated with ALL therapy. In addition, our data also confirmed previous reports demonstrating an association between Native American ancestry and poorer outcomes in children with ALL.8-10
Data on racial/ethnic differences in risk of fracture among children are sparse. Our study provides the first evidence that African ancestry may confer protection against fracture and osteonecrosis during pediatric ALL therapy. In healthy adults, African ancestry is associated with higher bone mineral density (BMD) and lower risk of fractures.14-18 Similar findings have also been reported among children and adolescents. In 2 pediatric studies, children of African descent as defined by genetic inheritance had significantly higher BMD than those of European and Asian groups.19 Furthermore, among the self-reported European group, the percentage of African admixture was also positively associated with BMD. In a longitudinal study of healthy youths between 9 and 25 years of age for whom BMD was measured annually, Black children had consistently higher BMD than children of non-African descent.20 In 1 US study of White and non-White children (a majority of whom were Black), White children had significantly higher risk of fracture.21 Similar findings were also reported in a United Kingdom study, which showed higher incidence of fracture among White vs Black children.22
The literature on racial differences in the risk of osteonecrosis is limited, with conflicting results in non-ALL settings.23,24 In a large United Kingdom study of children with ALL, although no difference was noted between Black and White patients, Asians were at significantly higher risk only after adjusting for covariates but not in the unadjusted model.25 In a previous study by Kawedia et al,26 among children with ALL, Blacks (genetically categorized) tended to have a lower risk of osteonecrosis than Whites, although the difference was not statistically significant. In another study in childhood ALL by Karol et al,27 African genetic ancestry was associated with lower risk of osteonecrosis. Our findings confirmed the previous results and also showed that the associations extended to risk of fracture as another bone toxicity and also to Hispanic children who usually had a much lower admixture of African ancestry.
Several studies have reported that Native American ancestry is associated with poor outcomes among Hispanic children with ALL.8-10 Because of the limited number of clinically identified Black patients in our study, we did not find them to be at a significantly lower risk of bone toxicities until we used genetically defined race/ethnicity instead of the groups defined by reported race/ethnicity. The signal became even clearer when analyzing genetic ancestries as numeric proportions in all patients in whom African ancestry was most significantly associated with bone toxicities among the 4 founder ancestries. Our findings demonstrate the power of genetic ancestry analysis in unraveling the underlying causes of phenotypes as complex as racial/ethnic disparities in cancer treatment outcomes.
A significant association with global genetic ancestry is usually interpreted as an indication of a possible role of genetic factors in shaping the racial/ethnic differences in the phenotype of interest. However, caution should be taken when interpreting such findings. Although genetic ancestry is inferred from genotype data, social, cultural, behavioral, and genetic factors are closely interconnected and co-segregate within a racial/ethnic group, which makes it challenging to separate the 2 forces at play. In the case of patients with treatment-related bone toxicities, adherence to chemotherapy (including mercaptopurine and dexamethasone) during the maintenance phase of ALL treatment must be considered. Corticosteroids such as dexamethasone are known to be detrimental to bone health. Adherence to maintenance therapy for ALL has been shown to be significantly lower in Hispanic children than in White children with ALL.28 Although we did not collect treatment adherence data in DFCI 05-001, it is possible that the lower adherence in Hispanic patients contributed to lower risk of bone toxicities. Interestingly, treatment adherence itself is influenced by both pharmacogenetic (possibly through influences on drug toxicities and intolerance) and health behavioral factors. Adherence to mercaptopurine was found to be significantly associated with East Asian ancestry in children with ALL,29 highlighting the complicated inter-relationships between genetic and non-genetic factors and the challenge of separating the 2 in the setting of health disparities.
One approach to more definitively determining the genetic contributions to health disparities is by performing genome-wide association studies (GWASs). They allow us to identify risk variants with large differences in allele frequency between racial/ethnic groups in the direction that is consistent with their risk differences. Additional fine-mapping and functional studies may be needed to understand biologic mechanisms. Successful examples of this approach can be found in the identification of genes underlying the poorer outcomes in Hispanic children and lower tolerance to mercaptopurine in East Asian children with ALL.8,29 As far as bone-related phenotypes are concerned, a previous study reported higher allele frequencies of genetic variants associated with BMD in Black children than initially identified in White children and provided some evidence of evolutionary selection pressure in shaping the racial differences in such allele frequencies. To our knowledge, no GWAS has been performed that focused on fracture among children treated for ALL. Two GWASs have been published on osteonecrosis in the White pediatric ALL patient population,26,27 and 1 genome-wide significant risk variant, rs10989692 near the glutamate receptor GRIN3A locus, was identified. Interestingly, the risk allele of this variant occurs at a much higher frequency in populations of African descent than in populations of European ancestry according to gnomAD data (0.31 vs 0.09), which is in the opposite direction of our finding of a lower risk of osteonecrosis associated with African ancestry. It should be noted, however, that the generalizability of GWAS findings from one population to another is highly uncertain. In a previous GWAS of fracture in Black women, only 1 of the previously identified variants for BMD or fracture in Whites could be replicated.30 Therefore, future GWASs of ALL treatment-related bone toxicities in racial/ethnic minority populations are warranted, which will likely require multi-institute collaborations to amass an adequately large sample size required for such analyses.
Our study has a few limitations. As mentioned above, we did not collect data on patient adherence to maintenance therapy in the DFCI 05-001 study, which might partially explain the lower risk of bone toxicities resulting from lower corticosteroid exposure in Hispanic children. In a previous study of relapse in ALL patients, Hispanic children still had higher risk of relapse when analysis was restricted to those with high adherence,28 suggesting other causes of ethnic disparities beyond treatment adherence.31 Furthermore, Black patients had lower risk of bone toxicities but not were not at higher risk of relapse, also suggesting causes of bone toxicities other than treatment adherence. Another limitation of our study is the relatively small number of patients from racial/ethnic minority groups. As a result, some of the risk estimates may have been inflated with a wide confidence interval. Nevertheless, the consistency between reported and genetic race/ethnicity in our study and the consistency between our results in ALL patients and the literature on African admixture in association with higher BMD and lower risk of fractures in healthy children and adults, support the validity and robustness of our findings.
In conclusion, Black and Hispanic children treated for ALL had lower risk of fracture and osteonecrosis than White children, which was attributed in part to the percent of African ancestry in their genetic admixture, whereas patients with a higher percentage of Native American ancestry had significantly poorer OS and EFS. Our findings provide suggestive evidence for protective effects of genetic factors associated with African descent against bone damage caused by ALL therapy, as well as clues for future studies to identify underlying biological mechanisms.
Presented at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7-10 December 2019.
The data reported in this article have been deposited in dbGaP (accession number phs002317.1.1).
Acknowledgments
The authors thank the patients, families, physicians, nurses, research coordinators, and all others who participated in the data and biospecimen collection associated with this study through the DFCI ALL Consortium.
This work was supported in part by grants from the National Cancer Institute (NCI), National Institutes of Health (R03 CA223730) (S.Y., K.M.K., and Q.Z), Rally Foudation (S.Y.), and by the Roswell Park Alliance Foundation (S.Y. and K.M.K.). J.M.K is supported by a KL2 Mentored Career Development Award Grant (KL2-TR001874) from the Irving Institute for Clinical and Translational Research at Columbia University Irving Medical Center. Roswell Park Data Bank and Biorepository and Genomic Shared Resource are shared resources supported by a Cancer Center Support Grant and NCI (P30 CA16056 [principal investigator: Candace Johnson]).
The patients described in this report were enrolled at the following DFCI ALL Consortium sites: DFCI/Boston Children’s Hospital (Boston, MA), Columbia University Irving Medical Center, Morgan Stanley Children’s Hospital of New York-Presbyterian (New York, NY), Hospital Sainte Justine (Montreal, QC, Canada), Le Centre Hospitalier de L’Universite Laval (Quebec City, QC, Canada), McMaster Children’s Hospital (Hamilton, ON, Canada), San Jorge Children’s Hospital (San Juan, PR), University of Rochester Medical Center (Rochester, NY), Hospital Ste. Justine (Montreal, QC, Canada), Hasbro Children’s Hospital (Providence, RI), and Inova/Fairfax Hospital for Children (Falls Church, VA).
Authorship
Contribution: S.Y. and K.M.K conceived and designed the study; P.D.C., K.S., M.H.H., U.H.A., L.A.C., C.L., J.-M.L., B.M., M.A.S, J.J.G.W., S.E.S., L.B.S., and K.M.K. provided study materials and/or patients; S.Y., Q.Z., K.S., L.B.S., and K.M.K. collected and assembled the data; S.Y., Q.Z., E.S., K.S., J.M.K., E.J.L. and K.M.K. analyzed and interpreted the data; and all authors helped write and gave their final approval to the manuscript.
Conflict-of-interest disclosure: L.B.S. has served on advisory boards for Sigma-Tau Pharmaceuticals and Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Song Yao, Department of Cancer Prevention and Control, Roswell Park Comprehensive Cancer Center, Elm and Carlton St, Buffalo, NY 14263; e-mail: song.yao@roswellpark.org.
References
Author notes
S.Y. and Q.Z are joint first authors.
The full-text version of this article contains a data supplement.