Key Points
Relatively small grants focused on an unmet medical need such as SCD can spur new and significant advances.
Follow-on grant funding was 4 times the original investment, and all but one team published results from the program.
Abstract
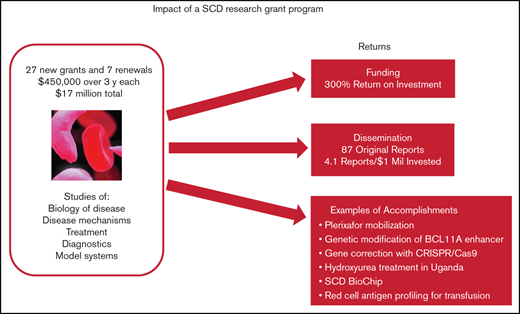
More than 20 years ago, clinical trials and federal grant support for sickle cell disease (SCD) research were not on par with support for other genetic diseases. Faced with the opportunity to spur research and advance treatments for SCD, and at the recommendation of advisors, the Doris Duke Charitable Foundation (DDCF) offered an SCD research funding opportunity starting in 2009 through its Innovations in Clinical Research Awards (ICRA) program. Twenty-eight new grants of $450 000 for direct costs over 3 years and 7 renewals were awarded, for a total investment of $17 million. Only about half the research teams garnered follow-on funding directly related to their ICRA projects, but the financial return on the research investment was substantial (∼4 times the original $17 million or 300%). All but 1 of the ICRA investigative teams published original research reports that acknowledged DDCF as a source of funding; the median number of publications per team was 3. Major innovations in the diagnosis and treatment of SCD included but were not limited to a demonstration that genetic modification of BCL11A enhancer is a potentially important treatment modality, establishment that plerixafor mobilization is safe and effective for those with SCD, development and validation of a new diagnostic called SCD BioChip, and evidence that hydroxyurea treatment is safe and efficacious in African children. These outcomes show that relatively small research grants can have a substantial return on investment and result in significant advances for a disease such as SCD.
Introduction
Important research in the 1940s and 1950s1,2 led to the finding in 19573 that a mutation in the gene encoding the β-hemoglobin protein is the cause of sickle cell disease (SCD). These studies placed the disease at the leading edge of investigations into the molecular basis of human disease.4 Unfortunately, for a long time, knowledge of the molecular biology did not translate into corresponding therapies. Until recently, there was only 1 drug treatment option—hydroxyurea—for individuals with SCD. Trials in the 1980s and 1990s confirmed that this drug enhanced fetal hemoglobin5 and reduced painful crises.6 Although it has been approved for use in adults since 1998, hydroxyurea was not authorized by the US Food and Drug Administration for pediatric use until 2017.
SCD is an inherited and often devasting blood disorder that occurs mostly in those from or whose ancestors are from sub-Saharan Africa, South America, the Caribbean, Central America, Saudi Arabia, India, Turkey, Greece, and Italy.7 It is characterized by production of an abnormal hemoglobin, which causes red blood cells to become rigid and sickle-shaped and can precipitate a cascade of microvascular events. Consequences include severe pain, stroke, organ damage, and even early death. The median life expectancy is only about 54 years.8 The disease affects millions worldwide, with more than 300 000 babies born with the disease each year.9
Several studies have raised the question of inequities in supporting SCD research in the United States compared with support for research in other genetic diseases such as cystic fibrosis and described gaps in government and private philanthropic funding for research.10-12 The Doris Duke Charitable Foundation (DDCF) analyzed the field in 2008 with the intent of developing a new grant program. Clinical trials and federal grant support were sparse and not on a par with those for other genetic diseases. Despite SCD being 3 times as prevalent as cystic fibrosis, there were only 10 registered clinical trials of drug interventions in SCD vs 42 for cystic fibrosis.13 Funding by the National Institutes of Health (NIH) in 2008 was only $80 million for SCD compared with $90 million for cystic fibrosis.14 Faced with the opportunity to spur research and advance treatments for SCD, and at the recommendation of advisors, staff at the DDCF Medical Research Program designed a research funding opportunity through its Innovations in Clinical Research Awards (ICRA) program.
To accelerate application of technologies pioneered in other fields such as cancer to problems in SCD, investigators new to the field of sickle cell research were encouraged to apply. Priority areas of research were identified to specifically advance innovations in treatment and disease management. These included drug discovery and development of new therapeutics, genetic and genomic approaches to studying disease severity, gene therapy and bone marrow transplantation, disease risk factors, development of end points for clinical trials, and diagnostics and treatments for use in low-resource settings. Although these research topics were clearly encouraged, other subjects for research were also eligible. Grants of $450 000 for direct costs over 3 years were offered in every other year from 2009 to 2013, and no-cost extensions and competitive renewals were allowed. The last set of renewals was awarded in 2015.
An evaluation of the ICRA program was conducted in the first quarter of 2021, 12 years from the start of the first grants, to see whether this funding mechanism was successful. The following research questions were included: Are the researchers continuing to work on SCD (particularly those who were new to the field)? Are the projects continuing with follow-on funding? Was the ICRA research disseminated through publications? and Were new treatments or techniques developed that have the potential to improve or are actively improving care for those with SCD?
Methods
Data on the ICRA competitions, including characteristics of the grantees and type of research, were collected from the applications and biographical sketches of the applicants. Yearly progress reports and a final report are collected for every biomedical research project that is supported by the DDCF. Information in the ICRA final reports was used in this evaluation and included publications, funding, and patents at the time of closing, summary of key findings, and contributions of the ICRA grant to the investigator’s research program and career advancement. There was also a question in the reports that asked whether SCD research was the investigator’s primary field.
An online survey was administered to principal investigators and co-investigators of the ICRA projects in March 2021 to capture more recent information about their progress (survey questions are included in the supplemental material). Respondents were asked to provide publications, grants, and patents and licenses related to their ICRA grants. To determine whether the investigators are still in the SCD field, they were asked to categorize time spent on SCD research. They were also asked to summarize the main findings from their studies. To help select stories of breakthroughs, survey participants were asked to describe any breakthroughs, new agents, improvements in patient care, etc, based on their ICRA project. To identify emerging trends that might represent new opportunities, survey participants were asked in an open-ended question to describe the most important breakthrough in SCD research in the last 10 years. Survey participants were informed that de-identified results would be used in a report to be disseminated to the public.
To confirm grants and publications for responders and to find data for nonresponders, searches were conducted of the NIH RePORTER, National Science Foundation (NSF) Award Search, the Health Research Alliance (HRA) Analyzer, and PubMed databases. All searches included the investigator’s name and were limited to years after the start date of the ICRA grant. Determination of the relationship between follow-on grants and ICRA was based on the survey responses or on analysis by the authors of this study if there was no survey response. In the latter case, each follow-on grant was reviewed for direct links to ICRA-specific aims and findings. If there was not a clear connection between a grant and ICRA, even if the topics were similar, the grant was considered unrelated and was not used to determine return on investment (ROI). ROI was calculated as the total follow-on grant dollars relevant to ICRA minus the original investment divided by the original investment, expressed as a percentage. It was based on both NIH and non-NIH grants.
Publications were considered related to ICRA if DDCF funding was acknowledged. Only original research reports are summarized here to capture whether ICRA research data and results were shared with the community. To adjust for investigators who received renewal grants and to compare with other studies, the number of publications per million dollars of grant funding was calculated.
In the database searches, it was difficult to differentiate which ICRA grant was linked to a publication or follow-on grant for investigators who received more than 1 ICRA grant. This is because the ICRA original and renewal projects often overlapped scientifically. In addition, many authors did not reference their specific DDCF grant number. Therefore, the unit of analysis for outcomes in Table 3 is the investigator or investigative team rather than the ICRA grant. Quantitative outcome analysis can miss the impact of individual projects. Consequently, case reports were selected to highlight accomplishments of specific projects and to complement the outcome data. Selection was based on review of final reports, findings disseminated at DDCF research meetings, survey responses, follow-on grant attainment, and published literature.
Results
Eighty to 85 ICRA proposals were received in each of the 3 competitions for new grants (see Table 1 for ICRA funding history) and were reviewed by expert panels. The review criteria focused on originality, inventiveness, and relevance to SCD clinical research (see Table 2). Twenty-eight new grants and 7 renewals were awarded to 27 unique principal investigators for a total investment of $17 million. To put this in context, the Cystic Fibrosis Foundation has been spending more than $100 million per year on research.15 The overall success rate for the ICRA program was 11% for new grants and 54% for competitive renewals. Twenty principal investigators (74%) were male. One of the teams switched principal investigators in a renewal, so there were 26 distinct investigative teams. Two-thirds of the 35 projects were led by teams of 2 or more. Almost half (17) of the 35 projects were led by 13 unique investigators who did not consider SCD research their primary field. The career stage of the lead investigators at the time of the award covered a broad range: among the 35 projects, 40% were led by instructors or assistant professors, 9% by associate professors, 46% by full professors, and 6% by researchers with nonacademic appointments. The type of research identified by applicants included basic biology of disease (26%), disease mechanisms (26%), or treatment (40%) and a few projects (8%) focused on diagnostics and model systems.
History of grant competitions for ICRAs in SCD
| . | 2009 . | 2011 . | 2012 . | 2013 . | 2015 . | All Years . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Type of competition | Open | Open | Limited renewal | Open | Limited renewal | |||||||
| ICRA applications | 80 | 85 | 5 | 83 | 8 | 262 | ||||||
| ICRA grants | 8 | 9 | 3 | 11 | 4 | 35 | ||||||
| Success rate | 10 | 11 | 60 | 13 | 50 | 11 (new), 54 (renewal) | ||||||
| Unique investigators or investigative teams | 8 | 9 | 0 | 9 | 0 | 26 | ||||||
| Early career lead investigators* | 3 | 38 | 4 | 44 | 1 | 33 | 4 | 44 | 2 | 50 | 14 | 40 |
| Total grant amount ($) | 3 888 000 | 4 374 000 | 1 458 000 | 5 346 000 | 1 944 000 | 17 010 000 | ||||||
| Grant projects led by teams of 2 or more | 4 | 50 | 8 | 89 | 1 | 33 | 7 | 64 | 3 | 75 | 23 | 66 |
| Grant projects led by investigators who did not consider sickle cell research their primary field | 5 | 63 | 4 | 44 | 3 | 100 | 5 | 45 | 0 | 17 | 49 | |
| . | 2009 . | 2011 . | 2012 . | 2013 . | 2015 . | All Years . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Type of competition | Open | Open | Limited renewal | Open | Limited renewal | |||||||
| ICRA applications | 80 | 85 | 5 | 83 | 8 | 262 | ||||||
| ICRA grants | 8 | 9 | 3 | 11 | 4 | 35 | ||||||
| Success rate | 10 | 11 | 60 | 13 | 50 | 11 (new), 54 (renewal) | ||||||
| Unique investigators or investigative teams | 8 | 9 | 0 | 9 | 0 | 26 | ||||||
| Early career lead investigators* | 3 | 38 | 4 | 44 | 1 | 33 | 4 | 44 | 2 | 50 | 14 | 40 |
| Total grant amount ($) | 3 888 000 | 4 374 000 | 1 458 000 | 5 346 000 | 1 944 000 | 17 010 000 | ||||||
| Grant projects led by teams of 2 or more | 4 | 50 | 8 | 89 | 1 | 33 | 7 | 64 | 3 | 75 | 23 | 66 |
| Grant projects led by investigators who did not consider sickle cell research their primary field | 5 | 63 | 4 | 44 | 3 | 100 | 5 | 45 | 0 | 17 | 49 | |
Instructor or assistant professor.
Criteria provided to reviewers for ICRA competitions
| Innovation | Originality and inventiveness of the research proposal. For this competition, the foundation seeks to support bold research into mechanisms, methods, technologies, or treatments with the potential for high impact. Please weigh this criterion heavily in your score |
| Significance | Importance of the question posed to the field of sickle cell disease research and the potential for clinical application |
| Approach | Appropriateness of the methodology and scope of the project. When assessing the approach, keep in mind that proposals may be for exploratory projects that do not have extensive preliminary data |
| Investigator | Evidence of the applicant’s potential to drive innovation in SCD clinical research. Please note that the foundation has encouraged applications from researchers in fields from other disciplines and specialties. Therefore, prior expertise in sickle cell disease research and its morbidities should not be weighed against the scores |
| Environment | Resources available to the applicant |
| Innovation | Originality and inventiveness of the research proposal. For this competition, the foundation seeks to support bold research into mechanisms, methods, technologies, or treatments with the potential for high impact. Please weigh this criterion heavily in your score |
| Significance | Importance of the question posed to the field of sickle cell disease research and the potential for clinical application |
| Approach | Appropriateness of the methodology and scope of the project. When assessing the approach, keep in mind that proposals may be for exploratory projects that do not have extensive preliminary data |
| Investigator | Evidence of the applicant’s potential to drive innovation in SCD clinical research. Please note that the foundation has encouraged applications from researchers in fields from other disciplines and specialties. Therefore, prior expertise in sickle cell disease research and its morbidities should not be weighed against the scores |
| Environment | Resources available to the applicant |
There were 23 responses to the 2021 survey with a response rate of 56% for principal investigators (15 of 27) and 67% for co-investigators (8 of 12). About one-third of the research time for these investigators was spent on SCD (31%). Among the 10 investigators who responded to the survey and previously identified that SCD was not their primary field, 8 (80%) still allocate some of their current research time to SCD. There were 3 nonresponders who had earlier reported that SCD was not their primary field, so their grant and publication records were examined to determine whether they still worked on SCD: 1 has an active NIH grant involving SCD, 1 published an SCD case report in 2021, and the third has no recent publications or active grants in the SCD field. Therefore, 10 of the 13 (77%) seem to have been retained in SCD research.
There were notable similarities in the survey participants’ open-ended descriptions of the most important breakthroughs in the last 10 years. Themes that emerged were gene editing or gene therapy (42%), fetal hemoglobin regulation (32%), and new drug therapies and new uses of hydroxyurea such as early intervention to prevent chronic disease (26%). The following are responses to a survey question about the impact of the ICRA program on individual research programs: (1) This funding was invaluable in allowing us to focus efforts on what seemed to be a relatively high-risk set of projects at the time, and (2) It provided initial funds to develop what began as a side project on SCD into a major, ongoing area of investigation in the laboratory.
Searches of the RePORTER database revealed that a total of 77 NIH grants were received by 22 of the 26 ICRA investigative teams after the start of their ICRA grants. Four did not obtain subsequent NIH funding. Among these 77 NIH grants, 14 were research projects directly related to ICRA with a total grant commitment of $55.6 million (Table 3). This follow-on NIH funding was concentrated in the laboratories of 8 investigators, and 4 of these were new to SCD research when they applied for ICRA funding. Eighteen investigative teams did not receive NIH funding directly related to their ICRA projects, although most were successful at obtaining NIH grants for other SCD studies or different disciplines such as cancer.
Follow-on grants, publications, and patents of ICRA grantees
| Characteristic . | No. . | % . | Median . | 1st quartile/3rd quartile . |
|---|---|---|---|---|
| NIH grants directly related to ICRA | 14 | |||
| NIH grants directly related to ICRA ($million) | 55.6 | |||
| Investigators or teams receiving NIH funding related to ICRA | 8 | 31 | ||
| Non-NIH grants directly related to ICRA | 13 | |||
| Non-NIH grants directly related to ICRA ($million) | 12.9 | |||
| Investigators or teams receiving non-NIH funding related to ICRA | 6 | 23 | ||
| Total grants related to ICRA | 27 | |||
| Total grants related to ICRA ($million) | 68.5 | |||
| Total investigators or teams receiving funding related to ICRA | 12 | 46 | ||
| ROI | ||||
| Based on total grants related to ICRA | 303 | |||
| For early career investigators* | 448 | |||
| For established investigators† | 238 | |||
| Original reports published by ICRA authors that acknowledge DDCF | 87 | |||
| Investigators/teams publishing original reports that acknowledge DDCF | 25 | 96 | ||
| Original reports per investigator or team | 3.0 | 1.0/4.8 | ||
| Years from ICRA start year to publication | 4.0 | 3.0/6.0 | ||
| Original reports per million dollars of ICRA grants | 4.1 | 2.1/7.7 | ||
| Patent applications filed by ICRA investigators or teams | 7 | |||
| Investigators or teams filing | 5 | 19 |
| Characteristic . | No. . | % . | Median . | 1st quartile/3rd quartile . |
|---|---|---|---|---|
| NIH grants directly related to ICRA | 14 | |||
| NIH grants directly related to ICRA ($million) | 55.6 | |||
| Investigators or teams receiving NIH funding related to ICRA | 8 | 31 | ||
| Non-NIH grants directly related to ICRA | 13 | |||
| Non-NIH grants directly related to ICRA ($million) | 12.9 | |||
| Investigators or teams receiving non-NIH funding related to ICRA | 6 | 23 | ||
| Total grants related to ICRA | 27 | |||
| Total grants related to ICRA ($million) | 68.5 | |||
| Total investigators or teams receiving funding related to ICRA | 12 | 46 | ||
| ROI | ||||
| Based on total grants related to ICRA | 303 | |||
| For early career investigators* | 448 | |||
| For established investigators† | 238 | |||
| Original reports published by ICRA authors that acknowledge DDCF | 87 | |||
| Investigators/teams publishing original reports that acknowledge DDCF | 25 | 96 | ||
| Original reports per investigator or team | 3.0 | 1.0/4.8 | ||
| Years from ICRA start year to publication | 4.0 | 3.0/6.0 | ||
| Original reports per million dollars of ICRA grants | 4.1 | 2.1/7.7 | ||
| Patent applications filed by ICRA investigators or teams | 7 | |||
| Investigators or teams filing | 5 | 19 |
Instructors or assistant professors.
Associate or full professors.
Several investigators were successful at getting non-NIH grants that stemmed from the ICRA program. This includes 2 grants from NSF, 2 from the California Institute for Regenerative Medicine, 3 from the Canadian Institutes of Health Research, and 6 from private foundations for a total of $12.9 million (Table 3). The non-NIH grants were concentrated in 6 laboratories. The results for private foundations are potentially incomplete for nonresponders because there could be grants from organizations that are not members of HRA that were missed.
If follow-on grants from NIH and non-NIH agencies that are directly related to ICRA are combined ($68.5 million), the financial ROI for the ICRA program is ∼4 times the original $17 million or 303% (Table 3). Early-career investigators had a higher ROI than more established researchers (448% vs 238%; Table 3). In all, 12 investigative teams (46%) attained ICRA-related follow-on funding (6 from NIH only, 4 from non-NIH only, and 2 from both NIH and non-NIH).
Twenty-five ICRA investigative teams published 87 original research reports that acknowledged DDCF as a source of funding (Table 3). The median number of publications per team was 3.0, and the median time to publish from the start of ICRA was 4 years. The median number of research publications per million dollars of grant funding was 4.1. Some of the specific publications are highlighted in the following impact cases.
There were 5 teams that filed 7 patent applications for products related to ICRA research (Table 3). To our knowledge, 4 have been licensed. Three of these are interrelated patents for a method that uses gene transfer by lentiviral vector to increase fetal hemoglobin. They were licensed to Aruvant Sciences. The fourth is for a technology that assesses blood cell adhesion and deformity and is licensed to BioChip Labs (the project is described below under “New technology to measure blood cell adhesion and deformity: SCD BioChip”). The other patent applications are for a technology to develop therapeutic red blood cells, an assay for neutrophil extracellular traps that protect against pathogens, and a systematic approach to sift through noncoding parts of the human genome to determine whether and how they affect traits.
Examples of lasting contributions to the field
Defining ROI as grant dollars attained as a percent of the ICRA investment is limited and does not describe other economic and human benefits. For this reason, we present the following case studies of projects that showed notable advances in the understanding and treatment of SCD. Many other projects yielded significant results; only 6 cases are presented because of space limitations.
A safe way to obtain blood stem cells for gene therapies: plerixafor mobilization in SCD.
Standard methods for obtaining stem cells from the bone marrow for gene modification were found to be dangerous for those with SCD16,17 or require an invasive bone marrow harvest procedure under anesthesia. One of the first tests of plerixafor for safety and efficacy in mobilizing stem cells in patients with SCD was conducted under an ICRA-supported research project. Plerixafor was well tolerated by the study participants and, although sufficient cells were obtained for gene therapies in some cases, there was variability in the amount of mobilized stem cells.18 A subsequent study by another research team has confirmed consistent, safe, and sufficient stem cell mobilization, collection, and processing with plerixafor,19 and the agent is currently the main method used to obtain stem cells in SCD gene modification trials.10
A novel therapeutic approach: fetal hemoglobin reactivation by disruption of BCL11A enhancer.
The transcription factor BCL11A has been shown to repress fetal hemoglobin levels.20 New work with ICRA support demonstrated that core sequences of the BCL11A enhancer are essential for fetal hemoglobin repression.21 CRISPR/Cas9-mediated gene modification of the enhancer resulted in clinically meaningful fetal hemoglobin induction.22 Targeting the BCL11A enhancer by genome editing is therefore a viable strategy for therapeutic genome editing for SCD. Continuation of this work is being supported by a $3 million commitment from the National Heart Lung and Blood Institute’s (NHLBI’s) Cure Sickle Cell Initiative to conduct a clinical trial of therapeutic gene editing of the BCL11A enhancer in patients with SCD.
Laying the groundwork for a gene editing clinical trial: correction of the sickle cell mutation with CRISPR/Cas9.
Ideal gene-editing strategies to correct the gene encoding the β-hemoglobin protein should have no off-target effects, cytotoxicity, or other deleterious effects on cell function.23 Site-specific editing strategies were developed with ICRA support,24 and a CRISPR/Cas9 endonuclease was found to produce high specificity for the β-hemoglobin target.19 On the basis of these findings, a clinical trial was designed and received $2.2 million in funding from the California Institute for Regenerative Medicine.25
Effective treatment of African children with SCD: clinical benefits of hydroxyurea.
Hydroxyurea is recommended for treatment of SCD in the United States, but its safety and efficacy in sub-Saharan Africa were unknown. A 12-month long, placebo-controlled trial of hydroxyurea in Ugandan children called Novel Use of Hydroxyurea in an African Region with Malaria (NOHARM) was conducted under the ICRA program. A safety concern was that hydroxyurea may directly affect the pathogenesis of malaria, with potentially harmful consequences. In the trial results, hydroxyurea significantly increased hemoglobin concentration and fetal hemoglobin, children receiving the drug had significantly fewer adverse clinical events such as severe pain or hospitalizations, and the incidence of malaria was the same in both treatment arms.26 On the basis of this study, Uganda’s Ministry of Health moved to approve hydroxyurea for use in children and adults with sickle cell anemia in March 2018, promising to increase access and availability. The research team is continuing their studies to determine optimal dosing and monitoring regimens for Africa and longer-term follow-up of the participants, studies that are also supported by DDCF. They recently reported that escalating the dose of hydroxyurea to a maximum tolerated level had superior clinical efficacy and equivalent safety to that of fixed-dose hydroxyurea.27
New technology to measure blood cell adhesion and deformity: SCD BioChip.
Abnormal adherence of red blood cells to vessel linings may be the initiating factor in the development of pain in SCD.28 A new technology called SCD BioChip to assess blood cell adhesion and deformity was developed with ICRA support. The chip was designed to model physiological flow of post-capillary venules.29 It allows rapid processing of whole blood samples and analyzes the adherence and aspect ratio of single cells under flow and no-flow conditions. Experiments with the SCD BioChip have demonstrated quantitative evaluation of red blood cell adhesion that could be used to characterize SCD and make assessments before and after therapeutic interventions.30 The technology has been licensed to BioChip Labs in Cleveland, Ohio, which is commercializing the assay. About $3.7 million from the NHLBI Cure Sickle Cell Initiative has been committed to support the use of the BioChip in assessing the Initiative’s multiple genome modification trials to cure SCD.
Improving transfusion therapy by using modern genomic approaches.
Blood transfusion is an important therapeutic intervention in patients with SCD, aiming to temporarily provide normal red blood cells that will reduce the complications of SCD.31 However, it can cause the patient to mount an immunologic response, which can result in clinical symptoms and reduce the effectiveness of subsequent transfusions. More detailed antigen typing of patients would improve the matching of donor blood and reduce the risk of alloimmunization. Potential risk factors for alloimmunization in patients with SCD were identified in an ICRA project. They found that variant RH genes are common in patients with SCD and contribute to Rh alloimmunization and transfusion reactions.32 They also demonstrated that personalized genotyping of patients and donors for Rh has the potential to reduce alloimmunization.33
Discussion
The goals of the ICRA program were to increase knowledge of the causes, severity, and outcomes of SCD, advance innovations, and attract new researchers and their knowledge to the field. The goal of encouraging researchers to move into SCD research was accomplished: almost half the projects were directed by investigators for whom SCD research was not their primary area and, among those who answered the survey, close to 80% are still dedicating some research time to the disease. One of these researchers stated: “Encouragement of cross-disciplinary work and crossover of researchers from other disciplines…was the reason I decided to apply for this highly competitive opportunity. DDCF ICRA was my first major grant as an early-career faculty member. This project has defined my academic research career, and it does so to this day.” Only 26 percent of ICRA principal investigators were women, which is similar to female representation among NIH research project grants over the same period (27-29%).34
Although most ICRA investigators were successful at getting subsequent grant funding, only 12 laboratories (46%) obtained grants directly related to their ICRA work. Nonetheless, the financial return on investment (303%) was similar to that in another study of research grants for innovations that found a 237% ROI.35 It was also similar to an evaluation of a grant portfolio focused on melanoma, which found a 3.6-fold or 260% ROI.36 It should be noted that follow-on grant funding is not the only measure of success. For example, a gene transfer technology was developed, and 2 patients were treated with ICRA support. This technology was successfully licensed to a company that will fund continuation of the clinical trial.
ICRA research was disseminated through publications by all but 1 investigative team, and the median number of original research reports per investigator or team was 3.0. To adjust for teams that received more than 1 grant and to compare with other studies, the number of publications per million dollars of grant funding was calculated. The median value was 4.1. This is greater than a study of NIH research grants, which found the median number of publications per million dollars to be 3.3.37 The NIH study followed 2009 grants through 2014, which is a shorter time frame than this evaluation, but they also counted all publications attributed to a grant, not just original research reports. The publication output for ICRA, therefore, seems to compare favorably with that for NIH.
There are some limitations to this evaluation. Only 56% of principal investigators responded to the survey. Nonresponses could affect the completeness of the data on grants from agencies other than NIH, NSF, and HRA members. If foundation grants obtained by ICRA investigators were missed, however, this would only increase follow-on funding. The identification of follow-on grants directly related to ICRA relied on an evaluation by the authors. One limitation is the lack of independent assessment by another evaluator that would help establish validity and reproducibility. The follow-up time frame varied for each set of grants with a maximum of 12 years for 2009 grants and a minimum of 6 years for 2015 grants. Publications could still be in the works for the more recent projects, although a median time to publication of 4.0 years and an interquartile range of 3.0 to 6.0 years suggests that most manuscripts were captured. Studies that map grants to publications can give false positives (claiming publications that are not related) and false negatives (missing publications).38 Because ours was a small study in a well-defined field, it is unlikely that there were false positives, but it is possible that publications were missed. There is selection bias in the cases presented; this was intentional to illustrate accomplishments. There are also projects that were not as effective, although all projects advanced knowledge and all but 1 published results.
The field of SCD research has made impressive strides since the ICRA program was started. Only 10 clinical trials testing drug therapies were registered in 2008, but the number almost tripled to 27 in 2020.10 In addition to hydroxyurea, there are now 3 new drug therapies approved and available: L-glutamine, crizanlizumab, and voxelotor. DDCF started an initiative in 2017 with larger research grants specifically targeting cures for SCD. After this DDCF Sickle Cell Disease/Advancing Cures program was established, NHLBI started a new Cure Sickle Cell Initiative in 2018, which aims to advance gene therapies to the bedside (2 ICRA investigators have received support from this initiative). On the basis of a search of RePORTER, the NIH Cure Sickle Cell Initiative paid out more than $366 million in 2020 and $168 million in 2021. Other NIH funding for sickle cell research has grown from $80 million in 2008 to $142 million in 2020.11 Work on gene therapies has moved forward at a rapid pace. There are at least 13 ongoing gene modification trials for people with SCD, including some that use CRISPR gene editing.10
The results from this program evaluation can inform future approaches. A focus on a specific unmet need and encouragement of new investigators can bring new knowledge and skills to an area that is underfunded and needs more research. If a goal is development of new treatments for diseases with a molecular component, support for projects rooted in rational understanding of disease mechanisms and underlying genetics can lead to tangible results that have clinical applications. Relatively small research grants can have a significant ROI, although it is likely that some projects, maybe even half, will not go on to achieve related funding. Finally, qualitative assessment of impact should be included in evaluation studies to complement quantitative information.
Acknowledgments
The authors thank the DDCF Program Director for Medical Research in 2008, Elaine Gallin, for getting the program off the ground and past DDCF President and CEO, Ed Henry, for his unwavering support for SCD research.
Authorship
Contribution: S.N.E.A. and E.R.M. designed the study; E.R.M. collected and analyzed the results; and S.N.E.A. and E.R.M. wrote the article, revised it critically for important intellectual content, and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sindy N. Escobar Alvarez, Doris Duke Charitable Foundation, 650 Fifth Ave, 19th Floor, New York, NY 10019; e-mail: mrp@ddcf.org.
References
Author notes
Results presented in this report were aggregated from public data sources. A list of specific projects supported with ICRA grants is available at https://www.ddcf.org/funding-areas/medical-research.
The full-text version of this article contains a data supplement.