Key Points
This study shows that treatment with eltrombopag can rapidly increase platelet counts and reduce bleeding for at least 3 months.
Eltrombopag treatment was well tolerated, and dose regimen may not differ between East Asian children and those of European heritage.
Abstract
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder with isolated thrombocytopenia and risk of hemorrhage. Treatment with eltrombopag increases and maintains hemostatic platelet counts; however, to date, long-term data are lacking on the outcome of children with ITP who are treated with eltrombopag. This prospective, observational, longitudinal cohort study evaluated the efficacy and safety of eltrombopag in pediatric patients with persistent or chronic ITP. For the 116 pediatric patients enrolled, duration of eltrombopag treatment was at least 3 months. Median effective dose was 25 mg/day, 50 mg/day, and 50 mg/day, respectively, for children age 5 years or younger, 6 to 11 years, or 12 years or older. In all, 89 patients (76.7%) achieved overall response, 53 (45.7%) achieved complete response, and 36 (31.0%) achieved response. Median platelet counts increased by week 1 and were sustained throughout the treatment period. During treatment with eltrombopag, the proportion of patients with grade 1 to 4 bleeding symptoms decreased from 83.61% at baseline to 9.88% at 6 months when only grade 1 was reported. Forty-three patients (37.1%) reported using concomitant medications at study entry, which was reduced to 1 patient (2.5%) who needed concomitant medications at 12 months. All adverse events were grade 1 or 2 according to Common Terminology Criteria for Adverse Events. No serious adverse events, cataracts, malignancies, or thromboses were reported during the study. Long-term treatment with eltrombopag was generally safe, well tolerated, and effective in maintaining platelet counts and reducing bleeding in most pediatric patients with persistent or chronic ITP. Combined with future studies, these findings will help establish how eltrombopag should best be used in the management of pediatric patients with East Asian ancestry.
Introduction
Immune thrombocytopenia (ITP) is an immune-mediated disorder characterized by increased platelet destruction in peripheral blood and decreased platelet production by megakaryocytes because of the loss of immune tolerance to platelet autoantigens.1,2 Primary ITP is defined as a platelet count <100 × 109/L in the absence of underlying causes.3 With an estimated incidence of 4.2 per 100 000 person-years, it is the most common type of thrombocytopenia in children.4 ITP can be classified by disease duration as newly diagnosed (<3 months), persistent (3-12 months), or chronic (>12 months).1,2 Severe bleeding mostly occurs at very low platelet counts. The probability of severe bleeding is higher in those with moderate bleeding manifestations.5,6
In general, a watch-and-wait policy is recommended for managing children newly diagnosed with ITP, given a high early spontaneous remission rate of more than 75% in this population.7 For patients with bleeding symptoms that require treatment, treatment strategies focus on increasing platelet counts to a safe level to achieve effective control of bleeding symptoms and simultaneously improving health-related quality of life and reducing parental burden associated with the disease.8,9 Corticosteroids and intravenous immunoglobulin G are regarded as first-line therapies.10 Recommended treatments for persistent or chronic ITP in children include thrombopoietin receptor agonists (TPO-RAs), immunosuppressive drugs (eg, rituximab, mycophenolate mofetil, and sirolimus), or splenectomy to prevent prolonged exposure to bleeding and a potentially reduced quality of life resulting from the fear of or burden of bleeding events and the restriction of physical activities.10,11
Eltrombopag was the first TPO-RA licensed for treatment of pediatric ITP by the US Food and Drug Administration (FDA) in August 2015. It is a small non-peptide agonist that noncompetitively binds within the transmembrane domain of the TPO receptor and activates the JAK/STAT and MAPK signal transduction pathways to promote proliferation and differentiation of megakaryocytes.12 Eltrombopag has proved to be an effective second-line therapy with acceptable tolerability for pediatric patients with ITP according to the findings of 2 international, multicenter, randomized, double-blind, placebo-controlled phase 3 trials (PETIT and PETIT2).5,13 However, eltrombopag exhibited different metabolic properties among patients with Asian ancestry in the pharmacokinetics/pharmacodynamics model, resulting in relatively high exposure to the drug.14 There are insufficient efficacy and safety data in PETIT2 to draw a suitable dosing recommendation for Asian children.
Because of the lack of sufficient evidence for efficacy in treating Asian children, eltrombopag was approved in China in 2018 for treating patients age 12 years or older with ITP. Published review articles with small cohorts demonstrated the efficacy of eltrombopag among children,15,16 but there is still a lack of prospective data from a large cohort for clinical references. Here we present our 12-month follow-up findings regarding the use of eltrombopag in a prospective, longitudinal cohort of 116 children with persistent or chronic ITP who received treatment in a real-world clinical practice in China, with the goal of characterizing clinical effectiveness, tolerability, and evidence-based dosing strategies of eltrombopag therapy among pediatric patients with East Asian ancestry.
Methods
This was a prospective, observational, longitudinal cohort study of eltrombopag use for pediatric patients with ITP who experienced ineffective first-line therapy or relapse. Participants were enrolled at Beijing Children’s Hospital, Capital Medical University in Beijing, China, between September 2018 and June 2020 after the study was approved by the Ethics Committee. The study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each enrolled patient or his or her legally authorized representatives.
Patients were eligible if they were younger than age 18 years with a confirmed diagnosis of persistent or chronic ITP1 and had a continuous platelet count of <30 × 109/L on 3 occasions within the last 3 weeks (once per week is recommended) before the initial dose of eltrombopag. Patients with other hematologic disorders (eg, platelet agglutination abnormality, Evans syndrome, nonimmune or hereditary thrombocytopenia) or secondary ITP were excluded.
Before the start of treatment with eltrombopag, all patients were informed about potential adverse events (AEs), appropriate administration of the drug, and possible interaction with certain foods. Clinical hematology and liver function tests were evaluated regularly throughout eltrombopag treatment, and the dosage regimen was adjusted based on the platelet count according to the instructions for use. The starting dose of eltrombopag was 1.5 mg/kg per day for patients age 5 years or younger, 37.5 mg per day for patients age 6 to 11 years, and 50 mg per day for patients age 12 years or older. Platelet count was monitored once per week for 2 weeks and then once per month. The dosing regimen for eltrombopag based on platelet counts was individualized with the goal of achieving and maintaining platelet counts of ≥50 × 109/L to <150 × 109/L (Table 1).
Dose adjustment of eltrombopag
| Platelet count . | Dose adjustment or response . |
|---|---|
| <50 × 109/L after treatment for at least 2 weeks | Increase the daily dose by to a maximum of 75 mg/day. |
| >150 × 109/L at 2-week intervals | Decrease the daily dose by 12.5 mg/day. |
| >250 × 109/L | Treatment was interrupted and resumed at next lowest dose based on 12.5-mg increments once the platelet count had decreased to <100 × 109/L. |
| Platelet count . | Dose adjustment or response . |
|---|---|
| <50 × 109/L after treatment for at least 2 weeks | Increase the daily dose by to a maximum of 75 mg/day. |
| >150 × 109/L at 2-week intervals | Decrease the daily dose by 12.5 mg/day. |
| >250 × 109/L | Treatment was interrupted and resumed at next lowest dose based on 12.5-mg increments once the platelet count had decreased to <100 × 109/L. |
Demographic and clinical characteristics were recorded for age, sex, time since diagnosis, number of previous ITP therapies, platelet counts, disease duration, concomitant medication, medication dose, treatment duration, bleeding scores, AEs (graded by using Common Terminology Criteria for Adverse Events [CTCAE] 5.0), and laboratory data. Bleeding was assessed with a validated measure for pediatric ITP patients (grade 1, minor; grade 2, mild; grade 3, moderate; grade 4, severe).10 Follow-up was based on real-life practice for up to 12 months.
Platelet counts were measured and recorded for response. Complete response (CR) was defined as a platelet count ≥100 × 109/L and absence of bleeding. Response (R) was defined as a platelet count between 30 × 109/L and 100 × 109/L, at least doubling of the baseline platelet count, and absence of bleeding. Overall response (OR) was the number of patients achieving either CR or R. No response (NR) was defined as platelet count <30 × 109/L, or less than doubling of the baseline count, or bleeding.17 Durable response (DR) was defined by the 2019 American Society of Hematology (ASH) guideline as a platelet count ≥30 × 109/L and at least doubling of the baseline count at 6 months.1
Statistical methods
Statistical analyses were performed using Excel (Microsoft, Redmond, WA) and SPSS 20.0 software for Windows (IBM Corp., Armonk, NY). Data were described as mean, standard deviation, median, minimum, maximum, interquartile range (IQR), or percentages. Normality of continuous variables was tested with the Kolmogorov-Smirnov test. Categorical variables were compared with Pearson χ2 or Fisher exact tests, and continuous data were compared using nonparametric tests if the data followed non-normal distribution. The confidence interval was 95%, and differences associated with P < .05 were considered statistically significant.
Results
This study started in August 2018 and is ongoing. In all, 116 patients who received at least 3 months of treatment with eltrombopag were selected. Of those, 81 patients received treatment for more than 6 months and 40 received treatment for at least 12 months. Patient are still in follow-up, and data up to month 12 after start of medication was used for analysis. No patients were lost to follow-up within the first 3 months, 3 patients were lost to follow-up between 3 and 12 months, and data at the corresponding time points were censored at the time of statistical analysis. No patient withdrew or discontinued treatment within the first 3 months. Seven patients withdrew from observation after 3 months, 4 of whom withdrew because they had NR, 3 for financial reasons, and none because of AEs.
Patient demographics
The mean age of patients was 6.7 years (range, 0.58-16.00 years), and 60.3% were males (Table 2). Of the 116 enrolled patients, 102 (87.9%) had chronic ITP, and 14 (12.1%) had persistent ITP. The mean time from ITP diagnosis to entry into the study was 2.91 years (range, 0.08-11.00 years). Before study entry, 94.0% of patients had received ≥2 previous ITP treatments, and 72.4% of them had received ≥3 previous ITP treatments. At baseline, 37.1% were receiving concomitant ITP medications, the most common of which were corticosteroids (76.7%) and rituximab (20.9%). The mean baseline platelet count was 10.16 × 109/L (range, 1 × 109/L to 29 × 109/L).
Baseline characteristics
| Characteristic . | Patients (n = 116) . | |
|---|---|---|
| Mean age, y | 6.70 ± 3.71 | 0.58-16 |
| Female sex | 46 | 39.7 |
| Mean weight, kg | 28.14 ± 14.84 | 7-73 |
| Mean baseline platelet count × 109/L | 10.16 ± 7.07 | 1-29 |
| Mean ITP duration, y | 2.91 ± 2.62 | 0.08-11 |
| ITP phase | ||
| Chronic | 102 | 87.9 |
| Persistent | 14 | 12.1 |
| Concomitant ITP medication use | 43 | 37.1 |
| No. of previous ITP treatments | ||
| 1 | 7 | 6.0 |
| 2 | 25 | 21.6 |
| ≥3 | 84 | 72.4 |
| Characteristic . | Patients (n = 116) . | |
|---|---|---|
| Mean age, y | 6.70 ± 3.71 | 0.58-16 |
| Female sex | 46 | 39.7 |
| Mean weight, kg | 28.14 ± 14.84 | 7-73 |
| Mean baseline platelet count × 109/L | 10.16 ± 7.07 | 1-29 |
| Mean ITP duration, y | 2.91 ± 2.62 | 0.08-11 |
| ITP phase | ||
| Chronic | 102 | 87.9 |
| Persistent | 14 | 12.1 |
| Concomitant ITP medication use | 43 | 37.1 |
| No. of previous ITP treatments | ||
| 1 | 7 | 6.0 |
| 2 | 25 | 21.6 |
| ≥3 | 84 | 72.4 |
Data are n (%) or mean ± standard deviation.
Dosing
The median initial daily dose was 25 mg for children age 5 years or younger, 37.5 mg for those age 6 to 11 years, or 50 mg for those age 12 years or older; the median effective doses and maximum doses for those age groups were 25 mg/day, 50 mg/day, and 50 mg/day, respectively (Table 3). During the study, 26 patients (22.4%) required a change in the dose of eltrombopag. Of these, 22 patients (19.0%) had a dose increase compared with 4 (3.4%) who had a dose decrease.
Dose of eltrombopag by age group
| Dose . | Age (y) . | P . | |||||
|---|---|---|---|---|---|---|---|
| 5 or younger (n = 42) . | 6 to 11 (n = 60) . | 12 or older (n = 14) . | |||||
| Median | IQR | Median | IQR | Median | IQR | ||
| Initial dose, mg | 25 | 25-25 | 37.5 | 25-50 | 50 | 50-50 | |
| Initial daily dose, mg/kg | 1.56 | 1.39-1.67 | 1.28 | 1.16-1.47 | 0.96 | 0.82-1.38 | .000 |
| Effective dose, mg | 25 | 25-25 | 50 | 31.25-50 | 50 | 50-59.38 | |
| Effective daily dose, mg/kg | 1.56 | 1.39-1.67 | 1.25 | 1.17-1.50 | 1.02 | 0.83-1.32 | .000 |
| Maximum dose, mg | 25 | 25-25 | 50 | 37.5-50 | 50 | 50-75 | |
| Maximum daily dose, mg/kg | 1.66 | 1.45-1.93 | 1.39 | 1.21-1.56 | 1.11 | 0.83-1.67 | .000 |
| Dose . | Age (y) . | P . | |||||
|---|---|---|---|---|---|---|---|
| 5 or younger (n = 42) . | 6 to 11 (n = 60) . | 12 or older (n = 14) . | |||||
| Median | IQR | Median | IQR | Median | IQR | ||
| Initial dose, mg | 25 | 25-25 | 37.5 | 25-50 | 50 | 50-50 | |
| Initial daily dose, mg/kg | 1.56 | 1.39-1.67 | 1.28 | 1.16-1.47 | 0.96 | 0.82-1.38 | .000 |
| Effective dose, mg | 25 | 25-25 | 50 | 31.25-50 | 50 | 50-59.38 | |
| Effective daily dose, mg/kg | 1.56 | 1.39-1.67 | 1.25 | 1.17-1.50 | 1.02 | 0.83-1.32 | .000 |
| Maximum dose, mg | 25 | 25-25 | 50 | 37.5-50 | 50 | 50-75 | |
| Maximum daily dose, mg/kg | 1.66 | 1.45-1.93 | 1.39 | 1.21-1.56 | 1.11 | 0.83-1.67 | .000 |
Efficacy
Platelet response.
In total, 89 patients (76.7%) achieved OR, 53 patients (45.7%) achieved CR, 36 patients (31.0%) achieved R, and 27 patients (23.3%) achieved NR. The CR rates were slightly lower in children in older age groups (age 5 years or younger, 47.6%; age 6-11 years, 46.7%; age 12 years or older, 35.7%). The overall median time to relapse (TTR) was 10 days, and there was no significant difference among the 3 age groups (13 days for patients age 5 years or younger, 9.5 days for patients age 6-11 years, and 20 days for patients age 12 years or older; P > .05). The responses to eltrombopag were consistent in all 3 age cohorts (Table 4). Platelet responses were not different irrespective of sex, ITP phase, concomitant medications, number of previous ITP treatments, or previous recombinant human TPO (rhTPO) (Table 5).
Platelet response overall and by age group
| Platelet response . | Age (y) . | Total . | P . | ||
|---|---|---|---|---|---|
| 5 or younger . | 6-11 . | 12 or older . | |||
| Complete response | 20 (47.6) | 28 (46.7) | 5 (35.7) | 53 (45.7) | .598 |
| Response | 12 (28.6) | 17 (28.3) | 7 (50.0) | 36 (31.0) | |
| No response | 10 (23.8) | 15 (25.0) | 2 (14.3) | 27 (23.3) | |
| Median days to response (IQR) | 13 (7-21) | 9.5 (6-18) | 20 (9-36) | 10 (7-23) | .086* |
| Platelet response . | Age (y) . | Total . | P . | ||
|---|---|---|---|---|---|
| 5 or younger . | 6-11 . | 12 or older . | |||
| Complete response | 20 (47.6) | 28 (46.7) | 5 (35.7) | 53 (45.7) | .598 |
| Response | 12 (28.6) | 17 (28.3) | 7 (50.0) | 36 (31.0) | |
| No response | 10 (23.8) | 15 (25.0) | 2 (14.3) | 27 (23.3) | |
| Median days to response (IQR) | 13 (7-21) | 9.5 (6-18) | 20 (9-36) | 10 (7-23) | .086* |
All data are n (%) unless otherwise specified.
Homogeneity of variance test, P = .001; missing variance with rank sum test.
Factors associated with efficacy data
| Factor . | OR (n = 116) . | P . | TTR (days) (n = 91) . | P . | ||
|---|---|---|---|---|---|---|
| No. . | % . | Median . | IQR . | |||
| Sex | .466 | .7695 | ||||
| Female | 36 | 78.3 | 13.5 | 7-27.5 | ||
| Male | 53 | 75.7 | 10 | 7-20 | ||
| ITP phase | .418 | .4227 | ||||
| Chronic | 79 | 77.5 | 10 | 7-23 | ||
| Persistent | 10 | 71.4 | 10 | 2.75-30.75 | ||
| Concomitant medications | .373 | .849 | ||||
| Yes | 31 | 72.1 | 10 | 6-24.5 | ||
| No | 58 | 79.5 | 10.5 | 7-18.5 | ||
| No. of previous ITP treatments | .170 | .4078 | ||||
| ≥3 | 62 | 73.8 | 10 | 7-22.25 | ||
| ≤2 | 27 | 84.4 | 15 | 7-26 | ||
| Previous rhTPO use | .077 | .4284 | ||||
| Yes | 40 | 70.2 | 10 | 7-21.5 | ||
| No | 49 | 83.1 | 11.50 | 7-24.75 | ||
| Factor . | OR (n = 116) . | P . | TTR (days) (n = 91) . | P . | ||
|---|---|---|---|---|---|---|
| No. . | % . | Median . | IQR . | |||
| Sex | .466 | .7695 | ||||
| Female | 36 | 78.3 | 13.5 | 7-27.5 | ||
| Male | 53 | 75.7 | 10 | 7-20 | ||
| ITP phase | .418 | .4227 | ||||
| Chronic | 79 | 77.5 | 10 | 7-23 | ||
| Persistent | 10 | 71.4 | 10 | 2.75-30.75 | ||
| Concomitant medications | .373 | .849 | ||||
| Yes | 31 | 72.1 | 10 | 6-24.5 | ||
| No | 58 | 79.5 | 10.5 | 7-18.5 | ||
| No. of previous ITP treatments | .170 | .4078 | ||||
| ≥3 | 62 | 73.8 | 10 | 7-22.25 | ||
| ≤2 | 27 | 84.4 | 15 | 7-26 | ||
| Previous rhTPO use | .077 | .4284 | ||||
| Yes | 40 | 70.2 | 10 | 7-21.5 | ||
| No | 49 | 83.1 | 11.50 | 7-24.75 | ||
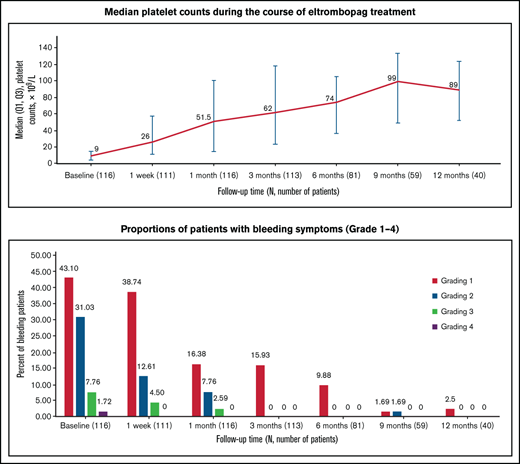
Median platelet counts increased from 9 × 109/L (IQR, 4.00 × 109/L to 14.75 × 109/L) at baseline to 26 × 109/L (IQR, 11 × 109/L to 58 × 109/L) after a week of treatment with eltrombopag. From month 1 throughout treatment with eltrombopag, median platelet counts increased to 51.5 × 109/L and remained consistently above ≥50 × 109/L (Figure 1). Eighty-one of the 116 enrolled patients received treatment for more than 6 months; among those patients, 78 achieved DR, accounting for 67.2% of the total 116 patients.
Median platelet counts during the course of eltrombopag treatment. Q1, first quartile.
Median platelet counts during the course of eltrombopag treatment. Q1, first quartile.
Bleeding.
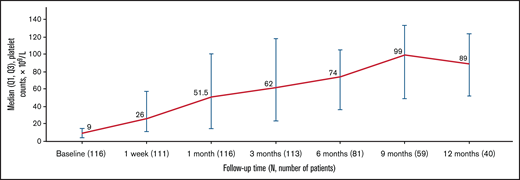
At baseline, 83.6% of patients reported grade 1 to 4 bleeding symptoms. The proportion of patients with any bleeding symptoms decreased to 55.6% after 1 week of treatment and remained lower than the baseline level throughout the study (P = .0014; Figure 2). Similarly, 40.5% of patients reported clinically significant bleeding (grade 2-4) at baseline compared with 17.1% at 1 week and 10.4% at 1 month, which reduced to 0.0% after 3 months (except 1.7% at 9 months). No patients had grade 4 bleeding after baseline.
Concomitant medication use.
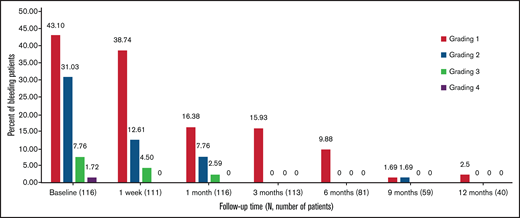
At baseline, 43 patients (37.1%) reported the use of concomitant medications, and that number nearly halved between 1 week and 6 months. In all, 2.5% of patients needed concomitant medications at 12 months (Figure 3). The most frequently discontinued or reduced medications were corticosteroids and rituximab.
Safety
No serious AEs were reported. All AEs were CTCAE grade 1 or 2; no patients treated with eltrombopag had grade 3 or 4 AEs (Table 6). In total, 22 patients reported 26 laboratory hepatobiliary abnormalities; all were grade 1, including 6 patients (5.2%) with increased indirect bilirubin, 2 (1.7%) with increased aspartate aminotransferase (AST), and 2 (1.7%) with increased alanine aminotransferase (ALT). Sixteen patients (13.8%) had increased alkaline phosphatase (ALP) (all grade 1). Thrombocytosis occurred in 3 (2.6%) of 116 children. No patients had new or worsening cataracts, thromboses, or malignancies during the study.
AEs
| AE . | No. . | % . | 95% CI . |
|---|---|---|---|
| Increased ALT | 2 | 1.7 | 0.31-5.24 |
| Increased AST | 2 | 1.7 | 0.31-5.24 |
| Increased indirect bilirubin | 6 | 5.2 | 2.30-9.61 |
| Thrombocytosis | 3 | 2.6 | 0.71-6.43 |
| Abdominal reactions | 3 | 2.6 | 0.71-6.43 |
| Rash | 1 | 0.9 | 0.04-3.96 |
| Upper respiratory tract infection | 14 | 12.1 | 7.61-17.68 |
| Increased ALP | 16 | 13.8 | 8.91-19.88 |
| AE . | No. . | % . | 95% CI . |
|---|---|---|---|
| Increased ALT | 2 | 1.7 | 0.31-5.24 |
| Increased AST | 2 | 1.7 | 0.31-5.24 |
| Increased indirect bilirubin | 6 | 5.2 | 2.30-9.61 |
| Thrombocytosis | 3 | 2.6 | 0.71-6.43 |
| Abdominal reactions | 3 | 2.6 | 0.71-6.43 |
| Rash | 1 | 0.9 | 0.04-3.96 |
| Upper respiratory tract infection | 14 | 12.1 | 7.61-17.68 |
| Increased ALP | 16 | 13.8 | 8.91-19.88 |
CI, confidence interval.
Discussion
Even though TPO-RAs have become the preferred second-line therapy for ITP according to the most recent international guidelines, real-world evidence for eltrombopag use among ITP children is insufficient. This is the first large prospective cohort of pediatric patients with ITP receiving eltrombopag. Our results regarding follow-up for as long as 12 months in East Asian children are available for discussion.
Effectiveness of eltrombopag for treating Chinese children with ITP was well demonstrated in this study: in total, 76.7% of our study cohort achieved either CR or R, and rates of OR were consistently observed among the 3 age groups. These findings are in line with registration in the PETIT and PETIT2 trials.13,18 None of the baseline covariates (sex, ITP phase, concomitant ITP medications, number of previous therapies, previous use of rhTPO) examined in our study were shown to significantly influence OR to eltrombopag, which is in agreement with data from the multicenter, randomized phase 3 study of Chinese patients with ITP by Yang et al.19 Even though statistical significance was not reached, rates of OR were ∼10% higher among patients who received ≤2 previous therapies and with no previous use of rhTPO. Similar to evidence from a real-world analysis of eltrombopag for treatment of ITP in adults,20 our findings suggest that use of eltrombopag earlier in the disease process may be more effective and therefore lower the risk of treatment failure. Because current indications for eltrombopag were limited to chronic ITP, more research is required to determine whether using eltrombopag in earlier phases of ITP improves treatment response.
We found a median TTR of 10 days after the start of eltrombopag use that seems to be shorter than that in the PETIT study, which reported TTRs of 20, 12, and 19 days among children ages 1-5, 6-11, and 12-17 years, respectively.18 Because eltrombopag was used for the first time among pediatric patients in the PETIT study, starting doses were relatively conservative, with as many as 86% of the patients having at least 1 dose increase during the double-blind phase. Again, these results indicate the importance of optimal dosing for pediatric patients with ITP. In this study, we also found that median TTR (20 days) was relatively longer for children age 12 years or older, which may be attributable to the longer duration of ITP observed for this age cohort (median, 7 years) when compared with our total cohort of patients (median, 2.91 years). Because there were fewer patients in the oldest age group, we need to further explore whether our findings are the consequence of inadequate dosing or whether pathogenesis of ITP may differ across age groups. As with OR, no baseline covariates were found to have a significant impact on TTR.
In this study, the median starting dose was 25 mg per day for children age 5 years or younger, 37.5 mg for children age 6 to 11 years, and 50 mg for children age 12 years or older. Owning to the differences in pharmacokinetics among races,14 the FDA recommends a reduced initial dose of eltrombopag for patients with East Asian ancestry. However, even with the limited data in PETIT2, we can see that the average daily dose for East Asian children did not seem to be lower than that for the total cohort.13 Compared with the dosing in the small cohort in our retrospective study,16 this large prospective cohort reported a higher starting dose than that recommended by the FDA. Therefore, compared with previous studies, our cohort illustrated a shorter TTR with less frequent dose adjustments. Notably, the median effective dose was 25 mg for children age 5 years or younger and 50 mg for older children in our study, of which an increment of 12.5 mg from median starting dose was observed for children age 6-11 years. On the basis of our dosing summary of the 116 Chinese children with ITP, we speculate that the eltrombopag dose regimen may not differ significantly between East Asian children and those with European heritage. The starting dose of eltrombopag may increase to 50 mg for pediatric patients age 6 years or older who have Asian ancestry, thus allowing children to reach a relatively safe platelet level, achieve bleeding control in a shorter time period, and reduce the number of dose adjustments before reaching the target response.
Elevating platelet counts to a relatively safe level as early as possible to reduce bleeding events is an important goal of ITP treatment.1,10 In our cohort of children, after 1 week of eltrombopag treatment, median platelet counts rebounded from 9 × 109/L at baseline to 26 × 109/L, a level at which severe bleeding is less common for children.1 The platelet counts increased continuously and were maintained at the level of >50 × 109/L throughout the remainder of the 12-month period. Meanwhile, we observed a rapid and sustained improvement in bleeding. The proportion of patients with bleeding symptoms was reduced by approximately one-third for any bleeding and more than a half for grade 2 to 4 bleeding during the first week of eltrombopag treatment. A decreasing trend was indicated, with only 15.9% of patients reporting a minor (grade 1) bleeding, 1.7% mild (grade 2) bleeding, and no grade 3-4 bleeding throughout the period of 3 to 12 months.
In our study, the proportion of children who need concomitant medication decreased from 37.1% at baseline to ∼20% in 1 month, and only 1 patient (2.5%) was receiving a reduced dose of sirolimus at 12 months. Corticosteroids were the most frequently discontinued or reduced ITP medications followed by rituximab and rapamycin. Immunosuppressive drugs have been available for a long time but are associated with relatively low efficacy and potential for significant AEs.11,21 The 2019 ASH guideline also stressed the importance of avoiding long-term immunosuppression in children.1 Given that the immune system in children is actively undergoing growth and development, acceptance of long-term immunosuppressants use is low for Chinese parents. None of the children in our study cohort had a splenectomy. Splenectomy is unacceptable to many parents because of cultural norms and the long-term risks associated with it.13,21 Treatment with eltrombopag led to a significant reduction in bleeding events along with a sustained increase in platelet counts that allowed patients to discontinue concomitant ITP medications and subsequently avoid relevant AEs, immunosuppression, or splenectomy because of treatment failure.
Eltrombopag was generally well tolerated because most of the AEs observed in our study were mild and were in accordance with the safety profiles of eltrombopag. Hepatotoxicity is a primary concern with eltrombopag treatment, especially for patients with East Asian ancestry. In total, 22 patients reported 26 AEs with increased ALT, AST, ALP, or indirect bilirubin. Among them, a 13-year-old patient had concurrent increased ALT and AST at a maximum dose of 75 mg/day, and the other 3 patients had simultaneous increases in ALP and indirect bilirubin. No patients had 3 or more AEs occurring simultaneously. Because all AST, ALP, and indirect bilirubin abnormalities were grade 1, no patient had to reduce or discontinue treatment for these reasons. During the open-label period of the PETIT 2 study, a total of 6 patients (7%) (including 5 with East Asian ancestry) had ALT levels ≥3 times the upper limit of normal, of whom 3 met the protocol-defined discontinuation criteria. Supplementary analysis of treatment-emergent AEs stratified by race and age revealed that increased ALT or AST concentrations were more common for East Asian patients and those age 6 to 11 years.13 All of these AEs were CTCAE grade 1 and <3 times the upper limit of normal for aminotransferases. The abnormalities resolved when the frequency of eltrombopag dosing was reduced. No patients required discontinuation of eltrombopag treatment. Our rates of elevated aminotransferase were lower than those in previous findings, suggesting the importance of standardized clinical management, individualized treatment regimen, close follow-up and monitoring, and timely dose adjustment to maintain the platelet counts at a safe level. Increased ALP was observed in some children in our cohort that was not reported in other studies, but it was CTCAE grade 1 and reversible, and it did not lead to reduction or discontinuation of eltrombopag. The elevated ALP decreased spontaneously in most patients during follow-up, and no other clinically significant symptoms or abnormalities were observed. ALP is a nonspecific liver enzyme that also originates in various tissues, including bone and the digestive tract; physiological increase can also occur during a child’s growth and development.22 In future studies, we will continue to assess tolerability of eltrombopag in a larger sample size with longer follow-up. Other clinically significant AEs such as cataracts, thrombosis, and iron deficiency anemia were not encountered in our patient cohort.
Our study has some limitations. One is that because of the real-world nature of the study cohort, data may not be uniformly presented; therefore, patients who had been treated for 3 to 12 months were selected to ensure meaningful analysis of the effectiveness and safety outcomes. In addition, patient-reported outcomes, such as health-related quality of life and parental burden, were not captured. Some of the patients did not reach the maximum dose recommended by the protocol, which might have influenced the efficacy and safety results discussed here.
In conclusion, this is the first large prospective cohort that received eltrombopag for the treatment of persistent or chronic ITP. Eltrombopag allows rapid and sustained increase of platelet counts, significant reduction of bleeding events and concomitant medications, and has acceptable tolerability throughout 12 months in real-world clinical practice. Effectiveness of eltrombopag has been demonstrated in all subgroups, and the responses seen in our cohort are consistent with those seen in the PETIT and PETIT2 trials and in some studies of adults with ITP treated with eltrombopag.13,18,19,23-25 Furthermore, our findings propose the rationale for initiating treatment for pediatric ITP patients from East Asia at dose levels comparable to those for non-Asians.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (no. 81970111), Beijing Natural Science Foundation of China (no. 7192046), the Pediatric Medical Coordinated Development Center of Beijing Municipal Administration of Hospitals (no. XTZD20180205), National Science and Technology Key Projects (no. 2017ZX09304029004), and Children’s Medicine Research Project of Beijing Children’s Hospital, Capital Medical University (no. YZYB202001).
Authorship
Contribution: X.C. helped critically analyze the clinical data and wrote the first draft of the paper; L.F. and J.M. helped collect clinical data; H.G. and Z.C. helped analyze the data; L.Z. and X.W. helped edit the paper; and R.W. developed the concept for the study, analyzed the data, supervised statistical analysis, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Runhui Wu, Comprehensive Care Team for Hemophilia, Department of Hematology, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, 56 Nanlishi Rd, West District, Beijing 100045, China; e-mail: runhuiwu@hotmail.com; and Xiaoling Wang, Department of Pharmacy, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, 56 Nanlishi Rd, West District, Beijing 100045, China; e-mail: wangxiaoling@bch.com.cn.
References
Author notes
To request data, please contact Xiaoling Cheng via ea-mail at chengxiaoling1224@163.com.