Key Points
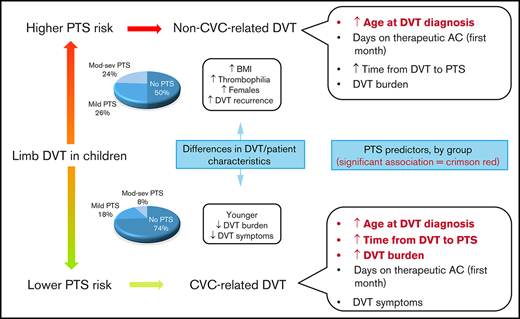
Pediatric PTS is more severe in non–CVC-related than in CVC-related DVT.
DVT burden, age at the time of DVT, and time from DVT diagnosis were predictors of PTS severity in children with CVC-related thrombosis.
Abstract
Our understanding of postthrombotic syndrome (PTS) predictors in children is evolving. The present study aimed to investigate differences in patient- and deep vein thrombosis (DVT)–related characteristics between central venous catheter (CVC)–related and non–CVC-related thrombosis in children, as well as early PTS predictors. Children aged 0 to 18 years were prospectively recruited ≥6 months after imaging-proven upper- or lower-extremity DVT. PTS was measured using CAPTSure. Early predictors included age at DVT diagnosis, DVT symptoms, DVT burden, and days on therapeutic anticoagulation within 30 days post-DVT diagnosis. Analysis of predictors was stratified by CVC-related and non–CVC-related thrombosis. Generalized estimating equations were used for data analyses. In total, 313 DVT-affected extremities of 256 patients were assessed; 275 (88%) DVT cases were CVC related. Patients with non–CVC-related thrombosis were older (median age, 5.8 years; 25th-75th percentile, 4.9-6.4 years vs 3.5 months; 25th-75th percentile, 0.7-18.7 months; P < .001) and more likely to have thrombophilia (64% vs 22%; P < .001) and obesity (30% vs 13%; P = .01) than patients with CVC-related thrombosis. CAPTSure scores were 9.5 points higher (standard error, 3.0; P = .02) in the non–CVC-related thrombosis stratum. Age at the time of DVT predicted PTS in both strata; DVT burden and time from DVT diagnosis to PTS assessment predicted PTS in CVC-related thrombosis. In sum, PTS severity was higher in non–CVC-related vs CVC-related thrombosis. Increasing age at the time of DVT was associated with higher PTS severity. DVT burden and time from DVT diagnosis to PTS assessment were significant PTS predictors in CVC-related thrombosis, indicating that long-term follow-up of these children is important.
Introduction
The occurrence of thrombotic events is increasing in the pediatric population1 as a result of advances in health care, including improved imaging techniques to detect thrombotic events, more aggressive treatments, and increased awareness among clinicians. Deep vein thrombosis (DVT) affecting the upper (UE) or lower extremity (LE) is the most common venous thrombotic event identified in children. Approximately 85% of DVT cases in the extremities develop as a result of the presence of central venous catheters (CVCs),2,3 which is the leading cause and most important single risk factor for thrombotic events in children.4
CVC-related thrombosis differs from non–CVC-related thrombosis in its pathophysiology (eg, the role of CVC mechanical damage to the vascular endothelium, venous stasis, and infections) and characteristics of the patient (eg, age of the affected patient and underlying conditions) at the time of diagnosis.2,3 These differences are expected to affect the management and outcomes of both types of DVT. For example, previous studies have shown that most children with CVC-related thrombosis are treated with anticoagulation alone, whereas those with non–CVC-related thrombosis are more likely to undergo thrombolysis.2,3
Postthrombotic syndrome (PTS) is the most common long-term complication of UE and LE DVT in children.2,3,5 As the frequency of DVT increases in children, the frequency of PTS is also expected to increase. Therefore, studies investigating characteristics, prognostic factors, and the impact of PTS after UE or LE DVT are relevant.
The study of PTS prognostic factors is complex, in part because of the variability in outcome and predictor assessment across studies. For example, 12 pediatric studies investigating predictors of pediatric PTS identified 15 different prognostic factors.2,3,6-15 A recent meta-analysis of these aforementioned pediatric studies identified 3 prognostic factors for the development of PTS: presence of a CVC, degree of venous occlusion at DVT diagnosis, and lack of thrombus resolution after treatment.16 Two of these prognostic factors, presence of CVC and degree of DVT occlusion, are typically known around the time of DVT diagnosis. It remains to be investigated whether these 2 and other early predictors that can be measured around the time of DVT diagnosis, such as adequacy of DVT anticoagulant, could guide management of the index DVT to prevent PTS. Importantly, adequacy of DVT anticoagulation has been reported to be associated with lower risk of PTS in adult patients.17,18
Studies regarding the impact of PTS on the different aspects of children’s health are emerging,15,19-21 and it is critical to continue our work to better understand the consequences of pediatric PTS.
The main objectives of the present study were to investigate differences in patient- and DVT-related characteristics between CVC-related and non–CVC-related thrombosis in children and to study early factors for PTS prediction in children with and without CVC-related thrombosis separately. A secondary objective was to explore the impact of PTS on patient functioning.
Methods
Study design and population
We analyzed the data of patients enrolled in 2 studies22,23 conducted for the development and testing of a clinical index to assess pediatric PTS (Index for the Clinical Assessment of Post-Thrombotic Syndrome in Children [CAPTSure]). In brief, these studies prospectively enrolled consecutive patients age 0 to 18 years seen in the Thrombosis Clinic at The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada, at least 6 months after a Doppler ultrasound–proven UE or LE DVT. Details on the design and inclusion and exclusion criteria of each study are provided in the data supplement. Ethics approval was given by The Hospital for Sick Children and the University of Toronto, and informed consent was obtained before study participation.
Outcomes
The main outcome was PTS, which was measured using CAPTSure. CAPTSure is a valid and reliable tool for the diagnosis and severity rating of pediatric PTS21,23 that assesses PTS symptoms and signs (data supplement). The scoring of each sign and symptom depends on its relative importance. The final CAPTSure score is expressed on a continuous scale that ranges from 0 (which represents no PTS) to 100 points (representing the worst possible PTS). In general, a change in >10 points in the final score is considered to be indicative of a true change in PTS severity.23 A score of 11 to 30.9 points corresponds to mild PTS, and a score ≥31 points corresponds to moderate to severe PTS (data supplement).
As a secondary outcome, the impact of PTS on patient functioning was explored using the Pediatric Outcomes Data Collection Instrument (PODCI).24 PODCI is a tool that measures aspects of health related to the Body Structures and Function, Activities, and Participation components of the International Classification of Functioning, Disability and Health for Children and Youth.25 The tool contains 5 subscales (UE, transfer and basic mobility, sports and physical functioning, pain and comfort, and happiness). The Global Functioning score is estimated from the first 4 subscales. The score ranges from 0 to 100 points; a score of 100 points corresponds to the best possible outcome. Normal values in healthy children are shown in the data supplement.26 PODCI was administered at the time of PTS assessment to parents of children enrolled in study 1 (data supplement).
DVT and patient characteristics
Collected data included age of the patient at the time of DVT, sex of the patient, underlying medical conditions, thrombophilia, triggering event (CVC-related vs non–CVC-related thrombosis) and DVT risk factors in non–CVC-related thrombosis, DVT-affected extremity and laterality, DVT burden, DVT symptoms, time from DVT symptoms or clinical suspicion to DVT treatment, body mass index (BMI) percentile at the time of PTS assessment, and DVT resolution and recurrence.
Thrombophilia was classified as none, minor, and major. Minor thrombophilia included heterozygous factor V Leiden (FVL) or prothrombin gene mutation (PTG) and elevated FVIII or elevated lipoprotein (a) levels documented on at least 2 occasions and at least 12 weeks apart. Major thrombophilia consisted of protein S, protein C, or antithrombin deficiency documented on at least 2 occasions and at least 12 weeks apart, positive anticardiolipin antibodies or lupus anticoagulant27 on at least 2 occasions and at least 12 weeks apart, and/or FVL or PTG homozygous mutation or double-heterozygous FVL/PTG mutation.
CVC-related thrombosis was defined as a venous thrombotic event involving the venous segment or segments in which a catheter was located.28
Burden of the index DVT at diagnosis included degree of occlusion, extension, and localization of the DVT. Degree of occlusion was classified as nonocclusive or occlusive (if ≥1 venous segments were found to be occlusive). Extension of DVT was expressed as the raw number of affected venous segments. Number of venous segments has been shown to highly correlate (Spearman r = 0.89; 95% confidence interval [CI], 0.83-0.93) with extension of vascular bed in the LE.29 DVT localization in the LE was classified as iliofemoral vs femoropopliteal.
Symptoms were defined as the presence of DVT-related UE or LE edema, pain, changes in skin color, temperature or perfusion, or catheter dysfunction, noted at the time of DVT diagnosis and leading to a request for a Doppler ultrasound to rule out DVT.
DVT treatment was defined as the number of days a patient was treated with anticoagulation during the first month after DVT diagnosis, from the time stable therapeutic levels were documented by laboratory monitoring. We focused on the first month of treatment, because we hypothesized that optimal anticoagulant management might be more relevant in the acute and subacute phases of DVT.
BMI percentile at the time of PTS assessment was determined using the World Health Organization growth standards for children age <2 years and the 2000 Centers for Disease Control and Prevention growth reference standards for children age ≥2 years. Following the Centers for Disease Control and Prevention guidelines, BMI percentiles between the 85th and 94th percentiles were classified as overweight and BMI percentiles ≥95th percentile were classified as obese.30
DVT resolution was determined according to follow-up Doppler ultrasound results ∼3 months post-DVT. Resolution was considered incomplete if there was wall thickening or worse.31 If extensions were documented between DVT diagnosis and the 3-month follow-up assessment, events were classified as extended. DVT recurrence was defined as per International Society on Thrombosis and Haemostasis guidelines.32
All of the above variables were compared between children with and without CVC-related thrombosis. For analysis of PTS prediction, independent variables were prespecified and chosen according to their clinical relevance, quality of measurement, and availability at the time of prediction (ie, around the time of diagnosis of the index DVT).33,34 Because patients with CVC-related and non–CVC-related thrombosis were expected to differ in several aspects, analysis of PTS predictors was stratified according to presence or absence of CVC. The stratified analysis also allowed investigation of the effect of age per stratum, which otherwise would not have been possible given that CVC-related thrombosis and age are intrinsically confounded.2 For the CVC-related stratum, early predictors included age of the patient at the time of DVT, DVT signs or symptoms at presentation, DVT burden, and DVT treatment, as described above. Given that the non–CVC-related thrombosis stratum was expected to be smaller, DVT symptoms were not included, because it was expected that most children in this stratum would be symptomatic. In these models, CAPTSure scores were analyzed as a continuous variable. Hypothesized relationships among the predictors included in the model and other known PTS predictors are shown in the data supplement.
Lastly, the association between CAPTSure scores and PODCI Global Functioning and Happiness scores was explored. CAPTSure scores and Global Functioning and Happiness scores were analyzed as continuous variables. The models were adjusted for presence or absence of underlying conditions, which could affect a patient’s activity and participation level.
Data analyses
Data were described using median and 25th to 75th percentile, or percentage, as appropriate. Characteristics of patients with and without CVC-related thrombosis were compared using the Wilcoxon rank sum test or χ2 test, as appropriate. Characteristics of DVT were compared between CVC-related and non–CVC-related thrombosis using generalized estimating equations (GEEs).35-37 GEEs were used to take into account the correlated nature of the data, because some patients had >1 DVT-affected extremity. The exchangeable matrix was used for modeling the correlation structure.37 When clinically relevant, DVT characteristics were compared by both CVC group and affected extremity.
PTS predictors in the 2 models (CVC-related and non–CVC-related thrombosis) were prespecified, as mentioned above. Predictors related to burden of the index DVT (degree of occlusion, localization, and extension) were combined in a weighted summary score for variable reduction33 (data supplement), particularly because the sample size of the non–CVC-related thrombosis stratum was expected to be small. Spike histograms were used to visualize the relationship between age and CAPTSure score to fit this predictor using splines if plots suggested a nonlinear relationship. Models were estimated using GEEs because of their correlated nature. In addition, the outcome was expected to be nonnormally distributed, because at least half the patients were expected to have scores of 0.2,3 In this context, GEEs were also suitable for analysis given their robustness to nonnormally distributed data.38 Missing data on predictors were handled using multiple imputation with predictive mean matching39,40 and 20 imputed data sets.
Lastly, the association between CAPTSure and PODCI Global Functioning scores and between CAPTSure and Happiness scores was explored using GEEs.
Analyses were carried out in R version 4.0.3 using the childsds, growthstandards, Hmisc, mice, and geepack packages.
Results
Between March 2014 and December 2017, 256 patients were recruited. Fifty-seven of these patients had >1 extremity affected by DVT, for a total of 313 DVT-affected extremities (UE, n = 150; LE, n = 163) assessed, 275 (88%) of which were CVC related. A total of 6 non–CVC-related cases of DVT (6 [16%] of 38) were diagnosed during hospital admission. Characteristics of the patients included in the study and of the index DVT in each group are shown in Tables 1 and 2.
Characteristics of included patients and PODCI scores according to group
| . | Patients with CVC-related thrombosis (n = 223) . | Patients with non–CVC-related thrombosis (n = 33) . | P . |
|---|---|---|---|
| Age at time of first DVT, mo | 3.5 (0.7-18.7) | 177.6 (149.6-194.8) | <.001 |
| Female sex | 102 (46) | 23 (70) | .02 |
| Underlying condition | — | ||
| CHD | 107 (48) | 0 (0) | |
| Infection | 24 (11) | 3 (9) | |
| Prematurity | 20 (9) | 0 (0) | |
| Congenital diaphragmatic hernia | 16 (7) | 0 (0) | |
| Cancer | 15 (7) | 5 (15) | |
| Organ transplantation | 13 (6) | 1 (3) | |
| Postsurgery | 10 (4) | 0 (0) | |
| Intestinal failure | 4 (2) | 0 (0) | |
| None | 0 (0) | 17 (52) | |
| Inflammation (IBD/lupus) | 0 (0) | 6 (18) | |
| Other | 14 (6) | 1 (3) | |
| Thrombophilia* | <.001 | ||
| None | 122 (78) | 12 (36) | |
| Minor | 28 (18) | 7 (21) | |
| Major | 6 (4) | 14 (43) | |
| PODCI Global Functioning score† | 94 (87-98) | 97 (92-99) | .4 |
| PODCI Happiness score‡ | 95 (81-100) | 91 (75-100) | .6 |
| . | Patients with CVC-related thrombosis (n = 223) . | Patients with non–CVC-related thrombosis (n = 33) . | P . |
|---|---|---|---|
| Age at time of first DVT, mo | 3.5 (0.7-18.7) | 177.6 (149.6-194.8) | <.001 |
| Female sex | 102 (46) | 23 (70) | .02 |
| Underlying condition | — | ||
| CHD | 107 (48) | 0 (0) | |
| Infection | 24 (11) | 3 (9) | |
| Prematurity | 20 (9) | 0 (0) | |
| Congenital diaphragmatic hernia | 16 (7) | 0 (0) | |
| Cancer | 15 (7) | 5 (15) | |
| Organ transplantation | 13 (6) | 1 (3) | |
| Postsurgery | 10 (4) | 0 (0) | |
| Intestinal failure | 4 (2) | 0 (0) | |
| None | 0 (0) | 17 (52) | |
| Inflammation (IBD/lupus) | 0 (0) | 6 (18) | |
| Other | 14 (6) | 1 (3) | |
| Thrombophilia* | <.001 | ||
| None | 122 (78) | 12 (36) | |
| Minor | 28 (18) | 7 (21) | |
| Major | 6 (4) | 14 (43) | |
| PODCI Global Functioning score† | 94 (87-98) | 97 (92-99) | .4 |
| PODCI Happiness score‡ | 95 (81-100) | 91 (75-100) | .6 |
Data are presented as median (25th-75th percentile) or n (%).
CHD, congenital heart disease; IBD, inflammatory bowel disease.
156 (70%) of 223 patients tested in CVC stratum, and 33 (100%) of 33 tested in non-CVC stratum.
Scores available in 97 patients in CVC stratum and 17 patients in non-CVC stratum.
Scores available in 90 patients in CVC stratum and 18 patients in non-CVC stratum.
Characteristics of index DVT and PTS scores according to group
| . | CVC-related thrombosis (n = 275) . | Non–CVC-related thrombosis (n = 38) . | P . |
|---|---|---|---|
| Extremity | .10* | ||
| Upper | 137 (50) | 13 (34) | |
| Lower | 138 (50) | 25 (66) | |
| Left side | .02* | ||
| Upper | 41 (30) | 6 (46) | |
| Lower | 53 (38) | 16 (64) | |
| No. of segments affected | 2 (1-2) | 3 (2-5) | <.001 |
| ≥1 completely occlusive segments at diagnosis | .03* | ||
| Upper | 76 (55) | 11 (95) | |
| Lower | 110 (80) | 23 (92) | |
| Edema in extremity | .004* | ||
| Upper | 41 (30) | 8 (63) | |
| Lower | 83 (60) | 21 (84) | |
| Pain in the extremity, n (%) | <.001* | ||
| Upper | 7 (5) | 6 (46) | |
| Lower | 6 (4) | 18 (72) | |
| Changes in skin color, temperature, or perfusion | .09* | ||
| Upper | 13 (9) | 0 (0) | |
| Lower | 41 (30) | 3 (12) | |
| Time from clinical suspicion or symptoms to DVT diagnosis, d | <.001* | ||
| Upper | 0 (0-0) | 0 (0-1) | |
| Lower | 0 (0-1) | 3 (2-6) | |
| CVC dysfunction | — | ||
| Upper | 13 (9) | — | |
| Lower | 3 (2) | — | |
| Treatment | <.001 | ||
| None | 43 (16) | 2 (5) | |
| Anticoagulation only | 232 (84) | 20 (53) | |
| Anticoagulation plus thrombolysis | 0 (0) | 16 (42) | |
| Length of treatment, mo | 3 (3-3) | 12 (3-26) | <.001 |
| No. of days on therapeutic anticoagulation in first month post-DVT | 27 (19-29) | 28 (27-29) | .03 |
| BMI percentile at time of PTS assessment | <.001 | ||
| Upper | 52.9 (17.5-80.4) | 80.5 (57.1-91.0) | |
| Lower | 52.1 (17.9-83.9) | 76.5 (63.2-96.9) | |
| CAPTSure score | .002* | ||
| Upper | 0.0 (0.0-9.1) | 13.0 (5.2-29.3) | |
| Lower | 3.6 (0.0-13.2) | 10.1 (3.8-32.0) | |
| Complete DVT resolution at 3 mo | 42 (17) | 7 (21) | .65 |
| Time of DVT resolution assessment, d | 88 (48-94) | 90 (30-95) | .99 |
| DVT recurrence in same DVT-affected extremity | 6 (2) | 3 (8) | .03 |
| Time to recurrence, d | 617 (390-903) | 94 (68-522) | .19 |
| . | CVC-related thrombosis (n = 275) . | Non–CVC-related thrombosis (n = 38) . | P . |
|---|---|---|---|
| Extremity | .10* | ||
| Upper | 137 (50) | 13 (34) | |
| Lower | 138 (50) | 25 (66) | |
| Left side | .02* | ||
| Upper | 41 (30) | 6 (46) | |
| Lower | 53 (38) | 16 (64) | |
| No. of segments affected | 2 (1-2) | 3 (2-5) | <.001 |
| ≥1 completely occlusive segments at diagnosis | .03* | ||
| Upper | 76 (55) | 11 (95) | |
| Lower | 110 (80) | 23 (92) | |
| Edema in extremity | .004* | ||
| Upper | 41 (30) | 8 (63) | |
| Lower | 83 (60) | 21 (84) | |
| Pain in the extremity, n (%) | <.001* | ||
| Upper | 7 (5) | 6 (46) | |
| Lower | 6 (4) | 18 (72) | |
| Changes in skin color, temperature, or perfusion | .09* | ||
| Upper | 13 (9) | 0 (0) | |
| Lower | 41 (30) | 3 (12) | |
| Time from clinical suspicion or symptoms to DVT diagnosis, d | <.001* | ||
| Upper | 0 (0-0) | 0 (0-1) | |
| Lower | 0 (0-1) | 3 (2-6) | |
| CVC dysfunction | — | ||
| Upper | 13 (9) | — | |
| Lower | 3 (2) | — | |
| Treatment | <.001 | ||
| None | 43 (16) | 2 (5) | |
| Anticoagulation only | 232 (84) | 20 (53) | |
| Anticoagulation plus thrombolysis | 0 (0) | 16 (42) | |
| Length of treatment, mo | 3 (3-3) | 12 (3-26) | <.001 |
| No. of days on therapeutic anticoagulation in first month post-DVT | 27 (19-29) | 28 (27-29) | .03 |
| BMI percentile at time of PTS assessment | <.001 | ||
| Upper | 52.9 (17.5-80.4) | 80.5 (57.1-91.0) | |
| Lower | 52.1 (17.9-83.9) | 76.5 (63.2-96.9) | |
| CAPTSure score | .002* | ||
| Upper | 0.0 (0.0-9.1) | 13.0 (5.2-29.3) | |
| Lower | 3.6 (0.0-13.2) | 10.1 (3.8-32.0) | |
| Complete DVT resolution at 3 mo | 42 (17) | 7 (21) | .65 |
| Time of DVT resolution assessment, d | 88 (48-94) | 90 (30-95) | .99 |
| DVT recurrence in same DVT-affected extremity | 6 (2) | 3 (8) | .03 |
| Time to recurrence, d | 617 (390-903) | 94 (68-522) | .19 |
Data are presented as median (25th-75th percentile) or n (%).
P value for CVC yes/no variable (see Table 3 for more details).
As shown in Table 1, patients with CVC-related thrombosis were younger than patients with non–CVC-related thrombosis at the time of their first event, with a slight predominance of male sex and congenital heart disease as an underlying condition. Thrombophilia was more common in children with non–CVC-related thrombosis. At the time of PTS assessment, 13% of children in the CVC-related thrombosis stratum (28 of 223) were overweight, and 13% (29 of 223) were obese. In the non–CVC-related thrombosis stratum, 9% of children (3 of 33) were overweight, and 30% (10 of 33) were obese. DVT risk factors in non–CVC-related thrombosis included anatomic variants in 12 patients (12 [36%] of 33), major thrombophilia in 6 patients (6 [18%] of 33), oral contraceptives in 5 female patients (5 [15%] of 33), systemic lupus erythematosus in 3 patients (3 [9%] of 33), and local infections in 3 patients (3 [9%] of 33).
When considering thrombotic events as the unit of analysis, 83% of CVC-related LE DVT cases (115 of 138) and 84% of non–CVC-related LE DVT cases (21 of 25) affected the iliofemoral segments. CVC-related thrombotic events had a lower thrombus burden, affecting fewer segments and being occlusive less frequently than non–CVC-related thrombotic events, as shown in Table 2.
A total of 169 (61%) of 275 CVC-related thrombotic events (UE, n = 61; LE, n = 108) and 31 (82%) of 38 non–CVC-related thrombotic events (UE, n = 9; LE, n = 22) had ≥1 symptoms at the time of DVT. Pain and edema were more frequent in the non–CVC-related thrombosis stratum, whereas changes in skin color, temperature, or perfusion were more common in the CVC-related thrombosis stratum. Treatment strategies differed between strata; thrombolysis was not used to treat any of the CVC-related thrombotic events.
Median time to PTS assessment was 58 months (25th-75th percentile, 35-107 months) for the CVC-related thrombosis stratum and 23 months (25th-75th percentile, 16.9-40.7 months) for the non–CVC-related thrombosis stratum. CAPTSure scores per stratum are shown in Table 2. Mild PTS (CAPTSure score between 11 and 30.9 points) was found in 18% (50 of 275) of CVC-related thrombotic events and in 26% (10 of 38) of non–CVC-related thrombotic events. Moderate to severe PTS (CAPTSure score ≥31 points) was found in 8% (23 of 275) of CVC-related thrombotic events and in 24% (9 of 38) of non–CVC-related thrombotic events. Considering patients as the unit of analysis, a total of 64 children (64 [29%] of 223) with CVC-related thrombosis and 18 (18 [55%] of 33) with non–CVC-related thrombosis had ≥1 extremities with CAPTSure scores >10 points.
Thrombus resolution was observed in 1 in 5 patients within 3 months, regardless of whether the thrombotic event was CVC related or non–CVC related. Nine local recurrent thrombotic events were documented in 8 patients, including 6 events in 5 children with CVC-related thrombosis and 3 events in 3 children with non–CVC-related thrombosis. The rate of DVT recurrence was higher among patients affected by non–CVC-related thrombosis than in patients with CVC-related thrombosis (0.04 vs 0.004 per patient-year, respectively).
Table 3 shows differences in DVT characteristics and in CAPTSure scores according to DVT pathophysiology (CVC-related vs non–CVC-related thrombosis) and affected limb (UE vs LE). Degree of occlusion of the index DVT, edema, and changes in skin color, temperature, or perfusion in the extremity at DVT presentation also differed between UE and LE (adjusted for CVC status).
Bivariable analysis of DVT characteristics and PTS CAPTSure scores by CVC status and affected extremity
| Variable . | Estimate (95% CI) . | P . |
|---|---|---|
| ≥1 completely occlusive segments at diagnosis | ||
| Non–CVC- vs CVC-related thrombosis | 3.4 (1.1-10.3)* | .03 |
| LE vs UE | 3.2 (1.9-5.5)* | <.001 |
| Edema in extremity | ||
| Non–CVC- vs CVC-related thrombosis | 3.5 (1.5-8.2)* | .004 |
| LE vs UE | 3.2 (2.0-5.2)* | <.001 |
| Changes in skin color, temperature, or perfusion | ||
| Non–CVC- vs CVC-related thrombosis | 0.3 (0.1-1.2)* | .09 |
| LE vs UE | 4.1 (2.1-8.3)* | <.001 |
| CVC dysfunction | ||
| LE vs UE | 0.2 (0.1-0.9)* | .03 |
| Pain in extremity | ||
| Non–CVC- vs CVC-related thrombosis | 32.2 (13.1-79.2)* | <.001 |
| LE vs UE | 1.4 (0.6-3.4)* | .43 |
| CAPTSure score | ||
| Non–CVC- vs CVC-related thrombosis | 9.2 (3.4-15.1)† | .002 |
| LE vs UE | 1.8 (−0.9 to 4.4)† | .19 |
| BMI percentile at time of PTS assessment | ||
| Non–CVC- vs CVC-related thrombosis | 19.2 (8.9-29.5)† | <.001 |
| LE vs UE | 1.4 (–0.5 to 3.2)† | .14 |
| Variable . | Estimate (95% CI) . | P . |
|---|---|---|
| ≥1 completely occlusive segments at diagnosis | ||
| Non–CVC- vs CVC-related thrombosis | 3.4 (1.1-10.3)* | .03 |
| LE vs UE | 3.2 (1.9-5.5)* | <.001 |
| Edema in extremity | ||
| Non–CVC- vs CVC-related thrombosis | 3.5 (1.5-8.2)* | .004 |
| LE vs UE | 3.2 (2.0-5.2)* | <.001 |
| Changes in skin color, temperature, or perfusion | ||
| Non–CVC- vs CVC-related thrombosis | 0.3 (0.1-1.2)* | .09 |
| LE vs UE | 4.1 (2.1-8.3)* | <.001 |
| CVC dysfunction | ||
| LE vs UE | 0.2 (0.1-0.9)* | .03 |
| Pain in extremity | ||
| Non–CVC- vs CVC-related thrombosis | 32.2 (13.1-79.2)* | <.001 |
| LE vs UE | 1.4 (0.6-3.4)* | .43 |
| CAPTSure score | ||
| Non–CVC- vs CVC-related thrombosis | 9.2 (3.4-15.1)† | .002 |
| LE vs UE | 1.8 (−0.9 to 4.4)† | .19 |
| BMI percentile at time of PTS assessment | ||
| Non–CVC- vs CVC-related thrombosis | 19.2 (8.9-29.5)† | <.001 |
| LE vs UE | 1.4 (–0.5 to 3.2)† | .14 |
OR, odds ratio. * Odds ratios; † coefficient estimates
The results of the regression model investigating early PTS predictors for the CVC-related and non–CVC-related thrombosis strata are shown in Table 4. Age at the time of DVT was a consistent predictor of PTS in both strata. Days on therapeutic anticoagulation within the first month after DVT did not predict PTS.
Regression models for early predictors of PTS per stratum
| . | Coefficient estimate (95% CI) . | P . |
|---|---|---|
| CVC-related thrombosis stratum | ||
| Age at DVT diagnosis (for every y) | 0.6 (0.1-1.2) | .02 |
| N of d on therapeutic anticoagulation in first month post-DVT | 0.0 (−0.1 to 0.1) | .44 |
| Presence of symptoms | 0.3 (−2.5 to 3.1) | .84 |
| Time from DVT diagnosis to PTS assessment (for every y) | 0.9 (0.4-1.3) | <.001 |
| DVT burden (for every point)* | 0.7 (0.1-1.4) | .05 |
| Non–CVC-related thrombosis stratum | ||
| Age at DVT diagnosis (for every y) | 1.7 (1.0-2.4) | <.001 |
| N of d on therapeutic anticoagulation in first month post-DVT | −0.2 (−0.6 to 0.2) | .26 |
| Time from DVT diagnosis to PTS assessment (for every y) | 0.9 (−1.2 to 2.9) | .41 |
| DVT burden (for every point)* | 3.6 (−1.9 to 9.1) | .21 |
| . | Coefficient estimate (95% CI) . | P . |
|---|---|---|
| CVC-related thrombosis stratum | ||
| Age at DVT diagnosis (for every y) | 0.6 (0.1-1.2) | .02 |
| N of d on therapeutic anticoagulation in first month post-DVT | 0.0 (−0.1 to 0.1) | .44 |
| Presence of symptoms | 0.3 (−2.5 to 3.1) | .84 |
| Time from DVT diagnosis to PTS assessment (for every y) | 0.9 (0.4-1.3) | <.001 |
| DVT burden (for every point)* | 0.7 (0.1-1.4) | .05 |
| Non–CVC-related thrombosis stratum | ||
| Age at DVT diagnosis (for every y) | 1.7 (1.0-2.4) | <.001 |
| N of d on therapeutic anticoagulation in first month post-DVT | −0.2 (−0.6 to 0.2) | .26 |
| Time from DVT diagnosis to PTS assessment (for every y) | 0.9 (−1.2 to 2.9) | .41 |
| DVT burden (for every point)* | 3.6 (−1.9 to 9.1) | .21 |
DVT burden score (see data supplement for details): occlusive DVT (yes = 2) + iliofemoral DVT (yes = 1) + n of affected venous segments (maximum score of 1 if entire UE or LE venous territory affected). Maximum score, 5 points.
Regarding the analysis of the impact of PTS on functioning as measured by PODCI, the results of the models showed that for every point increase in CAPTSure score, adjusted for the presence or absence of underlying conditions, PODCI Global Functioning scores decreased by 0.2 points (95% CI, −0.3 to −0.1; P = .001) and Happiness scores decreased by 0.3 points (95% CI, −0.6 to 0.0; P = .04).
Discussion
The main goals of the present study were to investigate patient-related and DVT-related differences between CVC-related and non–CVC-related thrombosis and determine PTS predictors in children with UE and LE DVT. In a sample including 256 children followed up at our institution, we found that children with non–CVC-related thrombosis were older at the time of their first DVT than children with CVC-related thrombosis. There was higher DVT burden, more DVT symptoms, higher PTS severity, and higher frequency of overweight/obesity in non–CVC-related thrombosis compared with CVC-related thrombosis. In terms of PTS predictors, age at the time of DVT predicted PTS in the CVC-related and non–CVC-related thrombosis strata. Time from DVT diagnosis to PTS assessment and DVT burden predicted PTS in the CVC-related thrombosis stratum.
CVC-related vs non–CVC-related thrombosis
Nearly 90% of the enrolled children had CVC-related thrombosis and, as expected,2,3 the children in this group were younger than those in the non–CVC-related thrombosis group at the time of DVT. We found a higher DVT burden in non–CVC-related thrombotic events, which can be explained by the underlying mechanism of DVT. Whereas mechanical factors are central to the development of CVC-related thrombosis, non–CVC-related events may be accompanied by a more marked multifactorial systemic hypercoagulable state, in addition to the presence of other underlying factors such as local compression and anatomic variations.41,42
The 39% frequency of overweight/obesity at the time of PTS assessment observed among patients with non–CVC-related thrombosis is higher than the 27% prevalence of overweight/obesity seen among Canadian children.43 Moreover, the 30% frequency of obesity seen in this group is more than twofold the estimated 13% frequency of obesity among Canadian children.43 Although high BMI is an established risk factor for PTS in the adult population,18,31,44-48 the fact that BMI percentile was measured at the time of PTS assessment in our study does not allow for studying the predictive role of BMI percentile in PTS severity. It must be pointed out that high BMI percentile could reflect a risk factor for DVT among children with non–CVC-related thrombosis. Data from a study conducted in the United States that enrolled children 3 months after pulmonary embolism and/or DVT showed a 57% prevalence of obesity/overweight among patients age 7 to 21 years screened for study participation at this early time point.49 The estimate is higher than the 41.5% reported prevalence of overweight, obesity, and severe obesity in children and adolescents in the United States in 2017 to 2018.50
The more symptomatic clinical presentation of non–CVC-related thrombosis compared with CVC-related thrombosis could be explained by higher thrombus burden and by the age of the patient, because older children are likely able to report pain or swelling in the UE or LE more effectively than younger patients. Indeed, research has shown that cognitive ability to self-report pain develops in the preschool years and that children are able to provide meaningful self-report of pain intensity by the age of 5 years.51-53
In line with previous reports using the Modified Villalta Scale (MVS) for PTS assessment, the frequency of PTS of any severity was higher in children with non–CVC-related thrombosis,2,3 with more than half of these patients showing a PTS CAPTSure score >10 compared with children with CVC-related thrombosis. The higher frequency of PTS among children with non–CVC-related thrombosis may be explained by the central role of inflammation in PTS.54-56 Non–CVC-related thrombosis is associated with different pathophysiologic mechanisms possibly including hypercoagulable states, which in turn may be accompanied by a distinct inflammatory process and more severe PTS compared with CVC-related thrombosis.
Early predictors of PTS
The stratified analysis carried out in the present study allowed investigation of the impact of age at DVT on the occurrence of PTS in the CVC-related and non–CVC-related thrombosis strata.
Age at the time of DVT diagnosis.
In line with studies in adults,46,57,58 increasing age at the time of DVT was associated with higher CAPTSure scores in both strata, regardless of DVT mechanism. The effect of age on PTS severity could be due to developmental changes in inflammatory response with aging, but research in this relatively new and very complex area is scarce.59,60 For example, although studies on inflammatory biomarkers in adult patients suggest that high interleukin-6 and intracellular adhesion molecule-1 levels may predict PTS,54,61,62 levels of these and other biomarkers change dynamically over time in children,63-65 making any research and inferences related to their role in pediatric DVT and PTS extremely challenging.
Therapeutic anticoagulation post-DVT.
We investigated the role of anticoagulation management in the first month post-DVT as a potential early predictor of PTS that could guide management of the index DVT. To minimize the impact of confounding by indication, given the observational study design, factors that could influence therapy management (eg, DVT mechanism, DVT burden, and presence of symptoms) were considered during the analysis. Studies in adults suggest that optimal anticoagulant management early on after DVT diagnosis,17,18 but not length of therapy,66,67 is associated with lower risk of PTS. Pediatric studies have already suggested that length of therapy is not associated with PTS risk.2,3 One of the potential mechanisms for the effect of anticoagulation on PTS risk comprises the anti-inflammatory properties of low molecular weight heparin,68 which could explain the higher benefit of heparinoids compared with warfarin for PTS prevention in adults.69 Such an anti-inflammatory effect would be particularly important within the first few weeks after DVT in view of the mounting evidence suggesting that the marked inflammatory response seen in early phases post-DVT plays an important role in subsequent PTS development.70-73 However, days spent on documented therapeutic anticoagulation (largely low molecular weight heparin) within the first month post-DVT did not show a significant effect on CAPTSure scores in this study. Another proposed mechanism for the preventive role of anticoagulation in PTS is through prevention of subclinical DVT recurrence.68 This hypothesis is supported by the reported higher risk of PTS in adult patients with poor international normalized ratio control in the first months post-DVT.17,18 However, recurrence in adults has been estimated at 3.3 events per patient-year in the presence of transient provoking factors and 7.4 events per patient-year in the absence of such factors. These rates are 180 to 800 times higher than the recurrence rates documented in the present study, suggesting that prevention of subclinical DVT recurrence may not be a critical aspect of PTS occurrence in pediatrics.
DVT signs and symptoms.
We found no evidence of an effect of DVT signs and symptoms on subsequent PTS severity in the CVC-related thrombosis stratum. Two observational pediatric studies prospectively screened children (median age, 0-2 years) with a newly placed CVC for DVT occurrence, and reported the outcomes of symptomatic and asymptomatic thrombotic events in these young patients. Sol et al74 studied 134 children admitted to the pediatric intensive care unit who were screened for symptomatic CVC-related femoral DVT. Whereas 5 (50%) of 10 survivors with symptomatic DVT developed mild PTS, none of the 3 children with asymptomatic DVT had PTS-related clinical findings at 2 years of follow-up. Similarly, Jones et al75 investigated 189 children admitted to the pediatric intensive care unit who had a CVC placed in either the jugular (82%) or femoral vein (18%). At 2 years, 7 (21%) of the 33 children with documented DVT had PTS. Two of these children had an MVS score of 3 points and clinically significant PTS as per the Manco-Johnson instrument; 1 of these 2 patients was symptomatic and the other was asymptomatic at the time of DVT. Thus, although PTS is more commonly seen in symptomatic CVC-related thrombosis, PTS can occur and be clinically significant in asymptomatic patients, even at a relatively early follow-up. As Sol et al point out, the follow-up period is relevant, and PTS may be recognized at later stages. For example, Kuhle et al8 reported the long-term follow-up of 13 patients found to have asymptomatic UE DVT during the PARKAA (Prophylaxis With Antithrombin Replacement in Kids With ALL Treated With L-Asparaginase) study. At 7 years of follow-up, 7 (54%) of 13 patients were found to have PTS. One of these 7 children had moderate PTS with pain during writing. The case would be considered clinically significant or important PTS whether using the Manco-Johnson Instrument, because of pain limiting activities, or CAPTSure, which assigns the highest relative importance to pain and low endurance.
Time from DVT diagnosis to PTS assessment.
Our study also showed that time from DVT diagnosis to PTS assessment was associated with a significant increase in CAPTSure scores over time (0.9 points per year) in the CVC-related thrombosis stratum. This is in line with retrospective pediatric studies reporting that it could take 5 to 10 years to document clinical findings of PTS in children with CVC-related thrombosis.2,3,76 The impact of time on PTS severity is likely related to the maturation of the cognitive capacity of children to conceptualize and report signs and symptoms of PTS as they grow older. Previous work found a very modest increase in MVS scores over time in LE DVT (0.05 points per year). However, the final score of the MVS is likely affected by floor effects,77 because the MVS only records 2 symptoms and relies on parental report. In contrast, CAPTSure allows the measurement of self- and proxy-reported symptoms of PTS in children. Importantly, testing of CAPTSure has shown that reliability of child self-reported symptoms is excellent, whereas reliability of proxy-reported symptoms is only moderate.23 This suggests that asking children about their symptoms is not only a reliable strategy, but also a critical aspect of PTS assessment and long-term follow-up. Furthermore, CAPTSure was shown to be reliable even when applied by nonclinicians. Therefore, nonspecialists can use the tool for PTS screening in their practices and refer patients to specialists if the CAPTSure score is >10 points. We found no significant impact of time on PTS severity in the non–CVC-related thrombosis stratum. This could be due to the relatively short follow-up time, but it could also be a result of the more mature age of the children in this group, which minimizes the influence of cognitive development, as mentioned.
PTS impact
Our study showed that for every 10-point increase in CAPTSure score, PODCI Global Functioning score decreased by 2 points and Happiness score decreased by 3 points, independently of the presence or absence of underlying conditions. Therefore, higher PTS severity was associated with lower overall functioning, as perceived by parents and caregivers. In a previous study, PTS was shown to be associated with parental dissatisfaction with the "blood clot condition" of their child. The study showed that for every 10-point increase in CAPTSure score, the odds of parental dissatisfaction increased by 75% in children with UE DVT and by 92% in children with LE DVT.21
There are limitations to this study. The small sample size of the non–CVC-related thrombosis stratum did not allow us to fit a more complex model. However, the variables included in the model allowed for the exploration of potentially relevant early predictors. In addition, height and weight were not typically available at the time of DVT, and therefore, the role of BMI as an early predictor of PTS could not be explored. Lastly, when analyzing the role of anticoagulation for PTS prevention, it must be acknowledged that there are other potential factors beyond symptoms, DVT burden, age at DVT diagnosis, and mechanism of DVT that could guide DVT therapy, and it is not possible to determine how subjective and intangible factors that affect prognosis, and on which clinicians heavily rely,78 could guide early DVT management. Therefore, confounding by indication, deemed the intractable bias of observational studies,79 cannot be ruled out.
Clinical practice strives to better outcomes in children affected by DVT. Therefore, research on prognostic factors to determine the risk of adverse outcomes, such as PTS, is pertinent. Our study shows that there are substantial differences between CVC-related and non–CVC-related thrombosis, including patient age, DVT burden, DVT symptoms, and PTS severity. Obesity is a health problem that needs to be addressed, particularly among children with non–CVC-related thrombosis. Children both with and without symptomatic CVC-related thrombosis can develop PTS. Children with non–CVC-related thrombosis have higher PTS severity than children with CVC-related thrombosis. Increasing age at the time of DVT predicted higher PTS severity, regardless of the pathophysiologic mechanisms of the underlying DVT. Among children with CVC-related thrombosis, DVT burden and time from DVT diagnosis to PTS assessment are relevant. The latter indicates that long-term follow-up of these children is important. Lastly, higher PTS CAPTSure scores were associated with lower functioning and happiness scores. Information in all the above aspects would allow better structuring for PTS follow-up and improve the clinical care of children who experience UE or LE DVT.
Acknowledgments
The authors thank Madeline Montoya and Celeste Lumia for their help conducting the study.
The study was funded in part by a health research grant from the Physicians’ Services Incorporated Foundation.
Authorship
Contribution: L.A. designed the study, assessed patients, entered and analyzed data, and wrote the manuscript; N.A. and R.D. entered and checked data for accuracy and reviewed the manuscript; J.V. and S.W. assessed patients and reviewed the manuscript; E.P. analyzed the data and wrote the manuscript; and L.R.B. assessed patients and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laura Avila, Division of Hematology-Oncology, The Hospital for Sick Children, Toronto, ON, M5G 1X8, Canada; e-mail: laura.avila@sickkids.ca.
References
Author notes
For data sharing, contact the corresponding author at laura.avila@sickkids.ca.
The full-text version of this article contains a data supplement.