Key Points
The outcome of patients with limited-stage NLPHL is excellent with 5-year PFS and OS >90%.
Patients with a negative PET2 scan have a 5-year PFS of 92%, and ABVD alone is a viable option in this population.
Abstract
Radiotherapy (RT) is typically incorporated into the treatment of limited-stage nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), although it remains unknown whether chemotherapy alone may be suitable in select patients. We evaluated outcomes of limited-stage NLPHL at BC Cancer on the basis of era-specific guidelines: routine RT era, 1995 to 2005 (n = 36), combined modality with 2 cycles of doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) chemotherapy followed by RT or RT alone; positron emission tomography (PET) era, after 2005 (n = 63), ABVD alone (4 cycles) if the PET scan after the second cycle of ABVD (PET2) is negative, or treatment is changed to RT if PET2 is positive. Median age of patients was 38 years (range, 16-82 years), 73% were male, and 43% had stage II. With a median follow-up of 10.5 years for all patients, 5-year progression-free survival (PFS) was 93% and was 97% for overall survival (OS), with no difference by treatment era (PFS, P = .13; OS, P = .35). For the 49 patients who had a PET2 scan, 86% were PET negative and 14% were PET positive by Deauville criteria with 5-year PFS rates of 92% and 80% (P = .70), respectively. This is the largest study of a PET-adapted approach in NLPHL and supports that ABVD alone may be a viable option in select patients with a negative PET2 scan, with consideration of acute and long-term toxicities.
Introduction
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare subtype of Hodgkin lymphoma (HL), distinguished from classic HL (cHL) by pathognomonic lymphocyte-predominant cells that are CD20+ but lack CD15 and CD30.1 Although the clinical course is generally favorable, late and occasionally multiple recurrences do occur, and there is an inherent higher rate of transformation with aggressive lymphomas.2-4
Because of the rarity of the disease and the resultant lack of prospective studies, management approaches vary across institutions and age groups, which reflect differences in treatment philosophies. In limited-stage disease, first-line strategies range from radiotherapy (RT) alone to combined modality therapy (CMT) and chemotherapy alone, with the latter used most commonly in pediatric patients.5,6 Historically, treatment approaches and regimens have often closely resembled that of cHL, but with mounting evidence of biological and clinical differences, the European Society of Medical Oncology and the National Comprehensive Cancer Network have recently established separate management guidelines for NLPHL.7,8 Noncurative approaches such as observation9,10 or rituximab monotherapy11,12 have also been explored, particularly given the excellent overall survival (OS) and the fact that patients rarely die as a result of NLPHL.
In British Columbia, past treatment guidelines in limited-stage NLPHL largely mirrored those of cHL. This study evaluates the outcome of patients with limited-stage NLPHL and the impact of a positron emission tomography (PET)–adapted approach.
Methods
Patient selection and treatment guidelines
Patients age 16 years or older diagnosed with limited-stage NLPHL from January 1995 to January 2019 were identified in the BC Cancer Lymphoid Cancer Database. All diagnoses were confirmed by central pathology review according to World Health Organization diagnostic criteria,1 and staging was performed by using computed tomography (CT). Although a centralized PET scan has been available since 2005 to assess response (“PET era”), the use of PET scans for staging purposes became routine in British Columbia only in 2011. Before that time, a staging PET scan was performed on a patient-by-patient basis. There were no staging PET scans performed in the RT era. Limited-stage was defined as stage IA (nonbulky lymph node regions <10 cm), IB (from 2001 onward), and IIA if the region could be encompassed within a reasonable radiation field.
Treatment approaches followed era-specific provincial guidelines for limited-stage HL at BC Cancer. RT, if used, consisted of involved field RT (IFRT) from 1995 to 2001 and involved nodal RT (INRT) since 2001.13,14 In the routine RT era (1995-2005), 2 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by RT was recommended for all patients. Select patients with stage IA high neck node disease received RT only. From 2005 onward (PET era), patients received 2 cycles of ABVD followed by interim PET scanning after the second cycle of ABVD (PET2). If the PET scan was negative, 2 more cycles of ABVD were given (AVD [ie, ABVD with bleomycin omitted] after 2016, similar to the approach taken in the RATHL trial for advanced-stage cHL15 ). Standard dosing of RT was 30 Gy in 17 fractions; if RT was administered for a positive PET scan with an INRT field that included all original sites of disease, the dose was 35 Gy in 20 fractions. PET scans were performed and centrally reviewed at BC Cancer, Vancouver Cancer Centre. Until 2014, scans were interpreted by using the International Harmonization Project (IHP) criteria.16 After 2014, interpretation followed the 5-point Deauville (D) scale. Similar to BC Cancer guidelines for limited-stage cHL14 and other early-stage studies,17 an interim PET2 scan was considered negative if the score was D1-2 or DX and positive if the score was D3-5. As a secondary analysis, PET scans performed in the earlier time period were re-reviewed according to Deauville criteria, blinded by outcome (R.P.T.).
After treatment completion, patients were observed clinically, and routine CT and/or PET scans were not performed according to our general lymphoma surveillance guidelines (www.bccancer.bc.ca). This study was approved by the University of British Columbia and the BC Cancer Research Ethics Board and performed in accordance with the Declaration of Helsinki.
Statistical analysis
OS was calculated from the date of diagnosis to the date of last follow-up or death as a result of any cause. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of last follow-up, lymphoma relapse, disease progression, or death as a result of any cause. Lymphoma relapse or progression included the development of aggressive B-cell lymphoma. The χ2 test was used to compare baseline clinical features between patient groups. Survival end points were estimated using the Kaplan-Meier method, and survival comparisons were made with the log-rank test. All statistical analyses were performed using SPSS v14.0.
Results
Patient characteristics and treatment strategies
In total, 99 patients were identified; 36 were managed in the routine RT era and 63 were managed in the PET era. Overall, slightly more than half the patients (50 of 99) were included in our previous study.18 For all patients, the median age was 38 years (range, 16-82 years), 72 patients (73%) were male, and the majority had supradiaphragmatic disease (86%) with a maximum number of 2 nodal regions involved (Table 1). Females were more likely to be older at diagnosis (median age, 50 years for females and 34.5 years for males) (age 40 years or older: 74% female vs 39% male; P = .002). Forty-three patients (43%) had stage II disease and for all patients, the median largest mass size was 4 cm (range, 2-9 cm). Patient characteristics did not differ between treatment eras (Table 1).
Baseline clinical characteristics at diagnosis by treatment era
| Characteristic . | All patients (N = 99) . | Routine RT era (n = 36) . | PET era* (n = 63) . | P . |
|---|---|---|---|---|
| Male sex | 72 (73) | 24 (67) | 48 (76) | .31 |
| Age, y | .85 | |||
| ≥40 | 48 (48) | 17 (47) | 31 (49) | |
| Median (range) | 38 (16-82) | 38 (17-82) | 39 (16-76) | |
| Stage | .57 | |||
| I | 56 (57) | 19 (53) | 37 (59) | |
| II | 43 (43) | 17 (47) | 26 (41) | |
| Disease location | .96 | |||
| Supradiaphragmatic | 85 (86) | 31 (86) | 54 (86) | |
| Infradiaphragmatic | 14 (14) | 5 (14) | 9 (14) | |
| Disease sites | ||||
| Cervical | 42 (42) | 16 (44) | 26 (41) | .76 |
| Axillary | 45 (45) | 17 (47) | 28 (44) | .79 |
| Mediastinal | 2 (2) | 2 (6) | 0 (0) | .06 |
| Intra-abdominal | 2 (2) | 0 (0) | 2 (3) | .28 |
| Pelvic | 3 (3) | 2 (6) | 1 (2) | .27 |
| Inguinal | 11 (11) | 4 (11) | 7 (11) | 1.0 |
| Performance status >1 | 4 (4) | 1 (3) | 3 (5) | .62 |
| B symptoms | 3 (3) | 0 (0) | 3 (5) | .18 |
| Extranodal involvement | 1 (1) | 1 (3) | 0 (0) | .19 |
| Mass, cm | .58 | |||
| ≥5 | 32 (33) | 13 (36) | 19 (31) | |
| Median (range) | 4 (2-9) | 4 (2-9) | 3 (2-9) |
| Characteristic . | All patients (N = 99) . | Routine RT era (n = 36) . | PET era* (n = 63) . | P . |
|---|---|---|---|---|
| Male sex | 72 (73) | 24 (67) | 48 (76) | .31 |
| Age, y | .85 | |||
| ≥40 | 48 (48) | 17 (47) | 31 (49) | |
| Median (range) | 38 (16-82) | 38 (17-82) | 39 (16-76) | |
| Stage | .57 | |||
| I | 56 (57) | 19 (53) | 37 (59) | |
| II | 43 (43) | 17 (47) | 26 (41) | |
| Disease location | .96 | |||
| Supradiaphragmatic | 85 (86) | 31 (86) | 54 (86) | |
| Infradiaphragmatic | 14 (14) | 5 (14) | 9 (14) | |
| Disease sites | ||||
| Cervical | 42 (42) | 16 (44) | 26 (41) | .76 |
| Axillary | 45 (45) | 17 (47) | 28 (44) | .79 |
| Mediastinal | 2 (2) | 2 (6) | 0 (0) | .06 |
| Intra-abdominal | 2 (2) | 0 (0) | 2 (3) | .28 |
| Pelvic | 3 (3) | 2 (6) | 1 (2) | .27 |
| Inguinal | 11 (11) | 4 (11) | 7 (11) | 1.0 |
| Performance status >1 | 4 (4) | 1 (3) | 3 (5) | .62 |
| B symptoms | 3 (3) | 0 (0) | 3 (5) | .18 |
| Extranodal involvement | 1 (1) | 1 (3) | 0 (0) | .19 |
| Mass, cm | .58 | |||
| ≥5 | 32 (33) | 13 (36) | 19 (31) | |
| Median (range) | 4 (2-9) | 4 (2-9) | 3 (2-9) |
Data are n (%) unless otherwise stated.
Staging PET in 30 of 63 patients; no staging PET scans in RT era.
Outcome of limited-stage NLPHL: routine RT era vs PET era and the impact of a PET-adapted approach
The median follow-up for all living patients was 10.5 years (range, 1.5-23.4 years), with a 5-year PFS of 93% and OS of 97% (Figure 1A-B). PFS was not affected by age (younger than age 40 years vs age 40 years or older; P = .65), stage at diagnosis (I vs II; P = .82), supra- or infradiaphramatic disease (P = .60), largest mass size (<5 cm vs ≥5 cm; P = .96), or sex (P = .13), but interestingly there has been only 1 NLPHL relapse in the female cohort. A multivariable analysis was not performed because there was a lack of identifiable risk factors. Of note, only 48% of patients (30 of 63) in the PET era had a staging PET/CT scan, but the proportion of patients with stage I or II was similar to that in patients who were staged using PET and those who were staged without PET (P = .22). Overall, 94 (95%) of 99 patients were actively treated, and the remaining 5 patients (all in the PET era) were either observed or declined therapy. Of interest, for all PET era patients, PFS was similar in patients staged with or without PET (P = .57), including those who received active treatment (P = .91). For all patients who received RT, the median dose was 35 Gy (range, 20-36 Gy).
Kaplan-Meier curves for all the patients from 1995 to 2019 are shown. PFS (A) and OS (B) in limited-stage NLPHL patients (n = 99).
Kaplan-Meier curves for all the patients from 1995 to 2019 are shown. PFS (A) and OS (B) in limited-stage NLPHL patients (n = 99).
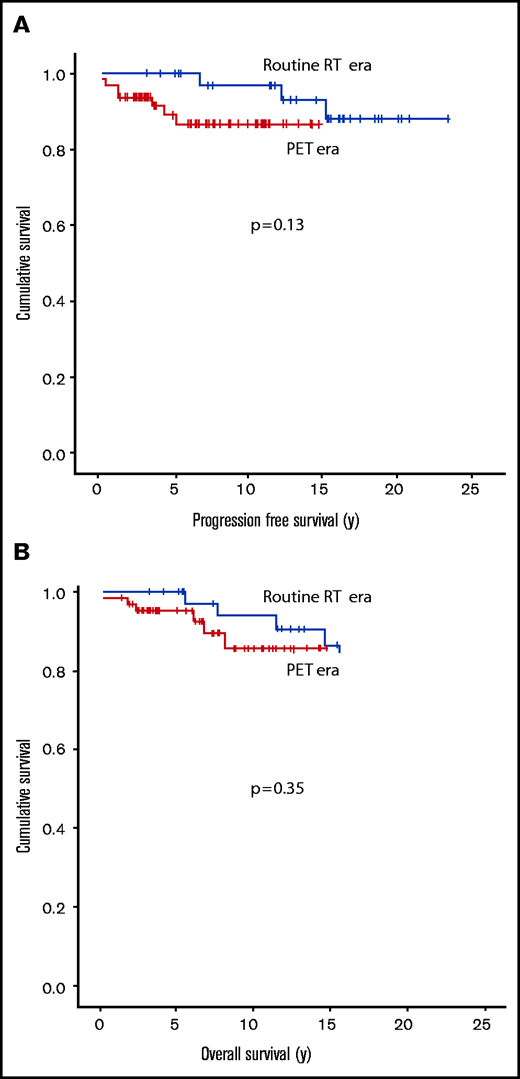
With a median follow-up of 16.7 years (range, 12.4-23.4 years) in the routine RT era and 7.3 years (range, 1.5-14.8 years) in the PET era, there was no difference in PFS (5-year PFS: 100% vs 89%; P = .13) or OS (5-year OS: 100% vs 95%; P = .35) by era (Figure 2A-B). Results were similar if only patients receiving active treatment were included (data not shown). Of interest, 42 patients had stage II disease and were managed with active therapy that incorporated RT (n = 25) (RT alone, n = 1; CMT, n = 24) or chemotherapy alone (n = 17) (ABVD-like, n = 16; rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP], n = 1). PFS was comparable in these 2 groups (RT/CMT vs ABVD alone; 5-year PFS: 96% vs 100%, P = .18) and interestingly, there were no lymphoma recurrences in the chemotherapy alone group although follow-up was shorter.
Kaplan-Meier curves for each treatment era are shown. PFS (A) and OS (B) in limited-stage NLPHL patients by treatment era (N = 99; routine RT era, n = 36; PET era, n = 63). There was no significant difference in PFS or OS by era comparison.
Kaplan-Meier curves for each treatment era are shown. PFS (A) and OS (B) in limited-stage NLPHL patients by treatment era (N = 99; routine RT era, n = 36; PET era, n = 63). There was no significant difference in PFS or OS by era comparison.
In the routine RT era, 6 (17%) of 36 patients with stage IA disease received RT alone because they had high neck node disease (n = 4) or cardiac disease (n = 1) or because they refused chemotherapy (n = 1). For the remaining 30 patients, 28 received CMT with ABVD for 2 cycles plus RT for 27 of those patients and ABVD for 4 cycles plus RT for 1 of them. One patient received ABVD alone because of a contraindication to RT (previously received craniospinal RT for brain tumor), and 1 received cyclophosphamide, vincristine, procarbazine, and prednisone (COPP)/doxorubicin, bleomycin, and vinblastine (ABV) alone by physician choice but only 2 cycles were administered because of patient noncompliance.
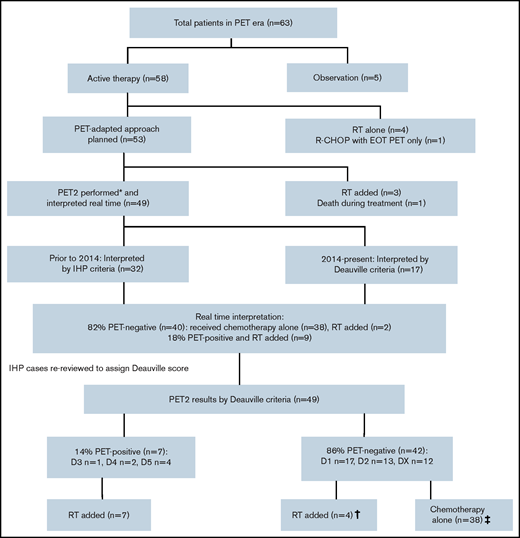
In the PET era, 5 (8%) of 63 patients were managed with observation or they declined therapy. Of the 58 actively treated patients, 4 received RT alone because of frailty (n = 2), patient preference (n = 1), or negative staging PET scan after lymph node excision (n = 1); 1 received 6 cycles of R-CHOP by physician choice, and the only scan performed was an end-of-treatment PET scan. The remaining 53 patients were planned for a PET-adapted approach (stage I, n = 30 [57%]; stage II, n = 23 [43%]). The majority received ABVD (n = 51): 1 patient was switched after 1 cycle of R-CHOP after pathologic review, 1 received modified cyclophosphamide, vinblastine, procarbazine, and prednisone/doxorubicin, bleomycin, and vincristine (CVPP/ABO; with bleomycin omitted [contraindication]), and 1 received R-CHOP at the treating physician’s discretion.
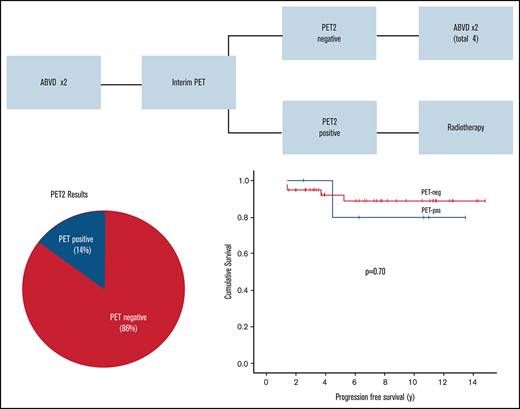
Four patients did not have a PET2 scan: 3 switched to RT after 2 cycles of ABVD because of toxicity, and 1 died of diabetic ketoacidosis after 1 cycle of ABVD. Thus, 49 patients had an interim PET2 scan with the exception of 1 patient for whom a PET3 scan was performed after R-CHOP (Figure 3). In the real-time interpretation (IHP, n = 32; Deauville, n = 17), 40 patients (82%) were PET negative and 9 patients (18%) were PET positive. All nine PET-positive patients received INRT (30-35 Gy), which encompassed all original sites of disease. Of the PET-negative patients, 2 proceeded to RT because of ABVD toxicity, and the patient who was treated with R-CHOP completed therapy after 3 cycles. Of the remaining 37 patients, all but 2 received 2 additional cycles (total of 4 cycles), including 5 patients who completed treatment with AVD after planned dropping of bleomycin; the remaining 2 patients declined further chemotherapy after 3 cycles of ABVD. Of interest, for all 51 patients who received bleomycin in the PET era, 2 (4%) developed pulmonary toxicity (1 after cycle 2 and 1 after cycle 4).
Overview of the PET-evaluated cohort and management by PET2 results. (*) A PET3 scan was performed after treatment with R-CHOP in 1 patient. (†) Two patients originally classified as positive by International Harmonization Project (IHP) interpretation were later changed to negative after re-review by Deauville criteria (DX, D2), accounting for 2 of 4 patients who received RT in the PET-negative group. The remaining 2 patients switched to RT because of chemotherapy toxicity. (‡) The majority of patients (35 of 38) received 2 additional cycles of ABVD (AVD in 5 patients). The remaining patients declined further chemotherapy after 3 cycles (ABVD in 2 patients and R-CHOP in 1 patient). EOT, end of treatment.
Overview of the PET-evaluated cohort and management by PET2 results. (*) A PET3 scan was performed after treatment with R-CHOP in 1 patient. (†) Two patients originally classified as positive by International Harmonization Project (IHP) interpretation were later changed to negative after re-review by Deauville criteria (DX, D2), accounting for 2 of 4 patients who received RT in the PET-negative group. The remaining 2 patients switched to RT because of chemotherapy toxicity. (‡) The majority of patients (35 of 38) received 2 additional cycles of ABVD (AVD in 5 patients). The remaining patients declined further chemotherapy after 3 cycles (ABVD in 2 patients and R-CHOP in 1 patient). EOT, end of treatment.
After the earlier scans were re-reviewed using Deauville criteria, 42 (86%) of 49 were PET negative (D1, n = 17; D2, n = 13; DX, n = 12) and 7 (14%) of 49 were PET positive (D3, n = 1; D4, n = 2; D5, n = 4) (Table 2). As above, all PET-positive patients were switched to RT, although 2 were reclassified as PET negative (D2 or DX) by Deauville criteria. Thus, of the 42 PET-negative patients, 38 completed treatment with chemotherapy alone and the remaining 2 patients switched to RT because of chemotherapy toxicity as described (Figure 3).
PET2 results and management of the cohort evaluated by PET
| PET feature . | PET-evaluated (n = 49) . |
|---|---|
| Real-time PET2 interpretation | |
| IHP criteria | 32 (65) |
| Deauville score | 17 (35) |
| Real-time PET2 result | |
| Negative | 40 (82) |
| Positive | 9 (18) |
| PET2 result by Deauville score | |
| Negative | 42 (86) |
| D1 | 17 |
| D2 | 13 |
| DX | 12 |
| Positive | 7 (14) |
| D3 | 1 |
| D4 | 2 |
| D5 | 4 |
| PET feature . | PET-evaluated (n = 49) . |
|---|---|
| Real-time PET2 interpretation | |
| IHP criteria | 32 (65) |
| Deauville score | 17 (35) |
| Real-time PET2 result | |
| Negative | 40 (82) |
| Positive | 9 (18) |
| PET2 result by Deauville score | |
| Negative | 42 (86) |
| D1 | 17 |
| D2 | 13 |
| DX | 12 |
| Positive | 7 (14) |
| D3 | 1 |
| D4 | 2 |
| D5 | 4 |
All data are n (%).
IHP, International Harmonization Project.
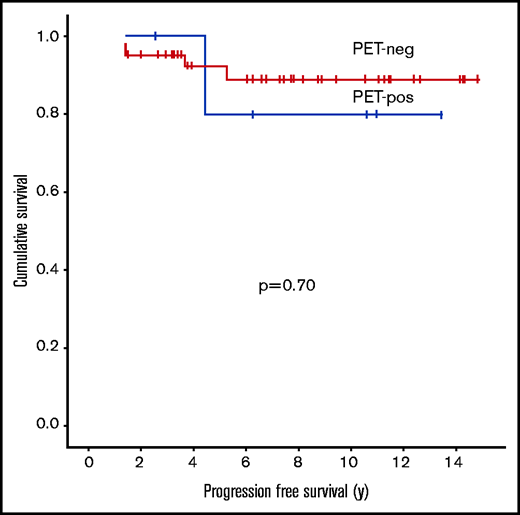
Within the cohort evaluated with PET, the 5-year PFS was 92% for PET-negative patients and 80% for PET-positive patients (P = .70) (Figure 4). Five patients had recurrences, 4 of which were in the PET2-negative group (DX, n = 1; D1, n = 1; D2, n = 2) and who had received ABVD alone. However, only 1 had a recurrence with NLPHL in a presumed RT field (in-field), and although 1 other patient had an in-field recurrence, repeat biopsy demonstrated nodular sclerosis cHL 5 years after the diagnosis of NLPHL, which was not clearly related. There was also an out-of-field recurrence that developed 4.5 years from diagnosis in a PET-positive patient (D5) who had received CMT.
PFS in limited-stage NLPHL patients by interim PET2 result (Deauville criteria) (n = 49; P = .70). neg, negative; pos, positive. There was no significant difference in PFS between PET-negative and PET-positive patients.
PFS in limited-stage NLPHL patients by interim PET2 result (Deauville criteria) (n = 49; P = .70). neg, negative; pos, positive. There was no significant difference in PFS between PET-negative and PET-positive patients.
Lymphoma recurrence, transformation, and causes of death in limited-stage NLPHL
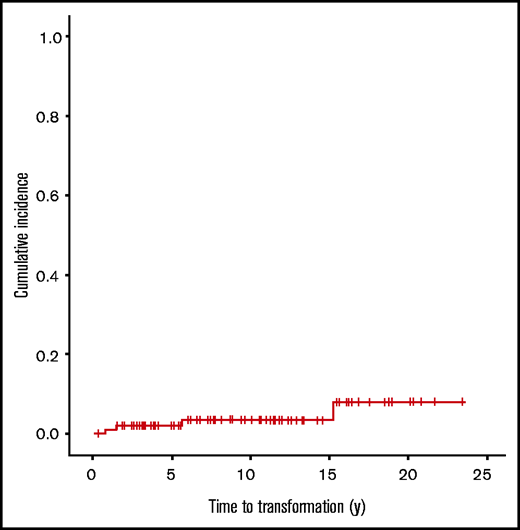
In the whole cohort, 9 patients had lymphoma recurrences (routine RT era treated with CMT, n = 3; PET era, n = 6 [5 actively treated]), 4 of which were transformation events to an aggressive lymphoma (routine RT era, n = 1 [biopsy-proven, T-cell-rich B-cell lymphoma]; PET era, n = 3 [2 developed splenic nodules 1 year after treatment completion; 1 patient refused all treatment and ultimately developed hepatic lesions]) for a 5-year time to transformation of 2% and a 10-year time to transformation of 3.5% (Figure 5). As described, a separate patient developed cHL. Overall, the median time to first lymphoma recurrence was 4.5 years (range, 0.5-15.3 years) from initial diagnosis. Three patients relapsed twice within the study period, including 1 with transformed T-cell-rich B lymphoma at first recurrence and diffuse large B-cell lymphoma at second recurrence. The remaining 2 patients relapsed with NLPHL both times.
In total, 12 patients died (routine RT era, n = 6; PET era, n = 6); however, only 1 died of NLPHL (PET era) 6 years after diagnosis, and that patient had declined all active therapies offered. One additional PET era patient died of diabetic ketoacidosis as described above, which may have been treatment related. The remaining 4 PET era patients died of gastrointestinal malignancy (n = 1), cardiac disease (n = 1), drug overdose (n = 1), and unknown cause (n = 1). The deaths in patients from the routine RT era were the result of cardiac disease (n = 1), renal failure (n = 1), lung cancer (n = 2), vulvar cancer (n = 1), and anaplastic lymphoma kinase–positive anaplastic large cell lymphoma (n = 1). Of note, 1 case of fatal lung cancer occurred within an irradiated field but the patient had a smoking history.
Discussion
We evaluated the long-term outcome of a real-world, population-based cohort with limited-stage NLPHL using a definition comparable to favorable early-stage disease in other classifications.19 Consistent with previous studies, outcomes were excellent with a 5-year OS of 97% and were maintained using a PET-adapted approach. Interestingly, females presented at an older age, which was noted in another study.20
Although NLPHL is highly curable, there is currently no consensus regarding the most appropriate initial therapy. RT is favored in adult patients with early-stage disease and has excellent results.21-24 However, concerns over late treatment toxicities have led to substantial modifications for RT but the impact on the secondary malignancy rate using modern techniques remains unknown. In this study, only 10 patients received RT alone (9 with stage IA), and there have been no lymphoma relapses in this subset. Current guidelines endorse RT alone for stage IA disease.7,8 Similarly, we have recently amended our guidelines to also endorse RT alone in this very favorable subgroup. However for those with stage II, the field may be large and there may be site-specific radiation toxicities that should also be considered.
CMT is typically used for stage IIA disease,6,7 but very few studies have evaluated chemotherapy alone, and some may have potentially introduced selection bias. The International Lymphoma Radiation Oncology Group recently compiled the largest multicenter retrospective study to date evaluating 559 patients with early-stage NLPHL, and they used a broader definition than we did in this study.25 Considering outcomes by treatment approach, the 5-year PFS was >90% in both RT alone and CMT groups compared with 77.8% in patients treated with chemotherapy alone. However, only 8.4% of patients in this study received chemotherapy alone and a greater proportion in this subset had unfavorable baseline prognostic factors, suggesting that this subset may be a higher-risk group. A previous study from BC Cancer showed improved PFS when systemic therapy was incorporated, although some of the patients who received RT alone were managed in an earlier era.18 We considered only patients with stage II NLPHL in our series, and we did not observe any lymphoma relapses when using chemotherapy alone. Although these patients likely represent a more favorable risk group by virtue of an interim negative PET2 scan, these results support that 4 cycles of ABVD alone is a viable option in select younger patients and in those with a contraindication to RT.
We previously evaluated the same PET-adaptive strategy in limited-stage cHL,14 which also demonstrated similar excellent results (5-year PFS, 89%; 5-year OS, 97%). To date, there have been few large studies dedicated to evaluating adaptive approaches or chemotherapy alone in NLPHL. A previous study in 37 patients with stage I to II disease demonstrated a similar PFS in those treated with a PET-adapted approach in which RT was either reduced or eliminated.26 More recently, Monteith et al27 performed a subgroup analysis of 29 patients with limited-stage (stage IA to IIA) NLPHL drawn from the larger Canadian Cancer Trials Group HD.6 trial who were randomly assigned to receive standard therapy (n = 15) with either RT alone (no risk factors) or 2 cycles of ABVD with RT (or CMT) (presence of risk factors) or ABVD alone (n = 14). With a median follow-up of 10 years, the 12-year OS for all NLPHL was 95%, with only 1 death in the NLPHL group that resulted from an unknown cause. By treatment arm, the 12-year event-free survival for CMT/RT compared with ABVD alone was 58% vs 85% (hazard ratio 0.46; 95% confidence interval, 0.09-2.37) and freedom from progression was 70% vs 85% (hazard ratio, 0.72; 95% confidence interval, 0.12-4.33). Although interim imaging was by CT scan and the RT doses and fields are not used today, this was the first prospective analysis to demonstrate highly favorable outcomes with ABVD alone in limited-stage NLPHL with very mature follow-up.
In this analysis, we found that patients with an interim negative PET2 scan (>90% of whom were treated with ABVD alone) have an excellent prognosis with a 5-year PFS of 92%. A PET-adapted approach has reduced the use of RT (94% vs 29% of patients) without compromising outcomes, which may be particularly relevant in younger patients and those with more challenging RT sites, such as the inguinal region in young women in whom scatter to the ovary may be a concern. Of importance, a minority of patients encountered chemotherapy toxicity, including pneumonitis resulting from the use of bleomycin, so they were switched to RT. Thus, ABVD should be avoided in older patients and those with a single nodal site. Recently, we have omitted bleomycin for subsequent cycles in PET2-negative patients, similar to the practice in advanced-stage patients.15 However, the impact of this approach in limited-stage HL is unknown. Of note, consistent with other similar studies, a Deauville score of 3 was considered PET positive, applying a more conservative criteria for switching to RT.17 All but 1 of the PET-positive patients had a Deauville score of 4 or 5 and 1 relapse (D5) was observed (14%). Our ability to make definitive conclusions was limited by sample size, but our data support that switching to RT maintains good outcomes in PET2-positive patients.
Although ABVD is still considered the standard chemotherapy in limited-stage NLPHL, R-CHOP has been increasingly used, given that lymphocyte-predominant cells are CD20+. A study of 27 patients treated with R-CHOP that included those with early-stage disease reported a 5-year PFS of 88.5%.28 R-CHOP is also preferred in advanced-stage patients with spleen or intra-abdominal involvement because of the high risk of transformation.2,12 Interestingly, in our series, 2 patients with stage IA disease who were treated with ABVD alone recurred with splenic lesions, suggesting occult transformed disease may have been present at diagnosis. Larger series are needed, ideally integrated with detailed pathology review to distinguish classic vs variant subtypes,29 which will help establish the role of R-CHOP in early-stage disease.
In light of the indolent clinical course of NLPHL, less aggressive initial management strategies have also been proposed. Rituximab alone yields high response rates, but the relapse risk is high and rituximab is typically favored in the relapse setting.11,12 Recent retrospective analyses have provided support for observation because survival is generally preserved even with delayed curative therapy, and a proportion of patients may be able to avoid treatment completely.10,30 In our study, 4 of 5 patients who did not receive active treatment have remained free from disease progression or recurrence. However, 1 patient did decline all treatment and ultimately died as a result of NLPHL, the only disease-related death in this series, which highlights the importance of treating symptomatic progression.
Our study has some important limitations. Although it represents the largest study using a PET-adapted approach, patient numbers are small and thus analyses are not adequately powered to detect small differences. In addition, longer follow-up will be important, given the propensity for late relapses in NLPHL. Although the data were retrospectively analyzed, a notable strength is that era-specific provincial treatment recommendations were applied prospectively and uniformly which minimizes selection bias.
In summary, we describe excellent outcomes in patients with limited-stage NLPHL who were treated primarily with a PET-adapted approach in which the majority of patients had 4 cycles of ABVD. RT alone is the preferred treatment modality in most stage I patients, but ABVD alone is an option for select younger patients with stage II disease that requires a large RT field. Longer follow-up will be required, given the potential for late recurrences in NLPHL.
Authorship
Contribution: P.T.M.C. and K.J.S. designed the study, collected and analyzed data, and wrote the paper; K.J.S. provided clinical care to patients; D.V., D.W.S., A.S.G., C.L.F., T.P., A.C.L., J.M.C., and L.H.S. provided clinical care and coauthored the paper; R.P.T., F.B., and D.W. reviewed PET scans and coauthored the paper; R.P.T. additionally performed re-review and reclassification of PET scans using Deauville criteria; and P.F., J.W.C., G.W.S., R.D.G., and B.S. reviewed pathology and coauthored the paper.
Conflict-of-interest disclosure: D.W.S. consulted for AbbVie, AstraZeneca, and Janssen, received research funding from Janssen and NanoString, and was named inventor on a patent related to subtyping of lymphomas with 1 patent licensed to NanoString. A.S.G. received research funding from AstraZeneca, Janssen, AbbVie, and Roche Canada, served on advisory boards for AstraZeneca, Janssen, AbbVie, and Sandoz, and received honoraria from Janssen. C.L.F. received honoraria from Seattle Genetics, Janssen, Amgen, Celgene, and AbbVie and received research funding from Roche and Teva. K.J.S. received honoraria from or consulted for Bristol Myers Squibb, Seattle Genetics, Merck, Novartis, Kyowa, Gilead, and AstraZeneca, consulted for Servier, and served on a steering committee for Beigene. The remaining authors declare no competing financial interests.
Correspondence: Kerry J. Savage, BC Cancer, 600 West 10th Ave, Vancouver, BC, Canada V5Z 4E6; e-mail: ksavage@bccancer.bc.ca.
References
Author notes
Presented in abstract form at the 61st American Society of Hematology Annual Meeting and Exposition, Orlando, FL, 8 December 2019.
For original data, please contact Kerry J. Savage via e-mail at ksavage@bccancer.bc.ca.