Key Points
CRISPR/Cas9 technology was used to generate the first alloantigen-specific mouse model of FNAIT.
This model recapitulates many clinically important features and can be used for preclinical evaluation of immunotherapy for FNAIT in humans.
Abstract
Fetal/neonatal alloimmune thrombocytopenia (FNAIT) is a life-threatening bleeding disorder caused by maternal antibodies directed against paternally inherited antigens present on the surface of fetal platelets. The human platelet alloantigen HPA-1a (formerly known as the PlA1 alloantigen), is the most frequently implicated HPA for causing FNAIT in Whites. A single Leu33Pro amino acid polymorphism residing within the ∼50-amino-acid plexin-semaphorin-integrin domain near the N-terminus of the integrin β3 subunit (platelet membrane glycoprotein IIIa [GPIIIa]) is responsible for generating the HPA-1a and HPA-1b epitopes in human GPIIIa and serves as the central target for alloantibody-mediated platelet destruction. To simulate the etiology of human FNAIT, wild-type female mice were pre-immunized with platelets derived from transgenic mice engineered to express the human HPA-1a epitope on a murine GPIIIa backbone. These mice developed a strong alloimmune response specific for HPA-1a, and when bred with HPA-1a+ males, gave birth to severely thrombocytopenic pups that exhibited an accompanying bleeding phenotype. Administering either polyclonal intravenous immunoglobulin G or a human monoclonal blocking antibody specific for the HPA-1a epitope into pregnant female mice resulted in significant elevation of the neonatal platelet count, normalized hemostasis, and prevented bleeding. The establishment of an alloantigen-specific murine model that recapitulates many of the clinically important features of FNAIT should pave the way for the preclinical development and testing of novel therapeutic and prophylactic modalities to treat or prevent FNAIT in humans.
Introduction
Fetal /neonatal alloimmune thrombocytopenia (FNAIT; also referred to as neonatal alloimmune thrombocytopenia [NATP] or neonatal alloimmune thrombocytopenia [NAIT]) is a clinical disorder characterized by maternal development of an immune response to an unfamiliar platelet antigen inherited from the father and present on the surface of fetal platelets.1 Estimated to affect nearly 1 in 1000 live births (∼3.9 million US births per year),2-4 FNAIT most commonly manifests as mild to severe neonatal thrombocytopenia, with the most severe cases resulting in spontaneous5 or trauma-induced intracranial hemorrhage with neurologic sequelae or a fatal outcome in nearly one-third of the most severe cases. An estimated 25% to 50% of the cases of FNAIT occur without warning during the gestation period of the first pregnancy, and the majority of cases affect subsequent pregnancies, the latter etiology being much the same as the better-known hemolytic disease of the newborn (Rh syndrome).
Naturally occurring amino acid polymorphisms in platelet surface membrane glycoproteins (GPs) are responsible for provoking the alloimmune response in most cases of FNAIT, with the HPA-1a/HPA-1b alloantigen system, historically known as PlA1/PlA2,6 accounting for more than 80% of the cases in Whites.7 The single Leu33Pro polymorphism in platelet GPIIIa (the integrin β3 subunit) underlying this syndrome was discovered more than 30 years ago7,8 and enabled the generation and use of molecular genetic technologies to diagnose FNAIT.9-11 But progress in treating or preventing this sometimes devastating alloimmune disorder has been hampered by the lack of a suitable animal model.
The Leu33 residue that controls formation of the epitope recognized by anti-HPA-1a alloantibodies is located within a small 55-amino-acid loop that extends out from the plexin-semaphorin-integrin (PSI) domain of GPIIIa12 (Figure 1A). The PSI domain itself is wedged between the so-called hybrid and epidermal growth factor 1 (EGF1) domains of the molecule, all located near the central hinge region of the integrin. Interestingly, the polyclonal immune response generated by Pro33 homozygous women to the Leu33 polymorphic amino acid located on fetal platelets is heterogeneous; some women produce type I alloantibodies that bind exclusively within the PSI domain, whereas others develop subpopulations of so-called type II antibodies whose binding requires amino acids in the linearly distant but conformationally close hybrid domain.13,14 Figure 1B depicts the binding of a prototypical type II monoclonal antibody (mAb), 26.4, which is a human immunoglobulin G (IgG) antibody cloned from the B cells of an alloimmunized woman who had given birth to an infant with FNAIT.15
Schematic of the three-dimensional structure of humanized murine GPIIIa. (A) The hybrid, PSI, and EGF1 domains of human GPIIIa. (B) Humanized murine GPIIIa. The positions of the murine amino acids that have been humanized (T30→A, S32→P, Q33→L, N39→D and M470→Q) are indicated in panel B, as are the amino acids required for the binding of the HPA-1a–specific human mAb 26.4. Ser469 is conserved in mouse and human GPIIIa.
Schematic of the three-dimensional structure of humanized murine GPIIIa. (A) The hybrid, PSI, and EGF1 domains of human GPIIIa. (B) Humanized murine GPIIIa. The positions of the murine amino acids that have been humanized (T30→A, S32→P, Q33→L, N39→D and M470→Q) are indicated in panel B, as are the amino acids required for the binding of the HPA-1a–specific human mAb 26.4. Ser469 is conserved in mouse and human GPIIIa.
We recently described the CRISPR-meditated generation of transgenic mice containing a series of mouse→human amino acid substitutions that enabled the binding of both human polyclonal and human and mouse monoclonal HPA-1a–specific antibodies.12 In this article, we demonstrate that immunization of wild-type (WT) female mice with such humanized mouse platelets results in their generation of high-titer anti-HPA-1a alloantibodies that can induce profound thrombocytopenia and bleeding in neonatal pups. Preliminary preclinical studies suggest that the destruction of circulating fetal platelets by maternal alloantibodies can be prevented by administering mAb 26.4 into the pregnant female. This notion paves the way for future clinical studies in humans to help prevent FNAIT in the subsequent pregnancies of women who have previously given birth to an FNAIT infant.
Materials and methods
Mice
C57BL/6 mice that express murine GPIIIa harboring T30→A, S32→P, Q33→L, N39→D, and M470→Q mouse-to-human amino acid substitutions within the PSI and adjacent EGF1 domains (hereafter referred to as APLDQ GPIIIa) were generated and maintained as previously described.12 Mouse genotypes were determined by polymerase chain reaction amplification of genomic tail DNA, and the APLDQ phenotype was confirmed by flow cytometry using mAb 26.4. Wild-type (WT) BALB/c mice (6 to 8 weeks of age) were purchased from Charles River Laboratories. All mice were maintained in the Biological Resource Center at the Medical College of Wisconsin. All animal protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee and were carried out in compliance with the human and animal experimentation guidelines of the US Department of Health and Human Services and in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Antibodies
Two different mAbs with specificity for the HPA-1a epitope were used in this study; murine mAb SZ2116 and mAb 26.4,15 a human mAb derived from an immortalized B cell from an HPA-1a alloimmunized woman who had an infant affected by FNAIT. A variant effector-silent form of mAb 26.4 that contained P329G, L235A, L234A, and N297A amino acid substitutions to render it incapable of binding either complement or Fcγ receptors was used where specified. The murine mAb PSI-B1, which binds both the human and the mouse β3 integrin PSI domains independent of Leu33Pro allotype,17 was kindly provided by Heyu Ni, PhD (University of Toronto). Pharmaceutical prescription-grade human intravenous immunoglobulin (IVIG) was obtained from a local pharmacy.
Immunization, blood collection, and platelet preparation
WT female BALB/c mice (8 weeks of age; Charles River Laboratories) were immunized by mixing 1 × 108 APLDQ platelets with Sigma Adjuvant and injecting it intraperitoneally. BALB/c mice were used because of the known poor responsiveness of C57BL/6 mice to murine alloantigens.18 Blood samples were collected from the submandibular vein into EDTA-coated capillary tubes at specified times after immunization. Platelet counts were determined by using an scil Vet ABC Animal Blood Counter (scil Animal Care Company, Gurnee, IL). Plasma was harvested by centrifugation, IgG and IgM isotypes were analyzed, and blood counts and APLDQ-specific antibody levels were determined. For larger blood samples, blood was drawn from the inferior vena cava of anesthetized mice into a syringe containing 3.8% sodium citrate diluted 1:1 with modified Tyrode–N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Tyrode-HEPES) buffer (10 mM HEPES [pH 7.4], 12 mM NaHCO3, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose, 0.25% bovine serum albumin), layered onto Fico/Lite for Platelets (Atlanta Biologicals, Flowery Branch, GA), and centrifuged for 15 minutes at 700g. Platelets were collected from the interface, washed once in Tyrode-HEPES buffer containing 50 ng/mL prostaglandin E1 (PGE1) and 1 mM EDTA (pH 7.4), resuspended in phosphate-buffered saline to a final concentration of 3 × 108/mL, and examined for their ability to form aggregates in the presence of several physiological stimuli. Plasmas from these samples were collected and stored at –20°C until use. Human blood samples were obtained from healthy volunteers as approved by the Institutional Review Board of the Medical College of Wisconsin after obtaining informed consent in accordance with the Declaration of Helsinki.
Western blotting
Washed platelets were solubilized in Triton X-100 in the presence of a protease inhibitor cocktail (Thermo Fisher Scientific), mixed with non-reducing sodium dodecyl sulfate sample buffer, incubated for 1 hour at 37°C, and then loaded onto a 4% to 20% polyacrylamide gradient gel. After transfer to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA), the membranes were incubated with mouse anti-APLDQ antisera, human anti-HPA-1a antisera, mAb SZ21, or native mAb 26.4. Immunoblot conditions described by Weiss et al16 were used to allow SZ21 to discriminate between the HPA-1a (PlA1) and HPA-1b (PlA2 ) forms of human GPIIIa. Bound antibodies were visualized by using species-specific peroxidase-conjugated donkey anti-human IgG (heavy and light chains [H+L]), or goat anti-mouse IgG (H+L) secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).
Flow cytometric analysis of platelet-specific, HPA-1a–specific, and Ig subtype–specific antibodies
Binding of murine anti-APLDQ antibodies to APLDQ platelets was detected by using fluorescein isothiocyanate (FITC)–labeled goat F(ab')2 anti-mouse IgG (Jackson ImmunoResearch Laboratories). Platelets were positively identified by using FITC-conjugated anti-CD41 and Alexa Fluor 647–conjugated anti-CD61 (Beckman Coulter, Brea, CA) antibodies. The Ig isotypes present in murine anti-APLDQ antisera were determined by using FITC-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgG2b, IgG3, and IgM (Southern Biotech, Birmingham, AL). The presence of the HPA-1a epitope was verified by using mAbs SZ21 and native 26.4. Binding of human maternal alloantibodies was detected by using FITC-labeled goat F(ab')2 anti-human IgG (Jackson ImmunoResearch Laboratories). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Antibody binding was evaluated and analyzed on an Accuri C6 flow cytometer (BD Biosciences) and analyzed using FlowJo software.
Induction of FNAIT and preclinical evaluation of polyclonal Ab and mAb therapy
Female BALB/c mice that had been pre-immunized with APLDQ platelets derived from C57BL/6 transgenic mice were bred with APLDQ C57BL/6 males. After the pups were delivered, the platelet count was determined and the bleeding phenotype in the pups was assessed. Matings between WT BALB/c females and C57BL/6 APLDQ males were set up as controls. In some instances, pregnant female mice were injected intravenously with ∼25 mg of IVIG (10% stock solution, Baxalta US Inc., Lexington, MA) to achieve a final dose of 1 g/kg or with 30 μg per mouse of the effector-silent variant of mAb 26.4 on days 10.5 and 17.5 of gestation to simulate potential treatment regimens for FNAIT.
Statistics
For platelet count, antibody levels and tail bleeding time, log transformation was applied to variables that were not normally distributed. The student t-test was used to compare the different groups. The post-hoc analyses were adjusted by the step-down Bonferroni method and P <0.05 was considered statistically significant. The Generalized Estimating Equation(GEE) with maximum likelihood estimation method was used to model the platelet count in mAb or mouse plasma treatment mice over time. The Pearson correlation was used to analyze the correlation of maternal alloantibody level with fetal platelet count.
Results
Mouse platelets expressing the human HPA-1a alloantigenic epitope function normally
Previous studies have shown that murine platelet membrane glycoprotein GPIIIa can be engineered to express the human HPA-1a epitope by simply humanizing 5 key amino acids (T30→A, S32→P, Q33→L, N39→D and M470→Q) within the PSI and adjacent EGF1 domains12,19 (Figure 1B). Murine platelets derived from these humanized APLDQ transgenic mice function normally, as confirmed by normal circulating platelet counts, normal tail vein bleeding times, and normal platelet aggregation responses to physiological platelet agonists (supplemental Figure 1).
APLDQ is immunogenic in mice and results in development of antigen-specific FNAIT
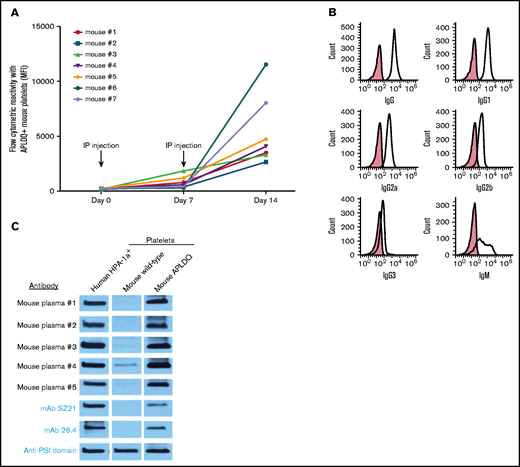
To evaluate the immunogenicity of the HPA-1a epitope presented within the context of the murine integrin β3 subunit, platelets from APLDQ C57BL/6 mice were mixed with adjuvant and introduced intraperitoneally into WT female BALB/c mice every 7 days for 2 consecutive weeks. Immunization in this manner was necessary, because persistent attempts to induce an antibody response via pregnancy alone never resulted in significant alloimmunization (see “Discussion” section). As shown in Figure 2A, recipient mice developed a robust, polyclonal immune response with all antibody subtypes represented (Figure 2B). There was also a small degree of reactivity against WT C57BL/6 platelets, as might be expected given the differences in class I major histocompatibility complex (MHC) alleles between the 2 strains; however, these antibodies were incapable of causing FNAIT (Control 2 in Figure 3A). Immunoblot analysis (Figure 2C) revealed that the antibodies that were generated bound to HPA-1a+ human GPIIIa and the APLDQ, but not to the WT allelic form of murine GPIIIa. Two well-characterized anti–HPA-1a mAbs, SZ2116,20 and 26.4,15 also bound to APLDQ, but not to WT murine GPIIIa (Figure 2C). When injected into APLDQ mice, both murine anti-APLDQ polyclonal antisera and native mAb 26.4 induced rapid platelet clearance (supplemental Figure 3). Conversely, the effector-silent form of mAb 26.4 did not induce thrombocytopenia in WT mice, as expected (not shown). Taken together, these data demonstrate that the HPA-1a alloantigenic epitope is immunogenic in BALB/c mice and that HPA-1a–specific alloantibodies can induce profound thrombocytopenia.
The APLDQ murine form of GPIIIa is immunogenic. (A) Seven WT female BALB/c mice were injected intraperitoneally (IP) with a Sigma adjuvant mixed with 1 × 108 washed platelets derived from APLDQ C57BL/6 mice on days 0 and 7. Note that all 7 recipients developed an IgG immune response specific for APLDQ platelets, although the titers varied approximately fourfold. There was little reactivity against WT C57BL/6 or BALB/c platelets (supplemental Figure 2). (B) IgM as well as all IgG subclasses were represented. (C) Five mouse plasmas were examined for alloantigen specificity and were demonstrated to bind GPIIIa derived from human HPA-1a+ platelets and GPIIIa from APLDQ platelets, but not WT murine platelets. Two different anti-HPA-1mAbs (SZ21 and 26.4) were used as controls, along with a broadly reactive mAb (kindly supplied by Heyu Ni, PhD, University of Toronto) that binds both the human and murine PSI domain irrespective of HPA allotype. MFI, mean fluorescent intensity.
The APLDQ murine form of GPIIIa is immunogenic. (A) Seven WT female BALB/c mice were injected intraperitoneally (IP) with a Sigma adjuvant mixed with 1 × 108 washed platelets derived from APLDQ C57BL/6 mice on days 0 and 7. Note that all 7 recipients developed an IgG immune response specific for APLDQ platelets, although the titers varied approximately fourfold. There was little reactivity against WT C57BL/6 or BALB/c platelets (supplemental Figure 2). (B) IgM as well as all IgG subclasses were represented. (C) Five mouse plasmas were examined for alloantigen specificity and were demonstrated to bind GPIIIa derived from human HPA-1a+ platelets and GPIIIa from APLDQ platelets, but not WT murine platelets. Two different anti-HPA-1mAbs (SZ21 and 26.4) were used as controls, along with a broadly reactive mAb (kindly supplied by Heyu Ni, PhD, University of Toronto) that binds both the human and murine PSI domain irrespective of HPA allotype. MFI, mean fluorescent intensity.
Maternal anti-HPA-1a alloantisera cause severe fetal and neonatal thrombocytopenia. (A) Pre-immunized WT BALB/c mice bred with APLDQ males give birth to severely thrombocytopenic pups. Shown are the platelet counts (mean ± standard error of the mean [SEM]; n = 3-10 mice per group) of neonatal pups 48 hours after birth from 3 representative BALB/c females that had been pre-immunized with APLDQ platelets. Although litter sizes were normal to near normal, all of the newborn pups were nearly devoid of circulating platelets. Control 1: platelet counts of pups derived from a female BALB/c mouse that had not been pre-immunized with APLDQ platelets before breeding with an APLDQ C57BL/6 male. Control 2: platelet count of pups derived from WT BALB/c females that had been pre-immunized with platelets from an APLDQ-expressing C57BL/6 mouse before being bred with a WT C57BL/6 male. Note that alloantibodies produced as a result of the difference in class I MHC molecules between the 2 strains of mice (supplemental Figure 2) result in neither thrombocytopenia nor FNAIT, much the same as in humans. (B) Maternal antibody levels correlate inversely with fetal platelet count. Maternal blood samples were collected on day 19.5 of gestation and subjected to flow cytometric analysis against APLDQ C57BL/6 murine platelets. Fetuses were collected in parallel, the blood of littermates was pooled, and the platelet count of each pool was determined. Each data point represents the average platelet count of a single litter. **P < .001.
Maternal anti-HPA-1a alloantisera cause severe fetal and neonatal thrombocytopenia. (A) Pre-immunized WT BALB/c mice bred with APLDQ males give birth to severely thrombocytopenic pups. Shown are the platelet counts (mean ± standard error of the mean [SEM]; n = 3-10 mice per group) of neonatal pups 48 hours after birth from 3 representative BALB/c females that had been pre-immunized with APLDQ platelets. Although litter sizes were normal to near normal, all of the newborn pups were nearly devoid of circulating platelets. Control 1: platelet counts of pups derived from a female BALB/c mouse that had not been pre-immunized with APLDQ platelets before breeding with an APLDQ C57BL/6 male. Control 2: platelet count of pups derived from WT BALB/c females that had been pre-immunized with platelets from an APLDQ-expressing C57BL/6 mouse before being bred with a WT C57BL/6 male. Note that alloantibodies produced as a result of the difference in class I MHC molecules between the 2 strains of mice (supplemental Figure 2) result in neither thrombocytopenia nor FNAIT, much the same as in humans. (B) Maternal antibody levels correlate inversely with fetal platelet count. Maternal blood samples were collected on day 19.5 of gestation and subjected to flow cytometric analysis against APLDQ C57BL/6 murine platelets. Fetuses were collected in parallel, the blood of littermates was pooled, and the platelet count of each pool was determined. Each data point represents the average platelet count of a single litter. **P < .001.
To determine whether these antibodies can induce FNAIT in vivo, pre-immunized BALB/c female mice were bred with C57BL/6 males that were homozygous for the APLDQ allele of murine GPIIIa, and the platelet counts of both fetal and 48-hour postpartum neonatal pups were determined. As shown in Figure 3A, neonatal pups born to such female mice were universally severely thrombocytopenic, regardless of the maternal alloantibody level shown in Figure 2A, likely because only ∼10% of cell surface GPIIb-GPIIIa needs to be antibody-occupied to target the platelet for clearance by the reticuloendothelial system (H.Z. and P.J.N., unpublished observations, December, 2020). The platelet counts of 19.5-day-old fetuses were also dramatically reduced, a phenomenon directly related to the titer of anti-HPA-1a antibodies present in the maternal circulation (Figure 3B). Control experiments (not shown) in which APLDQ pre-immunized females were bred with heterozygous males resulted in litters in which approximately half the pups had normal platelet counts but the remaining pups were severely thrombocytopenic. As an additional control, platelet counts of pups delivered by APLDQ pre-immunized BALB/c females that had been bred with WT males were also normal (not shown). Taken together, these data demonstrate that the HPA-1a alloantigen-specific murine model of FNAIT faithfully recapitulates several critical features of FNAIT, including the ability of pathogenic maternal HPA-1a–specific alloantibodies to cross the placenta, react selectively with HPA-1+ fetal platelets, and induce severe thrombocytopenia.
Interestingly, alloantibodies generated as a result of a single pre-immunization remained in the maternal circulation (Figure 4A), were readily detected in neonatal circulation (Figure 4B), and induced FNAIT (Figure 4C) and bleeding in internal organs and feet (Figure 4D) in at least 4 sequential pregnancies over a 24-week period. These findings phenocopy observations in humans in which children born after an initial case of FNAIT often suffer from severe thrombocytopenia, with a correspondingly increased risk of hemorrhage.21,22
Persistence of neonatal thrombocytopenia in singly alloimmunized female mice. (A) Maternal anti-APLDQ alloantibody (n = 3 mice per group) titer persists for the duration of 4 serial matings (over an ∼24-week period). Control is plasma from nonimmunized mice. (B) Circulating maternal anti-APLDQ alloantibody levels in the blood of neonatal pups (n = 11-30 mice per group) is present in all 4 serial litters of pre-immunized BALB/c female mice. Control is anti-APLDQ antibody levels in the pups of nonimmunized females. (C) Platelet counts (n = 11-30 mice per group) of all neonates in each litter born to 3 female BALB/c mice that had been pre-immunized with APLDQ murine platelets and then serially bred 4 times with APLDQ homozygous males. Note that the platelet counts of the neonatal mice was as low after the fourth litter as it was in the first litter. Control is neonatal platelet count of pups of nonimmunized females. (D) Representative bleeding in pups born to APLDQ pre-immunized WT BALB/c females crossed with APLDQ homozygous males. The pups are thus heterozygous for the paternally derived APLDQ humanized form of GPIIIa, with the other allele being maternally derived WT murine GPIIIa. Data are presented as mean ± SEM. *P < .05; **P < .001.
Persistence of neonatal thrombocytopenia in singly alloimmunized female mice. (A) Maternal anti-APLDQ alloantibody (n = 3 mice per group) titer persists for the duration of 4 serial matings (over an ∼24-week period). Control is plasma from nonimmunized mice. (B) Circulating maternal anti-APLDQ alloantibody levels in the blood of neonatal pups (n = 11-30 mice per group) is present in all 4 serial litters of pre-immunized BALB/c female mice. Control is anti-APLDQ antibody levels in the pups of nonimmunized females. (C) Platelet counts (n = 11-30 mice per group) of all neonates in each litter born to 3 female BALB/c mice that had been pre-immunized with APLDQ murine platelets and then serially bred 4 times with APLDQ homozygous males. Note that the platelet counts of the neonatal mice was as low after the fourth litter as it was in the first litter. Control is neonatal platelet count of pups of nonimmunized females. (D) Representative bleeding in pups born to APLDQ pre-immunized WT BALB/c females crossed with APLDQ homozygous males. The pups are thus heterozygous for the paternally derived APLDQ humanized form of GPIIIa, with the other allele being maternally derived WT murine GPIIIa. Data are presented as mean ± SEM. *P < .05; **P < .001.
Utility of the APLDQ mouse model for preclinical evaluation of therapeutic antibodies
IVIG has been used for more than 30 years for antenatal management of pregnant women to suppress the immune response after a previous case of FNAIT,23,24 and it represents the current standard of care. More specific treatments using modified anti-HPA-1a antibodies have also been proposed.15,25-27 To validate the potential usefulness of APLDQ mice in the preclinical evaluation of compounds designed to treat FNAIT, human IVIG or an effector-silent variant of mAb 26.4 was administered to pregnant BALB/c mice with preexisting high-titer anti-HPA-1a antibodies (Figure 5A). As shown in Figure 5B, administration of IVIG on days 10.5 and 17.5 of gestation resulted in a significant elevation of neonatal platelet counts, likely as a result of its ability to reduce anti-APLDQ antibody levels in both pregnant females (Figure 5C) and their newborn pups (Figure 5D). Litter sizes were also improved (Figure 5E). In contrast, administering an Fc-modified effector-silent variant of mAb 26.4 into pre-immunized pregnant female mice (litter #4; Figure 5B) elevated neonatal platelet counts and reduced bleeding incidence approximately fourfold (Table 1), but had little ability to reduce the levels of either maternal or neonatal levels of circulating anti-APLDQ (not shown), as might be expected when specifically targeting the effector arm of the immune response (ie, prevention of antibody-mediated platelet clearance). The maternal and neonatal antibody levels in the fourth and fifth pregnancies remained variable, as in litters 1 to 3 (Figure 5C-D). The neonatal platelet count was markedly rescued by mAb 26.4 treatment, returning to near zero levels one untreated pregnancy later (columns 4 and 5 of Figure 5B). Taken together, these data demonstrate the potential utility of this animal model for the preclinical evaluation of therapeutic modalities to treat FNAIT.
Administering human IVIG or the HPA-1a–specific mAb 26.4 into pregnant females elevates the platelet count of pups born to APLDQ alloimmunized mothers. (A) Four WT BALB/c female mice that had been pre-immunized with APLDQ murine platelets were bred 5 successive times with APLDQ males. (B) In the first pregnancy, the female mice received no treatment, and as a result, all the pups born were severely thrombocytopenic (first column). On days 10.5 and 17.5 of the second pregnancy, all 4 females received 25 mg of human pharmaceutical-grade IVIG (∼1 g/kg) administrated via tail vein injection. As a result, the platelet counts of the resulting pups were normal to near normal. No additional drug was administered during the third pregnancy, resulting in thrombocytopenic pups and demonstrating that the beneficial effects of IVIG treatment do not persist for more than ∼2 months. For the fourth pregnancy, the effector-silent variant of the HPA-1a–specific mAb 26.4 was administered at 30 mg per female on days 10.5 and 17.5 of gestation, resulting in rescue of platelet counts in the pups of 3 of the 4 females. Similar to IVIG treatment, the beneficial effects of treatment with mAb 26.4 did not persist in subsequent pregnancy #5, even though it was <8 weeks after delivery of litter #4. (C-E) IVIG treatment suppressed the maternal immune response (C), decreased the corresponding anti-APLDQ antibody levels in pups (D), and increased litter sizes (E). Although mAb 26.4 improved neonatal platelet counts (B), it had no effect on maternal or neonatal anti-APLDQ antibody levels (not shown), as might be expected of a treatment whose mechanism of action differs from that of IVIG, which acts as a generalized immunosuppressive agent.
Administering human IVIG or the HPA-1a–specific mAb 26.4 into pregnant females elevates the platelet count of pups born to APLDQ alloimmunized mothers. (A) Four WT BALB/c female mice that had been pre-immunized with APLDQ murine platelets were bred 5 successive times with APLDQ males. (B) In the first pregnancy, the female mice received no treatment, and as a result, all the pups born were severely thrombocytopenic (first column). On days 10.5 and 17.5 of the second pregnancy, all 4 females received 25 mg of human pharmaceutical-grade IVIG (∼1 g/kg) administrated via tail vein injection. As a result, the platelet counts of the resulting pups were normal to near normal. No additional drug was administered during the third pregnancy, resulting in thrombocytopenic pups and demonstrating that the beneficial effects of IVIG treatment do not persist for more than ∼2 months. For the fourth pregnancy, the effector-silent variant of the HPA-1a–specific mAb 26.4 was administered at 30 mg per female on days 10.5 and 17.5 of gestation, resulting in rescue of platelet counts in the pups of 3 of the 4 females. Similar to IVIG treatment, the beneficial effects of treatment with mAb 26.4 did not persist in subsequent pregnancy #5, even though it was <8 weeks after delivery of litter #4. (C-E) IVIG treatment suppressed the maternal immune response (C), decreased the corresponding anti-APLDQ antibody levels in pups (D), and increased litter sizes (E). Although mAb 26.4 improved neonatal platelet counts (B), it had no effect on maternal or neonatal anti-APLDQ antibody levels (not shown), as might be expected of a treatment whose mechanism of action differs from that of IVIG, which acts as a generalized immunosuppressive agent.
hIVIG and effector-silent mAb 26.4 ameliorate bleeding in the APLDQ murine model of FNAIT
| Pre-immunized BALB/c female bred with APLDQ male . | Treatment . | Total pups born from 4 females . | No. of dead or bleeding pups . | % Affected . |
|---|---|---|---|---|
| 1st litter | No treatment | 16 | 5 | 31 |
| 2nd litter | Treated with hIVIG on days 10.5 and 17.5 | 36* | 0 | 0 |
| 3rd litter | No treatment | 26 | 3 | 11.5 |
| 4th litter | Treated with effector-silent mAb 26.4 on days 10.5 and 17.5 | 38* | 1 | 2.6 |
| 5th litter | No treatment | 25 | 2 | 8 |
| Pre-immunized BALB/c female bred with APLDQ male . | Treatment . | Total pups born from 4 females . | No. of dead or bleeding pups . | % Affected . |
|---|---|---|---|---|
| 1st litter | No treatment | 16 | 5 | 31 |
| 2nd litter | Treated with hIVIG on days 10.5 and 17.5 | 36* | 0 | 0 |
| 3rd litter | No treatment | 26 | 3 | 11.5 |
| 4th litter | Treated with effector-silent mAb 26.4 on days 10.5 and 17.5 | 38* | 1 | 2.6 |
| 5th litter | No treatment | 25 | 2 | 8 |
Pups in the % Affected column had both major and minor bleeding.
hIVIG, human IVIG.
There was a modest increase in litter size in females treated with either IVIG or mAb 26.4.
Discussion
The purpose of our investigation was to develop and characterize an authentic alloantigen-specific animal model of FNAIT to facilitate basic, translational, and clinical studies aimed at better understanding, treating, and preventing this alloimmune disorder, which affects up to 1 in 1000 live births annually in the United States and Europe. Previous animal models of this disease have added importantly to our understanding of this disorder. Ni and colleagues28 immunized GPIIIa-deficient mice (whose platelets lack surface expression of the GPIIb-GPIIIa complex) with WT platelets and demonstrated that the resulting polyclonal isoantibodies were capable of inducing FNAIT-like symptoms in the offspring of female mice pre-immunized in this way. The investigators showed that administration of IVIG to pregnant female mice with preexisting anti-GPIIb-GPIIIa polyclonal antibodies decreased the maternal isoantibody titer and dampened the severity of thrombocytopenia and neonatal bleeding. This model was also used to demonstrate that the neonatal Fc receptor FcRn is required for transfer of the pathogenic antibodies from mother to neonate,29 and that co-stimulatory inflammatory agents such as bacterially derived lipopolysaccharide or polyinosinic:polycitidylic acid further exacerbate the maternal iso-immune response.30 Despite the advances provided by using this model of isoimmunity, it is limited by its inability to help determine the efficacy of alloantigen-specific reagents to prevent or treat FNAIT.
A very different model involving the administration of human platelets into the circulation of NOD/SCID mice was developed at about the same time,31 and it was used to investigate the efficacy of antiplatelet therapeutics and the molecular basis of platelet immunologic disorders.32 Bakchoul et al26 exploited this model to demonstrate the therapeutic potential of mAb SZ21 as either an F(ab′)2 fragment or as a deglycosylated effector-silent form to inhibit maternal anti-HPA-1a–mediated destruction of circulating human platelets.33 The latter reagent has the advantage of retaining transplacental transport into the fetus and eliminating recognition of opsonized platelets by phagocytes, thus providing, at least in concept, a novel treatment strategy for antenatal management of FNAIT in pre-alloimmunized women. This model is limited, however, by the short half-life of human platelets circulating in mice and the inability to examine the etiology of the alloimmune response.
To overcome some of these limitations, Ghevaert et al25 transduced the bone marrow of GPIIIa-deficient mice with a lentiviral vector encoding the Leu33 allelic isoform of human GPIIIa under the control of a platelet-specific promoter and transplanted the transduced bone marrow cells into lethally irradiated GPIIIa-deficient mice. They found that platelets in the resulting transplanted mice expressed sufficient levels of the hybrid integrin complex to be able to examine the ability of F(ab′)2 and effector-silent variants of the recombinant HPA-1a–specific mAb B2G1 to prevent anti-HPA-1a–mediated destruction of platelets circulating in an adult mouse. Although this model provided additional support for future therapeutic modalities for treating FNAIT, the model was limited by the need to perform bone marrow transplantation each time HPA-1a+ mice are needed, by the incomplete penetrance of the human allele into the murine platelet population, by the relatively low expression of the hybrid mouse-human GPIIb-GPIIIa complex on the platelet surface, and by the inability to recapitulate important clinical features of FNAIT.
The major contribution of this study is the generation and use of a CRISPR/Cas9 gene-edited mouse that harbors humanized residues within the PSI and EGF1 domains of murine GPIIIa sufficient to drive expression of the human HPA-1a epitope within the context of an intact murine GPIIIa subunit. Selection of the amino acids necessary to recreate the epitopes recognized by both type I and type II anti-HPA-1a human monoclonal and polyclonal alloantibodies was guided by the previous findings of Barron-Casella et al19 and Zhi et al12 that T30→A, S32→P, Q33→L, N39→D, and M470→Q mouse-to-human amino acid substitutions are all that are necessary to support their binding. Unlike previous models of FNAIT, all of the circulating murine platelets express HPA-1a on the platelet surface.12 The resulting APLDQ-allelic form of GPIIIa supports normal hemostasis (supplemental Figure 1), is immunogenic (Figure 2A), and elicits production of an HPA-1a alloantigen-specific polyclonal antibody response (Figure 2B-C). WT BALB/c female mice pre-immunized with APLDQ murine platelets (when subsequently bred with APLDQ males) give birth to pups suffering from profound thrombocytopenia and bleeding (Figures 3 and 4). These conditions could be mitigated by administering a generalized immunosuppressive agent such as IVIG,23,24 which lowers the anti-APLDQ antibody titers, or a modified effector-silent human mAb that inhibits antibody-mediated platelet destruction by blocking the binding of maternal alloantisera in an alloantigen-specific manner (Figure 5). The latter has the distinct potential, at least theoretically, to rescue fetal and neonatal thrombocytopenia without compromising the rest of the maternal immune system. This attribute could not have been examined in any of the previous animal models of FNAIT described above.
mAb 26.4 is unique in that it is the only HPA-1a antibody produced to date that contains a heavy and light chain pair derived from a single, naturally occurring B cell from an HPA-1a alloimmunized woman.15 Together with its property of being highly specific for the HPA-1a allelic isoform of GPIIIa (the β subunit of integrin αIIbβ3), mAb 26.4 has been shown to be effective in masking the HPA-1a epitope expressed on both αIIbβ3 and αvβ3 integrins.15 Thus, mAb 26.4 offers the therapeutic advantage of preventing binding of maternal alloantibodies to both fetal platelet GPIIb-GPIIIa and fetally derived αvβ3 expressed on the surface of placental syncytiotrophoblasts,34 the latter of which has been proposed to potentially affect trophoblast functions crucial for placental development.35 Adopting a similar strategy previously used by Ghevaert et al,25 the Fc region of a recombinant IgG1 isoform of native mAb 26.4 was modified to render it incapable of binding Fc receptors. Injecting native mAb 26.4 into APLDQ mice induces rapid platelet clearance, but the effector-silent Fc variant form has no effect on circulating platelet count (supplemental Figure 3), which allows it to be used for reducing the severity of neonatal thrombocytopenia and bleeding in this animal model of FNAIT (Figure 5B; Table 1) and to potentially be used in the future in human participants.
The availability of APLDQ mice allows a number of outstanding issues relevant to the clinical etiology and treatment of FNAIT to be investigated, but notable differences remain regarding issues in human FNAIT. For example, female BALB/c mice were immunized with APLDQ murine platelets via intravenous injection in the absence of adjuvant or via intraperitoneal injection in the presence of adjuvant. Although neither truly mimic the transplacental route of maternal immunization by fetal platelets that occurs during a 9-month pregnancy in humans, there was a much more robust alloimmune response using the intraperitoneal plus adjuvant injection route (supplemental Figure 2), which led to a pronounced FNAIT phenotype that was desired to examine the potential efficacy of anti-FNAIT therapeutics. Overt immunization of WT mice with APLDQ platelets was necessitated by the inability of naïve female mice to generate an alloimmune response to the APLDQ alloantigen solely as a result of pregnancy, even after 3 sequential matings with APLDQ homozygous males (data not shown). It is not known whether this is a result of genetic and/or environmental factors unique to BALB/c mice housed under pathogen-free conditions or the possibility that pregnant female mice are ignorant of or tolerant to the HPA-1a antigen presented by the fetus. The fact that female mice are exposed to the fetal alloantigens for only a 3-week gestation period, compared with the rather lengthier exposure that can take place during a 40-week gestation period in humans, may also contribute to the inability of mice to generate an APLDQ-specific alloimmune response during pregnancy, which remains an unfortunate limitation of this murine model. Experiments designed to examine and distinguish between these possibilities are in progress, with the hope of developing a second-generation model with the additional attribute of acquiring alloimmunization during pregnancy. Whereas severe thrombocytopenia is found in only ∼25% of neonates born to alloimmunized women (3% to 5% of whom experience severe bleeding problems such as intracranial hemorrhage36 ), we observed severe thrombocytopenia in nearly 100% of neonates born to overtly immunized mice, ∼20% to 30% of whom experienced severe bleeding (Figures 3-5), perhaps because of the rather harsher environmental conditions to which neonatal mice are exposed.
In humans, transmission of passive humoral immunity from the mother to the fetus is primarily mediated by the neonatal Fc receptor FcRn, which is expressed in fetally derived syncytiotrophoblasts37 where it transports immunoglobulins, primarily of the IgG subclass, from the maternal into the fetal circulation.38 Alloantibodies present in milk have also been found to prolong platelet clearance in newborns of women with immune thrombocytopenia,39 and ongoing red cell destruction in cases of hemolytic disease of the newborn.40 The clinical importance of milk-supplied HPA-specific alloantibodies in prolonging neonatal thrombocytopenia is less certain. A single case report of a breast-fed infant (despite breast milk that was positive for anti-HPA-1a antibodies by enzyme-linked immunosorbent assay) found no evidence for persistent thrombocytopenia.41 In contrast, in rodents, the majority of maternal IgG is delivered to pups postnatally in milk where, once ingested, it is transported by FcRn located on the surface of epithelial cells of the proximal small intestine.42 FcRn is notably absent from the placenta, with only small numbers of receptors present in the yolk sac endoderm,43 which results in inefficient antenatal transfer of Ig’s from mother to fetus.44 That maternal anti-platelet antibodies can cause thrombocytopenia during gestation, before this report, was somewhat of an unresolved issue. Chen et al29 were able to detect anti-GPIIb-GPIIIa isoantibodies in neonatal mice shortly after birth, suggesting that maternally derived antibodies were already present in the pups during gestation. Our finding (Figure 3B) of thrombocytopenia in post-coitus day 19.5 fetuses of mothers with high-titer anti-HPA-1a antibodies demonstrates that alloantibody levels sufficient to cause FNAIT can be transferred transplacentally in mice. Whether milk-derived anti-HPA-1a alloantibodies are able to mediate persistent postnatal thrombocytopenia in this animal model of FNAIT is an important, and perhaps clinically relevant, area for future investigation.
The development of an immune response to HPA-1a is one of the most MHC-restricted examples of alloimmunity in humans4,45-49 ; >92% of HPA-1b/b mothers who develop anti-HPA-1a antibodies express at least 1 HLA DRB3*01:01 class II allele. It is worth noting that BALB/c mice (H-2 I-Ad, I-Ed) dependably developed a robust response to the APLDQ humanized form of murine GPIIIa (Figure 2), but C57BL/6 mice (H-2 I-Ab, I-Enull) barely responded at all (not shown). This was similar to the findings of Sayeh et al18 that C57BL/6 mice are generally poor responders to alloantigenic polymorphisms. More studies that use additional strains of mice will be required to determine whether the immune response to the HPA-1a alloantigen is similarly MHC-restricted in mice.
Although FNAIT can occur without warning in first pregnancies, the incidence and severity is higher in second and third pregnancies, especially if the father is homozygous for the HPA-1a allele.50 Treatments to lessen the severity of FNAIT in secundigravida and beyond have ranged from in utero transfusion of maternal platelets51 to low-dose steroids52 to suppression of the maternal immune system by administering IVIG.24,53 The availability of the first alloantigen-specific animal model of FNAIT should provide investigators with a valuable new tool for evaluating agents designed to treat,36 and perhaps prevent, 54,55 alloimmunization to the HPA-1a epitope, and facilitate contemplated clinical trials in humans aimed at managing this too often serious alloimmune platelet disorder affecting fetuses and neonates.
Acknowledgments
The authors thank Ke Yan, PhD, from the Quantitative Health Sciences Program at the Medical College of Wisconsin for assistance with statistical analysis, and Gestur Vidarsson who generated and kindly supplied recombinant WT and effector-silent forms of mAb 26.4.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL-130054 and R35 HL139937) (P.J.N.) and grants from Helse Nord Regional Health Authority (HNF1354-17) (B.S.) and (SFP1281-16) (M.T.A.).
Authorship
Contribution: H.Z. designed and performed experiments, interpreted results, and wrote the paper; M.T.A. designed experiments, interpreted results, and edited the paper; B.S. edited the paper; D.K.N. designed experiments, interpreted results, and edited the paper; and P.J.N. designed experiments, interpreted results, and wrote the paper.
Conflict-of-interest disclosure: P.J.N. is a consultant for Rallybio, Inc. B.S. is an owner and co-founder of Prophylix Pharma. The remaining authors declare no competing financial interests.
Correspondence: Peter J. Newman, Blood Research Institute, Versiti Blood Center of Wisconsin, 8727 W. Watertown Plank Rd, Milwaukee, WI 53226; e-mail: pjnewman@versiti.org.
References
Author notes
All data associated with this study are available in the main text or the supplemental Data. Materials and mice are freely available to academic and not-for-profit investigators with appropriate material transfer agreements in place.
The full-text version of this article contains a data supplement.



![Maternal anti-HPA-1a alloantisera cause severe fetal and neonatal thrombocytopenia. (A) Pre-immunized WT BALB/c mice bred with APLDQ males give birth to severely thrombocytopenic pups. Shown are the platelet counts (mean ± standard error of the mean [SEM]; n = 3-10 mice per group) of neonatal pups 48 hours after birth from 3 representative BALB/c females that had been pre-immunized with APLDQ platelets. Although litter sizes were normal to near normal, all of the newborn pups were nearly devoid of circulating platelets. Control 1: platelet counts of pups derived from a female BALB/c mouse that had not been pre-immunized with APLDQ platelets before breeding with an APLDQ C57BL/6 male. Control 2: platelet count of pups derived from WT BALB/c females that had been pre-immunized with platelets from an APLDQ-expressing C57BL/6 mouse before being bred with a WT C57BL/6 male. Note that alloantibodies produced as a result of the difference in class I MHC molecules between the 2 strains of mice (supplemental Figure 2) result in neither thrombocytopenia nor FNAIT, much the same as in humans. (B) Maternal antibody levels correlate inversely with fetal platelet count. Maternal blood samples were collected on day 19.5 of gestation and subjected to flow cytometric analysis against APLDQ C57BL/6 murine platelets. Fetuses were collected in parallel, the blood of littermates was pooled, and the platelet count of each pool was determined. Each data point represents the average platelet count of a single litter. **P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/18/10.1182_bloodadvances.2021004371/3/m_advancesadv2021004371f3.png?Expires=1771039926&Signature=DIwaJN-0Ig24MROLytboNbiPgVhfce7e79upE7hh4FJXKZyLcTYiqbev-MQ973nZuqJ3rh3mk~tjvZwZCnNflgZdee-frBqCoeRBT1Flk7etfeCGFIHdV7BpfXMdgbLTdF091kzDU3dDpHQKVMcaH4ByyR6vYOk54AXREz6VfL2iU2unUI0bmcUhz8tNQkBMoNngG~BaHJUjHJvp5VOvkpjC4~ZiRlrlt4~ShHvw63zoiwGRxcwAiZil92L8N1itTi7NK9ckRuUkREK1NQTvFHfxzMSpQDoFuPn7lRy-hzuWVE8AfoJrpUt7OrcaiK0~UH1z64zKJxN6Hz2Ywo4~tA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

