Abstract
Since the introduction of imatinib, the management of chronic myeloid leukemia (CML) has changed considerably. Tyrosine kinase inhibitors (TKIs) are the mainstay of CML treatment; however, the high financial burden of TKIs can be problematic for both the patients and health care systems. After the emergence of generics, reimbursement policies of many countries have changed, and generics offered an alternative treatment option for CML patients. There are many papers published on the use of generics in CML patients with conflicting results regarding both efficacy and safety. In this paper, we systematically reviewed the current literature on generic imatinib use in CML, and 36 papers were evaluated. Both in vitro and in vivo studies of generic imatinib showed comparable results with branded imatinib in terms of bioequivalence and bioavailability. In most studies, generics were comparable with the original molecule in terms of efficacy and safety, both in newly diagnosed patients and after switching from Gleevec. Some generic studies showed contradictory findings regarding efficacy and toxicity, and these differences can be attributed to some factors including the use of different generics in different countries. Both in hypothetical models and in real life, introduction of generic imatinib caused significant reduction in health care costs. In conclusion, generics are not inferior to original imatinib in terms of efficacy with an acceptable toxicity profile. Notwithstanding the generally favorable efficacy and safety of generics worldwide to date, we most probably still need more time to draw firmer conclusions on the longer-term outcomes of generics.
Introduction
After the approval of first BCR-ABL1 tyrosine kinase inhibitor (TKI), imatinib, the management of chronic myeloid leukemia (CML) has changed considerably. Currently, TKIs are the mainstay of CML treatment1-3; however, the high financial burden of these therapies can be a serious problem for both the patients and health care systems.
With the emergence of generic imatinib, reimbursement policies of many countries have been changed, and generics became an alternative treatment option for CML patients.4 Besides the possible positive impacts of generic imatinib use on health care systems, there are concerns about these drugs including bioequivalence, efficacy, safety, tolerability, adherence, persistence, and health care costs.
In a survey consisting of 1518 CML patients and 259 hematologists, TKI reimbursement policies, prices of TKIs and new drug development were the 3 main concerns raised by the patients, whereas most hematologists had focused on other issues including new drug development, dose adjustments, and monitoring of patients.5 Both patients and physicians carried important level of concern regarding the use of generics and the quality of these drugs; however, these concerns were significantly higher among hematologists than that of patients.5
Although most of the studies reported comparable efficacy and safety results in CML patients receiving generics and the original molecule,4 there are some papers displaying inferior outcomes in patients receiving generics.6-9
In this paper, we systematically reviewed the current literature, showing the available data mainly on the efficacy and safety of generic imatinib in the management of CML patients. In addition, the impact of generics on health care costs were also evaluated.
Methods
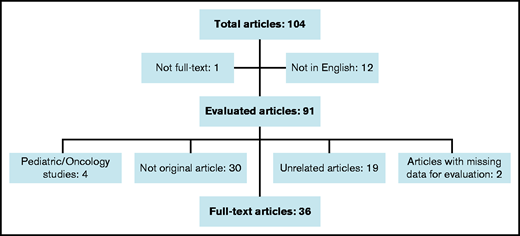
We used the PubMed database for a systematic literature search for full-text articles by using the terms “generic” and “imatinib.” We accessed 91 articles in English through December 2020. Reviews, case reports, correspondences, duplicates, studies reporting pediatric CML patients, and papers unrelated to this article were excluded. In the end, 33 full-text articles and 3 letters were included in this review (Figure 1).
Results
All studies are classified under 3 headlines: pharmacologic properties and bioequivalence; efficacy and safety; and impact on health care costs. These studies are summarized in Tables 1 to 3.
Studies of generic imatinib evaluating the pharmacologic properties and bioequivalence
| Authors . | Country . | Study type . | Sample size . | Results and comments . |
|---|---|---|---|---|
| Grillo et al10 | Argentina | In vitro | NA | Original imatinib was produced in β-crystal form while generics mostly in α-crystal form which was observed as less stable than β-form in room temperature |
| Yokoo et al11 | Japan | In vitro | NA | Comparable OS rates with original and generic imatinib in CML cell lines |
| Parrillo-Campiglia et al19 | Uruguay | In vivo | 30 | Mean test/reference ratios for AUC and Cmax:95% and 97% (CI: 90%), respectively |
| Ostrowicz et al18 | Poland | In vivo | 80 | Comparable Tmax, Cmax, AUC0-24 values with original and generic imatinib. Acceptable therapeutic limits (90-111.11%) for generic product |
| Malhotra et al14 | India | In vivo | 131 | Comparable mean imatinib trough levels with original imatinib (n = 84) and generic (n = 47; P = .079) |
| Ostojic et al12 | Croatia | In vivo | 24 | 75% of branded and 89% and 100% of generic pts achieved IPC ≥ 1000 ng/mL |
| Arora et al17 | India | In vivo | 42 | Mean test/reference Cmax and AUC0-24 ratios: 99% and 99.2%, respectively (P = .78 and P = .99; CI: 90%) |
| Natarajan et al13 | India | In vivo | 206 | All patients (original = 130, generics = 76) reached plasma concentration above 1000 ng/ml (P = .964). |
| Authors . | Country . | Study type . | Sample size . | Results and comments . |
|---|---|---|---|---|
| Grillo et al10 | Argentina | In vitro | NA | Original imatinib was produced in β-crystal form while generics mostly in α-crystal form which was observed as less stable than β-form in room temperature |
| Yokoo et al11 | Japan | In vitro | NA | Comparable OS rates with original and generic imatinib in CML cell lines |
| Parrillo-Campiglia et al19 | Uruguay | In vivo | 30 | Mean test/reference ratios for AUC and Cmax:95% and 97% (CI: 90%), respectively |
| Ostrowicz et al18 | Poland | In vivo | 80 | Comparable Tmax, Cmax, AUC0-24 values with original and generic imatinib. Acceptable therapeutic limits (90-111.11%) for generic product |
| Malhotra et al14 | India | In vivo | 131 | Comparable mean imatinib trough levels with original imatinib (n = 84) and generic (n = 47; P = .079) |
| Ostojic et al12 | Croatia | In vivo | 24 | 75% of branded and 89% and 100% of generic pts achieved IPC ≥ 1000 ng/mL |
| Arora et al17 | India | In vivo | 42 | Mean test/reference Cmax and AUC0-24 ratios: 99% and 99.2%, respectively (P = .78 and P = .99; CI: 90%) |
| Natarajan et al13 | India | In vivo | 206 | All patients (original = 130, generics = 76) reached plasma concentration above 1000 ng/ml (P = .964). |
CI, confidence interval; NA, not available.
Summary of studies evaluating the outcomes of generics after switching from the original molecule
| Authors . | Country . | Sample size, n . | Line of treatment (switched from branded IM/frontline) . | Median duration of branded IM before switch, years (range) . | Response level at the time of switch . | Median follow-up duration after switch, mo (range) . | Molecular response after switch (%) . | New or worsening adverse events at all grades after switch . | Name of generic and/or manufacturer . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCyR (%) . | ≥MMR (%) . | Stable . | Improved . | Worsened . | ||||||||
| Alwan et al9 | Iraq | Generics: 126 | Switched | 4 (0.5-7) | NR | NR | ≥9* | NR | NR | NR | NR | Imatib (Cipla) |
| Eskazan et al20 | Turkey | Generics: 80; Branded IM: 65 | Switched (n: 76) Frontline (n: 4) | ∼5 | 100 | 77 | 12 (4-16) | 83.75 | 8.75 | 7.5 | NR | Imatis (Deva): ● n = 63 (switched) ● n = 2 (frontline) Imatenil (Logus) ● n = 13 (switched) Imavec (Koçak) ● n = 2 (frontline) |
| Bonifacio et al21 | Italy | Generics: 294 | Switched | 7.4 (0.5-16.7) | NR | 92 | 7.5 (0-12.2) | 61 | 25 | 14 | 17% | Imatinib Sandoz |
| Lejniece et al22 | Latvia | Generics: 25 | Switched | ≥ 2 | NR | 100 | 24 | 100 | 0 | 0 | NR | Tibaldix Meaxin Imatinib Teva Itivas Imatinib Accord Imatinib Sano Swiss |
| Abou Dalle et al23 | United States | Generics: 38 | Switched | 2 (1.5-17) | 100 | 95 | 19.4 (3.4-46.3) | 89 | 8 | 3 | 39% | Sun Pharmaceuticals Apetex Teva Mylan |
| Scalzulli et al24 | Italy | Generics: 168 | Switched | 12 (1-16) | 100 | 100 | 19 (4-22) | 84 | 6 | 10 | 20% (15% ≥grade 3) | Imatinib Accord |
| Gemelli et al25 | Italy | Generics: 200 | Switched | 8.9 (3-17.4) | 100 | 100 | 20 | 69 | 25.5 | 5.5 | NR† | Accord (n = 117) Sandoz (n = 41) Teva (n = 109) Dr. Reddy (n = 1) Mylan (n = 1) |
| Authors . | Country . | Sample size, n . | Line of treatment (switched from branded IM/frontline) . | Median duration of branded IM before switch, years (range) . | Response level at the time of switch . | Median follow-up duration after switch, mo (range) . | Molecular response after switch (%) . | New or worsening adverse events at all grades after switch . | Name of generic and/or manufacturer . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCyR (%) . | ≥MMR (%) . | Stable . | Improved . | Worsened . | ||||||||
| Alwan et al9 | Iraq | Generics: 126 | Switched | 4 (0.5-7) | NR | NR | ≥9* | NR | NR | NR | NR | Imatib (Cipla) |
| Eskazan et al20 | Turkey | Generics: 80; Branded IM: 65 | Switched (n: 76) Frontline (n: 4) | ∼5 | 100 | 77 | 12 (4-16) | 83.75 | 8.75 | 7.5 | NR | Imatis (Deva): ● n = 63 (switched) ● n = 2 (frontline) Imatenil (Logus) ● n = 13 (switched) Imavec (Koçak) ● n = 2 (frontline) |
| Bonifacio et al21 | Italy | Generics: 294 | Switched | 7.4 (0.5-16.7) | NR | 92 | 7.5 (0-12.2) | 61 | 25 | 14 | 17% | Imatinib Sandoz |
| Lejniece et al22 | Latvia | Generics: 25 | Switched | ≥ 2 | NR | 100 | 24 | 100 | 0 | 0 | NR | Tibaldix Meaxin Imatinib Teva Itivas Imatinib Accord Imatinib Sano Swiss |
| Abou Dalle et al23 | United States | Generics: 38 | Switched | 2 (1.5-17) | 100 | 95 | 19.4 (3.4-46.3) | 89 | 8 | 3 | 39% | Sun Pharmaceuticals Apetex Teva Mylan |
| Scalzulli et al24 | Italy | Generics: 168 | Switched | 12 (1-16) | 100 | 100 | 19 (4-22) | 84 | 6 | 10 | 20% (15% ≥grade 3) | Imatinib Accord |
| Gemelli et al25 | Italy | Generics: 200 | Switched | 8.9 (3-17.4) | 100 | 100 | 20 | 69 | 25.5 | 5.5 | NR† | Accord (n = 117) Sandoz (n = 41) Teva (n = 109) Dr. Reddy (n = 1) Mylan (n = 1) |
IM, imatinib; NA, not available; NR, not reported.
Patients switched back to branded imatinib after at least 9 months of generic use but exact median duration under generic is not reported.
Fatigue, muscle cramps, myalgia, edema of limbs and periorbital area, diarrhea, and rash were significantly lower with generics.
Studies evaluating the outcomes generics in the frontline setting
| Authors . | Country . | Sample size, n . | Median follow-up duration (mo) . | Cumulative CCyR rates (%) . | Cumulative MMR rates (%) . | Adverse events (≥grade 3) (%) . | Results and comments . | Name of generic and/or manufacturer . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Branded IM . | Generics . | P . | Branded IM . | Generics . | P . | Branded IM . | Generics . | ||||||
| Eskazan et al, 2014,40 | Turkey | Generics: 26; Branded IM: 36 | Generic arm: 8.5; Branded IM arm: 20 | 56 | 52 | .818 | 33 | 33 | 1 | NR | NR | Rates of switching to second generation TKIs due to resistance and dose reduction because of AEs were comparable | NA |
| Chikkodi et al, 2015,31 | India | Generics: 28; Branded IM: 103 | 12 for both arms | NA | NA | NA | NR | NR | NR | NR | NR | No significant differences were observed at CHR or molecular responses at 3, 6, and 12 mo of therapy between arms | NA |
| Eskazan et al, 2017,28 | Turkey | Generics: 43; Branded IM: 47 | Generic arm: 13; Branded IM arm: 32.5 | 93 | 83 | .25 | 89 | 59 | .009 | NR | NR | Comparable EMR rates at 3 mo, OR rates at 6 mo; however, MMR rate at 6 mo is superior in branded IM arm | NA |
| Entasoltan et al, 2017,29 | Algeria | Generics: 355 | 46 | NA | NR | NA | NA | 67 | NA | NA | 27* | Similar efficacy and safety profile comparing IRIS trial | Imatib (Cipla) |
| Danthala et al, 2017,26 | India | Generics: 144; Branded IM: 1067 | ∼46 for both arms | 70 | 69 | NR | 23 | 15 | NR | 0 | 0 | Comparable CCyR, MMR, DMR, EFS, FFS, TFS, OS and adherence rates between arms | Veenat (Natco) |
| Nekoohesh et al, 2020,30 | Iran | Generics: 177 | 34.8 | NA | NR | NA | NA | 61 | NA | NA | NR | Lower MMR rates at 6 and 12 mo of generic imatinib comparing IRIS data (25.2% vs 33.3% and 44.2% vs 50.3%, respectively, P = not reported) | NA |
| Phukan et al, 2020,32 | India | Generics: 76 | 12 | NA | 52 | NA | NA | 44 | NA | NA | 28.9† | 44.7% and 41.3% of patients achieved optimal response at 6 and 12 mo of therapy, respectively | NA |
| Dou et al, 2020,27 | China | Generics: 210; Branded IM: 238 | Generic arm: 30; Branded IM arm: 34 | 88.8 | 89.4 | .782 | 72.8 | 64.8 | .138 | 8.3 | 7.7 | 4-y probabilities of achieving CCyR for branded and generic imatinib: 97.0% vs 97.3%; P = .736, MMR: 87.8% vs 90.1%; P = .113, respectively | Xinwei (Hansoh); genike (Chiatai Tianqing) |
| Authors . | Country . | Sample size, n . | Median follow-up duration (mo) . | Cumulative CCyR rates (%) . | Cumulative MMR rates (%) . | Adverse events (≥grade 3) (%) . | Results and comments . | Name of generic and/or manufacturer . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Branded IM . | Generics . | P . | Branded IM . | Generics . | P . | Branded IM . | Generics . | ||||||
| Eskazan et al, 2014,40 | Turkey | Generics: 26; Branded IM: 36 | Generic arm: 8.5; Branded IM arm: 20 | 56 | 52 | .818 | 33 | 33 | 1 | NR | NR | Rates of switching to second generation TKIs due to resistance and dose reduction because of AEs were comparable | NA |
| Chikkodi et al, 2015,31 | India | Generics: 28; Branded IM: 103 | 12 for both arms | NA | NA | NA | NR | NR | NR | NR | NR | No significant differences were observed at CHR or molecular responses at 3, 6, and 12 mo of therapy between arms | NA |
| Eskazan et al, 2017,28 | Turkey | Generics: 43; Branded IM: 47 | Generic arm: 13; Branded IM arm: 32.5 | 93 | 83 | .25 | 89 | 59 | .009 | NR | NR | Comparable EMR rates at 3 mo, OR rates at 6 mo; however, MMR rate at 6 mo is superior in branded IM arm | NA |
| Entasoltan et al, 2017,29 | Algeria | Generics: 355 | 46 | NA | NR | NA | NA | 67 | NA | NA | 27* | Similar efficacy and safety profile comparing IRIS trial | Imatib (Cipla) |
| Danthala et al, 2017,26 | India | Generics: 144; Branded IM: 1067 | ∼46 for both arms | 70 | 69 | NR | 23 | 15 | NR | 0 | 0 | Comparable CCyR, MMR, DMR, EFS, FFS, TFS, OS and adherence rates between arms | Veenat (Natco) |
| Nekoohesh et al, 2020,30 | Iran | Generics: 177 | 34.8 | NA | NR | NA | NA | 61 | NA | NA | NR | Lower MMR rates at 6 and 12 mo of generic imatinib comparing IRIS data (25.2% vs 33.3% and 44.2% vs 50.3%, respectively, P = not reported) | NA |
| Phukan et al, 2020,32 | India | Generics: 76 | 12 | NA | 52 | NA | NA | 44 | NA | NA | 28.9† | 44.7% and 41.3% of patients achieved optimal response at 6 and 12 mo of therapy, respectively | NA |
| Dou et al, 2020,27 | China | Generics: 210; Branded IM: 238 | Generic arm: 30; Branded IM arm: 34 | 88.8 | 89.4 | .782 | 72.8 | 64.8 | .138 | 8.3 | 7.7 | 4-y probabilities of achieving CCyR for branded and generic imatinib: 97.0% vs 97.3%; P = .736, MMR: 87.8% vs 90.1%; P = .113, respectively | Xinwei (Hansoh); genike (Chiatai Tianqing) |
NA, not available; NR, not reported; TFS, transformation-free survival.
Highest grade 3 to 4 AE rate (bone and joint pain). Cumulative rate of grade 3 and 4 AEs was not reported.
Highest grade 3 to 4 AE rate (anemia). Cumulative rate of grade 3 to 4 AEs was not reported.
Pharmacologic properties and bioequivalence of the generics
The crystal form of an active component of a drug may cause differences in solubility, stability, density, melting point, processability, and so on. Original imatinib was produced in β-crystal form, whereas generics are mostly in α-crystal form, which was observed to be less stable than the β-form in room temperature.10 However, after several in vitro and in vivo studies comparing the pharmacologic properties of reference molecule and generics, both forms were proved to be equal.
Yokoo et al11 investigated the antileukemic effects of one generic imatinib on CML cell lines, and they reported comparable growth inhibitory and proapoptotic effects for both branded and generic imatinib. The authors also studied mouse models of BCR-ABL1–induced leukemia, and they showed no significant difference in terms of overall survival (OS) rates for the original and generic imatinib.11
There are 3 in vivo studies evaluating the plasma trough levels of generic imatinib.12-14 Ostojic et al12 compared plasma imatinib concentrations (IPCs) in 24 patients with CML in chronic phase (CML-CP), who were switched from branded imatinib to 2 generics (imakrebin and neopax). An adequate IPC for an optimal response was defined to be ≥1000 ng/mL, as was previously shown in the original imatinib studies.15,16 After consuming 400 mg generic imatinib for at least 1 month, blood samples were collected 21 to 24 hours after the last dose. No significant difference was shown between the original and 2 generic molecules, with 89% of all patients achieving an adequate IPC. Interestingly, suboptimal IPC measurements because of suboptimal adherence were more common with original imatinib compared with generics (33%, 13%, and 0% of the patients receiving Gleevec, imakrebin, and neopax, respectively; P value was not reported).12
Imatinib plasma trough levels were also evaluated among 206 CML-CP patients.13 One hundred thirty patients were under original imatinib, whereas the remaining 76 were treated with 2 different generics. Imatinib plasma trough levels were above 1000 ng/mL in all patients, and there was no significant difference between the mean plasma trough levels of original and generic drugs.13
The study by Malhotra et al14 also demonstrated similar findings, in which the authors compared 84 and 47 patients receiving branded and generic imatinib regarding imatinib plasma trough levels, respectively. Consistent with the previous studies, patients with plasma trough levels higher than 988 ng/mL had superior molecular and cytogenetic response rates. In addition, plasma trough levels of imatinib did not differ between cases receiving branded imatinib and generics.14
In a multicenter, randomized, open-label, single dose bioequivalence study, peak plasma concentration (Cmax), time to reach peak plasma concentration (Tmax), and area under the plasma concentration vs time curve from time zero to 24 hours (AUC0-24) values of 40 CML-CP patients receiving original and generic imatinib were evaluated.17 Patients received imatinib 400 mg daily for 8 days, and blood samples were collected at days 1 and 8. The results were comparable between Gleevec and generic imatinib, and mean test/reference Cmax and AUC0-24 ratios were 99% and 99.2%, with 90% confidence intervals, respectively.17
Tmax, Cmax, and AUC results in 80 healthy volunteers receiving both original (n = 37) and generic (n = 43) imatinib were shown to be comparable in a single dose study.18 In a study from Uruguay, similar results were displayed among 30 male CML-CP patients.19
Overall, it is not wrong to say that the available in vitro and in vivo studies appear to reach a consensus on the bioequivalence and bioavailability of generics compared with the original molecule (Table 1).
Efficacy and safety of generic imatinib
Some studies showed contradictory findings regarding the efficacy and toxicity of generics. In a patient with deep molecular response (DMR, molecular response level better than MR4.0) under original imatinib, this response was lost after 3 months of generic imatinib, and DMR was reachieved after rechallenging the original molecule.6 Another CML-CP patient from India experienced loss of complete hematologic response (CHR) detected 3 months after switching from branded imatinib to generic.7 This patient also reachieved CHR shortly after switching back to branded imatinib and complete cytogenetic response (CCyR) was gained thereafter. Both patients received the generic imatinib named imatib (manufactured by Cipla, India).
Similarly, loss of response was detected in 5 of 8 CML-CP patients from Columbia after switching to generic imatinib (name of generic was not given), whereas another 4 newly diagnosed patients failing to achieve major cytogenetic response after 3 to 5 months of generic imatinib.8 In a prospective study from Iraq, CHR was lost in one-third of 126 patients with CML-CP after switching to generic imatinib (imatib).9
Besides these papers,6-9 no additional data reporting inferior outcomes with generics are available in the literature to date (Tables 2 and 3). Authors might have had a tendency to report patients experiencing inferior outcomes, especially when there was not enough evidence on the use generics in the real-life setting. In 3 of these articles,7-9 the authors stated that financial support for medical editorial assistance was provided by the pharmaceutical company that produces branded imatinib, which raises some questions. In addition, these differences can be caused by other factors including intra- and interpatient variability in the pharmacokinetics of generics and possibly patients’ adherence.
Six studies evaluated the efficacy of generics after switching from original imatinib,20-25 whereas another 7 compared Gleevec with generics in the upfront setting.26-32 Additionally, 4 studies reported the combination of both frontline and subsequent uses of generics.9,33-35 All studies evaluated treatment responses according to the European LeukemiaNet recommendations36,37; however, 1 study9 assessed patients according to hematologic response level only, and the authors declared results favoring original imatinib in terms of both efficacy and safety (Table 2).
From the safety perspective, although no statistical analysis was made, 2 studies23,24 reported considerably worsening adverse events (AEs) following switching to generic imatinib treatment, which were mostly classified as mild or moderate. Two additional studies showed significantly worsening toxicities under generics emerging after switching from the original molecule.38,39 Most of these AEs were nausea, diarrhea, edema, muscle cramps, and fatigue, but only a couple of patients switched back to branded imatinib because of gastrointestinal toxicities in both studies. On the other hand, a recently published study displayed significantly improved muscle cramps, edema, fatigue, and diarrhea after switching to generics.25 Other studies demonstrated comparable results between the original molecule and generics regarding safety.26,27
Generics after switching from frontline original imatinib.
In our center, we retrospectively evaluated 145 CML-CP patients, 65 of which only received original imatinib during the entire follow-up, whereas the remaining 80 had generics.20 Of these 80 patients, 76 were switched to generic imatinib after receiving the original molecule with a median follow-up of 55 months. The percentages of patients who maintained their responses in both groups were comparable, and the cumulative major molecular response (MMR) rates of original and generic groups were similar (77% vs 75%). During the follow-up, 10 and 7 cases with CCyR achieved MMR from the patients receiving original imatinib and patients with a switch, respectively, whereas 5 patients in both patient groups lost their responses.20
In an Italian retrospective study, the authors analyzed the sustainability of the achieved responses after switching to generic from branded imatinib. They included 140 CML patients of whom 27% were in MMR and 73% were in DMR at the time of switch.24 With a median follow-up of 19 months after switch, 84% of patients maintained their responses, whereas 6% improved their level of responses. Molecular fluctuations were detected in 10% of the cases, but none of these patients, except one, lost their responses during follow-up. Following the switch, AEs worsened in 20% of the cases, but only 15% of these AEs were recorded as grade ≥3. Two patients were switched back to branded imatinib because of toxicities.24
In 2019, the researchers from the MD Anderson Cancer Center published a retrospective analysis consisting of 38 patients switching to generic imatinib after the original molecule.23 With a median follow-up of 19.4 months under generic imatinib, 89% of the patients maintained their molecular response levels, whereas improvement and worsening of molecular responses were detected in 8% and 3% of the cases, respectively. Fifteen patients (39%) reported newly developed or worsened AEs after the switch.23 Three patients were switched back to branded imatinib because of AEs, whereas second-generation TKIs (2GTKIs) were started in 2 additional cases because of increased serum creatinine levels under generic imatinib.
In a study with 200 CML-CP patients who switched to generics after achieving stable CCyR at least for 18 months with at least 36 months of original imatinib use, a median of 3 polymerase chain reaction results performed within the 12-month period before and within 20 months after the switch were separately used to evaluate efficacy. The median of 3 polymerase chain reaction results before the switch was 0.4 × 10−4, which was remarkably decreased to 0.1 × 10−4 after switching to generics (P = .003). Sustained, improved, and worsened molecular response rates under generics were 69%, 25.5%, and 5.5%, respectively.25 Interestingly, the rate of AEs at any grade was 54.5% under generic imatinib therapy, whereas this was 85% with branded imatinib. In addition, the rates of grade 3 to 4 toxicities under branded imatinib and generics were 8% and 3.5%, respectively. Fatigue, muscle cramps, myalgia, edema, diarrhea, and rash were significantly lower under generic imatinib, and only 3 patients switched back to branded imatinib because of intolerance.25 There were 2 possible explanations for these findings: first, better tolerability resulting from long-term generic use and second, but more importantly, possible underestimation of AEs because of extraction of the toxicity data directly from the physicians but not from the patients.
Generic imatinib in newly diagnosed patients.
We conducted a study on the efficacy and tolerability of branded and generic imatinib in 62 de novo CML-CP patients.40 Thirty-six patients received original imatinib, whereas 26 were treated with a generic. CCyR and MMR rates at 6 months were comparable between groups. In addition, the percentages of patients switching to a 2GTKI because of resistance and imatinib dose reduction because of toxicities were similar in 2 groups.40
Our group also assessed the frontline use of generic imatinib in 43 cases with CML-CP and compared the findings with 47 patients receiving first-line Gleevec in 2017.28 Early molecular response (EMR) rates at 3 months and the cumulative CCyR and MMR rates were comparable between groups.
Danthala et al26 retrospectively analyzed 1067 CML-CP patients receiving upfront Gleevec and 144 patients with generics in terms of both efficacy and safety. Cumulative CCyR rates were comparable between groups (70% vs 69%, respectively; P value was not reported), whereas DMR rates in patients receiving generics were slightly superior than that observed in cases with frontline Gleevec (26% vs 17%, respectively; P value was not given). Event-free survival (EFS), failure-free survival (FFS), transformation-free survival, and OS rates were also similar for both patient groups. There were no grade 3 to 4 AEs reported in both groups, but grade 1 to 2 edema was more frequent in patients with branded imatinib than that of patients receiving generics (12% vs 5%, respectively).26
A multicentric retrospective study from Algeria with 355 CML-CP patients who received generic imatinib showed that, with a median follow-up of 46 months, 21% and 35% of patients achieved MMR at 3 and 6 months of therapy, respectively, whereas the 5-year OS rate was 83%.29 In another study from India, molecular response rates at 3 and 6 months were similar in 103 and 28 newly diagnosed patients receiving branded imatinib and generics, respectively.31
Supporting previous findings, in a study consisting of 255 Iranian CML patients receiving frontline generic imatinib, MMR rates at 3, 6, and 12 months of TKI therapy were 15.38%, 25.18%, and 44.1%, respectively, whereas the 7-year probability of OS was reported as 94.3%.30
In the study by Dou et al,27 the investigators retrospectively evaluated 442 CML-CP patients receiving branded (n = 206) or generic (n = 236) imatinib in the frontline setting. Thirty-one (15%) and 34 (14.4%) patients from the branded and generic imatinib groups experienced treatment failure and switched to 2GTKIs, respectively. Cumulative CCyR, MMR, and DMR rates were comparable between groups. Hematologic and nonhematologic grade ≥ 3 AEs were also similar in both arms. In addition, probability of FFS, PFS, and OS at 4 years also showed no significant difference between patients receiving the original molecule and generics.27
Generic imatinib as both upfront and subsequent lines of therapy.
Ćojbašić et al34 presented the data of 43 patients receiving generic imatinib as frontline therapy with 40 patients switching from original imatinib. Among the switching cases, 95% and 87.5% of the patients were in CCyR and MMR at the time of switch, respectively. Median follow-up of frontline generic group and switching patients were 28.1 and 43.8 months, respectively. CCyR rates were 67.4% and 76.7% for patients receiving upfront generic at 6 and 12 months of TKI therapy, respectively, and 25 of 43 patients (58.1%) achieved MMR at 12 months. After 24 months of generic imatinib, the percentages of patients who maintained, achieved, and lost MMR among cases with a switch were 72.5%, 12.5%, and 15%, respectively. There were no significant differences in cumulative MMR rates, hematologic and nonhematologic AE rates between the frontline generic group and the switching patients (P = .053, P = .097, and P = .151, respectively). Estimated 10-year OS, PFS, and EFS rates for the switch patients were 93.8%, 97.1%, and 41.2%, respectively, whereas the 5-year estimations of these parameters among cases with frontline generic therapy were 86.1%, 89.8%, and 48.8%, respectively.34 Although recruiting lower number of patients, other generic imatinib studies reported similar results in terms of efficacy.22,33,35
Most studies did not specifically investigate the topic of adherence and persistence, and there are only 2 studies evaluating these concepts in CML patients receiving generic imatinib.38,41 Klil-Drori et al38 conducted a prospective matched cohort study in Canada and they enrolled 167 CML-CP patients for both frontline generic and branded imatinib arms. In this study, persistence was defined as staying under the same TKI for at least 45 days without switching to an alternative treatment. After 3 years of follow-up, persistence rate for branded imatinib was 88.2%, whereas this was 72.8% in the generic arm (P = .03). The main reason for this nonpersistence was mostly linked to intolerance because of gastrointestinal AEs, which resulted in a twofold higher probability of switching to other TKIs in generic arm compared with patients receiving original imatinib.
However, Cole et al41 reported contradictory results. They designed a retrospective database study and enrolled 119 patients receiving frontline generic and 737 cases with upfront branded imatinib. Authors defined adherence as the percentage of days, which a patient had a TKI during 180 days of follow-up. Persistence was evaluated in both groups, and it was defined as the percentage of patients who used a TKI regularly without a gap for more than 30 and 60 days.41 After 6 months of follow-up, adherence rate in the generic arm was higher than that of branded imatinib arm (89% vs 81%, P value was not given). Persistence was evaluated in 2 separate parts as ≥30 and ≥60 days without TKI therapy, and persistence rates were also slightly higher for patients with generic imatinib compared with patients receiving original imatinib. Authors concluded that these results were most probably because of the lower cost of generic imatinib.
From a general point of view, in almost all studies, efficacy and safety profiles of both generic and original imatinib were similar (Tables 2 and 3). In the light of these results, it is possible to say that generics appear to be noninferior to the original molecule in terms of efficacy with a generally manageable toxicity profile. On the other hand, there are different generics available in different countries, and moreover, there are more than one generic in some countries. Therefore, although rarely observed, at least 1 of the reasons for these contradictory results may be because of the differences between these different generics.
Impact of generics on health care costs
A generic drug must contain the same active ingredients as the originator. Conducting preclinical and clinical studies for showing the safety and efficacy data are not required for the approval of generics, because these studies were already conducted by the brand-name company. Because a generic drug manufacturer does not bear the burden of proving the efficacy and safety of the drug by performing these studies, this enables the generic drug to be sold for a considerably lower price than its branded equivalent. It was shown that, the introduction of generic products with affordable treatment costs led to an increase in the number of patients who benefited from these therapies in 699 Chinese hospitals between 2011 and 2016.42
There are some papers exploring the potential impact of generic imatinib use on health care costs among CML patients.42-47 In 2015, just before the introduction of generic imatinib in the United States, Bloudek et al43 developed a model for the estimation of reduced treatment costs with generic imatinib. Among a hypothetical 1 million members of commercial and Medicare plans, calculated numbers of patients receiving any TKIs in 1 year were 103 and 347 for commercial and Medicare plans, respectively. According to this model, 95% of these patients were switched to generic imatinib at the end of second year, and the cost of generic product was reduced by 47.8% compared with branded. Based on these calculations, the authors estimated 28.8% cost saving at 2 years, which were equal to $6.8 and $22.9 million for commercial and Medicare plans, respectively. Estimated pharmacy cost of generic imatinib per box was nearly half of the original imatinib ($4403 vs $9211), whereas the estimated copayment was 4 times higher per package for the original product ($110 vs $25).43
The same group then updated their predictions for 5 years with real-life savings after 2 years of generic imatinib availability in the United States.44 According to their calculations, in the first 2 years, generic imatinib saved $2.5 billion for US payers and cumulative projected savings from years 3 to 5 were reached to 39%, which was approximately $12.2 billion.44
Hill et al45 performed multiple analysis to assume a target price for generic imatinib. Authors considered all production costs, including active pharmaceutical ingredient, excipients, packing, and shipping. According to their calculations, with a 50% profit margin, generic imatinib’s estimated annual cost was $128 to $216 per patient, whereas the lowest real-life annual cost of original imatinib in the United States was $107 799 in 2015.
Also, Padula et al46 created a model for the comparison of 2 treatment strategies: (1) imatinib-first strategy, which is not allowing 2GTKIs in frontline settings and (2) physicians’ choice strategy, which allows all TKIs in every line. With the administration of generic products, the authors estimated a saving of $91 163 per patient in direct medical costs over 5 years in the imatinib-first strategy compared with the physicians’ choice.
A similar economic model of an oncology care model practice with 1000 cancer patients during a 6-month period was developed.47 Four CML patients were estimated for that 1000-cancer patient oncology care model practice, and the changes in costs associated with substitution of branded imatinib and 2GTKIs (dasatinib and nilotinib) with generic imatinib in CML patients were analyzed. If the use of branded TKIs was restricted, in a 6-month episode, there would be a total reduction of $38 220 in health care costs, and $25 250 of this net cost reduction would come from a branded to generic imatinib shift.47
The first generic drug manufacturer to submit an abbreviated new drug application, which challenges to the patent of brand-name drug and meets certain regulatory and legal requirements may be eligible for a 180-day exclusivity.48 During that period, the Hatch-Waxman Act gives temporary protection to the first generic manufacturer to sell the only generic in the market before the patent expiration of brand-name drug and other companies producing generic versions of the drug. Despite these promising expectations, most probably because of this fact, the effects of generic imatinib on health care costs were found to be lower than expected in real life.49 The price of generic imatinib was only 8% and 10% lower than branded imatinib in 2016 and 2017 in the US market, respectively. It was stated that the price reduction observed in generic imatinib was less than expected comparing with the historical generic pricing examples of other drugs, most probably because of the lack of competitor generics.49
As of March 2021, it is possible to get 30 tablets of 400 mg generic imatinib for $80 without health insurance, whereas the lowest price for branded imatinib is still $11 641 per 30 tablets of 400 mg in the US market.50 On the contrary, in many countries other than the United States, for example in Turkey, patients with CML are able to reach more than 1 generic imatinib, and this competitive environment generally results in important amount of cost reductions. Currently, there are 8 different generics in Turkey, and the price per box (400 mg, 30 tablets) of most generics is equal to approximately $416, which is the same as Gleevec. The price gap between Gleevec and lowest priced generic ($396) is only $20.51
Also, citizens of Turkey, Canada, and European Union countries are able to reach both generic and branded imatinib without an out-of-pocket cost.52 Competitive market conditions and reimbursement policies of these countries forced the price of Gleevec to be close to that of generics.51,53-55 However, none of the health insurance systems in the United States are covering the price of original imatinib, which is approximately $11 000 per box (400 mg, 30 tablets).56
Discussion
Regarding the data available in the literature, both in vitro and in vivo studies showed that generics are comparable with branded imatinib in terms of bioequivalence and bioavailability. In most studies, generics showed similar results regarding efficacy and safety, both in newly diagnosed patients and after switching from Gleevec. Knowing the fact that different generics are available in different countries, some contradictory findings regarding efficacy and toxicity can be attributed to manufacturer variability. In countries where more than one generic is available, it is not recommended to switch from one generic to another to avoid newly emerging AEs mainly because of the changes in drug structure other than the active ingredient.
Regarding the impact of generics on health care costs, both in hypothetical models and in real life, emergence of generic imatinib resulted in significant benefits. The launch of generics had an effect on reducing the price of branded imatinib globally, and this resulted in imatinib being more affordable and accessible, and generic imatinib was recently considered to be the most cost-effective frontline treatment option in patients with CML-CP by the European LeukemiaNet 2020 recommendations.2
The long-term efficacy and safety data of Gleevec was recently published,57 and notwithstanding the generally favorable efficacy and toxicity profiles of generics worldwide to date, most probably we still need more time to draw firmer conclusions on the longer-term outcomes of generics.
Authorship
Contribution: A.E.E. designed the study; A.E. and D.S.E. collected and analyzed the data; and A.E.E., A.E., and D.S.E. wrote the paper.
Conflict-of-interest disclosure: A.E.E. has received advisory board honoraria from Novartis, and Pfizer and received speaker bureau honoraria from Novartis, Bristol-Myers Squibb, and Pfizer outside the present study. The remaining authors declare no competing financial interests.
Correspondence: Ahmet Emre Eşkazan, Istanbul University-Cerrahpaşa, Cerrahpaşa Faculty of Medicine, Department of Internal Medicine, Division of Hematology, Fatih, Istanbul, Turkey 34303; e-mail: emre.eskazan@iuc.edu.tr.
References
Author notes
Data can be obtained by contacting the corresponding author: emre.eskazan@iuc.edu.tr.