Key Points
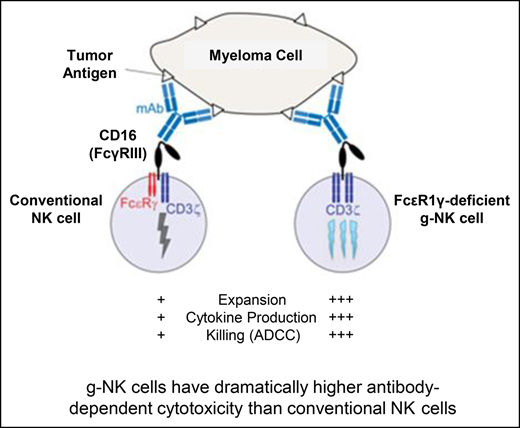
g-NK cells have superior antibody-dependent cellular cytotoxicity and effector functions compared with conventional NK cells.
g-NK cells enhance in vivo efficacy of daratumumab while demonstrating improved persistence vs conventional NK cells.
Abstract
Monoclonal antibodies (mAbs) are a central component of therapy for hematologic malignancies. Widely used mAb agents in multiple myeloma (MM) include daratumumab and elotuzumab. However, not all patients respond to these agents, and resistance is a significant clinical issue. A recently discovered subset of human natural killer (NK) cells lacking expression of FcεRIγ (g-NK cells) was found to have a multifold increase in antibody-dependent effector functions after CD16 crosslinking. In this study, we tested the capacity of g-NK cells to enhance the efficacy of therapeutic mAbs against MM. In vitro, we found that g-NK cells have strikingly superior anti-myeloma cytotoxicity compared with conventional NK (cNK) cells when combined with daratumumab or elotuzumab (∼sixfold; P < .001). In addition, g-NK cells naturally expressed minimal surface CD38 and SLAMF7, which reduced the incidence of therapeutic fratricide. In tumor-naïve murine models, the persistence of g-NK cells in blood and spleen was >10 times higher than that of cNK cells over 31 days (P < .001). In vivo efficacy studies showed that the combination of daratumumab and g-NK cells led to a >99.9% tumor reduction (by flow cytometry analysis) compared with the combination of daratumumab and cNK cells (P < .001). Moreover, treatment with daratumumab and g-NK cells led to complete elimination of myeloma burden in 5 of 7 mice. Collectively, these results underscore the unique ability of g-NK cells to potentiate the activity of therapeutic mAbs and overcome limitations of current off-the-shelf NK cell therapies without the need for cellular irradiation or genetic engineering.
Introduction
The monoclonal antibodies (mAbs) daratumumab targeting CD38 and elotuzumab targeting signaling lymphocytic activation molecule F7 (SLAMF7) have been approved by the US Food and Drug Administration for treating multiple myeloma (MM). Initial clinical responses have generally been encouraging, particularly for daratumumab, but almost all patients eventually develop progressive disease.1 Thus, there is a significant need for new strategies to either drive deeper remissions or overcome resistance to these agents.
For MM therapeutic mAbs, potential mechanisms of antitumor action include complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis, and antibody-dependent cellular cytotoxicity (ADCC).2-4 Although it has not yet been proven which primary mechanism is active in MM patients, laboratory studies have confirmed that ADCC, mediated by natural killer (NK) cells, can potently eliminate mAb-bound MM tumor.3,4
NK cells are activated when the Fc portion of an antibody binds their Fc receptor (FcγRIIIa or CD16a) and triggers activation and degranulation through a process involving the adapter proteins CD3ζ and FcεRIγ. Efforts to enhance the clinical ADCC response to MM mAbs have been challenging because NK cells also express CD38 and SLAMF7 (the targets for daratumumab and elotuzumab, respectively).3,4 High CD38 expression results in rapid depletion of NK cells early in the daratumumab treatment course,3,5 largely eliminating this source of innate immune cells which could potentially drive even more complete tumor eradication.
Recent studies have led to the discovery of a novel subset of human NK cells characterized by the lack of expression of the FcεRIγ adapter protein that have a multi-fold increase in ADCC activity after CD16 crosslinking.6-8 Herein, we refer to these FcεRIγ-deficient NK cells as g-NK cells. This special subset is relatively rare because g-NK cells are detectable at levels of ∼3% to 10% of total NK cells in only 25% to 30% of cytomegalovirus (CMV)-seropositive individuals6,7; thus, expansion is required for in vivo use.
In this study, we show that this novel subset of g-NK cells can be used for cancer therapy. Specifically, we demonstrate that g-NK cells have markedly enhanced antimyeloma ADCC and effector functions, and adoptive transfer of expanded g-NK cells eliminates myeloma tumor burden in vivo when combined with a therapeutic mAb (daratumumab). Importantly, because adoptive transfer of allogeneic NK cells does not result in severe graft-versus-host disease (GVHD),9 we propose that this antibody-directed NK cell therapy could be given in an off-the-shelf manner for clinical use.
Methods
Determination of g-NK frequency by flow cytometry
All human cell research was approved by the University of Houston Institutional Review Board. De-identified cryopreserved peripheral blood mononuclear cell (PBMC) samples were obtained from Bloodworks Northwest (Seattle, WA), washed with phosphate-buffered saline (PBS), and counted. After the cell count, a volume corresponding to 2.0 × 105 cells was aliquoted to flow tubes for determination of g-NK frequency by flow cytometry. Specifically, 2 µL each of 7-aminoactinomycin D (7-AAD) (viability dye) and fluorescent antibodies for the following extracellular markers were added: CD45, CD3, and CD56. All antibodies and 7-AAD were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). After a 10-minute incubation in the dark at 4°C, the cells were washed with PBS and fixed with 175 µL of 4% paraformaldehyde (10-minute incubation at room temperature in the dark). After fixation, the cells were washed with 0.2% saponin before being resuspended in 150 µL of 0.2% saponin to permeabilize cell membranes. After a brief 5-minute incubation at room temperature in the dark, 2 µL of fluorescently conjugated anti-FcεRIγ antibody (Millipore Sigma, Burlington, MA) was added. After an additional 10-minute incubation at 4°C, the percentage of g-NK cells was assessed using 4-color flow cytometry (Miltenyi MACSQuant Analyzer 10). Specifically, the percentage of g-NK cells (CD45+7-AAD–CD3–CD56+FcεRIγ– lymphocytes) was assessed directly, and the percentage of conventional NK (cNK) cells (CD45+7-AAD–CD3–CD56+FcεRIγ+ lymphocytes) was also assessed (supplemental Figure 1).
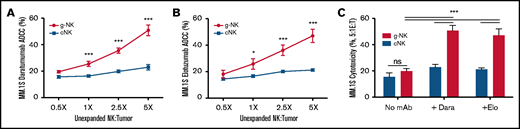
Unexpanded g-NK cells demonstrate superior ADCC activity in vitro. Comparison of the cytotoxicity of freshly isolated (unexpanded) g-NK and cNK cells against MM.1S cells at multiple NK:myeloma cell ratios (0.5X, 1X, 2.5X, and 5X) with daratumumab (A), elotuzumab (B), or no mAb present (C) (n = 16 unique donors). To compare cytotoxicity against MM cells between unexpanded g-NK and cNK cells, a maximum likelihood linear mixed model was built that included main effects for NK cell category (g-NK vs cNK), antibody category (daratumumab, elotuzumab, or no mAb), and E:T ratio (0.5X, 1X, 2.5X, 5X) as well as interaction effects of NK cell category × antibody category and NK cell category × E:T ratio. Bonferroni post hoc analyses were performed to determine the locations of the significant effects for NK cell category (ie, at what E:T ratios or antibody conditions the g-NK effects were present). Values are mean ± standard error of the mean (SEM). *P < .05; ***P < .001. Dara, daratumumab; Elo, elotuzumab; ns, not significant.
Unexpanded g-NK cells demonstrate superior ADCC activity in vitro. Comparison of the cytotoxicity of freshly isolated (unexpanded) g-NK and cNK cells against MM.1S cells at multiple NK:myeloma cell ratios (0.5X, 1X, 2.5X, and 5X) with daratumumab (A), elotuzumab (B), or no mAb present (C) (n = 16 unique donors). To compare cytotoxicity against MM cells between unexpanded g-NK and cNK cells, a maximum likelihood linear mixed model was built that included main effects for NK cell category (g-NK vs cNK), antibody category (daratumumab, elotuzumab, or no mAb), and E:T ratio (0.5X, 1X, 2.5X, 5X) as well as interaction effects of NK cell category × antibody category and NK cell category × E:T ratio. Bonferroni post hoc analyses were performed to determine the locations of the significant effects for NK cell category (ie, at what E:T ratios or antibody conditions the g-NK effects were present). Values are mean ± standard error of the mean (SEM). *P < .05; ***P < .001. Dara, daratumumab; Elo, elotuzumab; ns, not significant.
g-NK cell expansion
g-NK cells were preferentially expanded from cryopreserved PBMCs (Bloodworks Northwest) using a proprietary method that includes a 100 Gy gamma-irradiated lymphoblastoid cell line (available upon request) and 500 IU/mL interleukin-2 (IL-2) (Peprotech, Rocky Hill, NJ) in GMP stem cell growth media (CellGenix, Freiburg, Germany) supplemented with 5% AB serum (Millipore Sigma). g-NK cells were expanded for 2 weeks at a seeding and subculture density of 2 × 105 cells per mL at 37°C in a 5% CO2 incubator. Media and cytokines were changed every 2 to 5 days. Cells were counted each time the media was changed, and the percentage of g-NK cells was assessed by flow cytometry at days 0 and 14. Donors were CMV-seropositive (n = 8) or CMV-seronegative (n = 6) (age 37.8 ± 10.6 years; 8 males, 6 females). The proportion of g-NK cells in the CMV-seropositive donors was 30.8% ± 3.1% (% of total NK cells), whereas the proportion of g-NK cells was only 1.8% ± 0.3% (% of total NK cells) in the CMV-seronegative donors. After expansion, the proportion of g-NK cells was increased to 84.0% ± 1.4% for CMV-seropositive donors but was unchanged for CMV-seronegative donors (1.5% ± 0.4%) (supplemental Figure 1A). Expanded NK cells were cryopreserved with CS-10 (Biolife Solutions, Bothel, WA) at a concentration of 1 × 107 cells per mL for later use in functional assays. A successful expansion increased the proportion of g-NK cells from any CMV-seropositive donor with a detectable g-NK population and achieved at least a 400-fold increase in the overall number of NK cells (supplemental Figure 1B). Representative flow cytometry dot plots and histograms depicting the proportion of g-NK cells in CMV-seropositive and CMV-seronegative donors are provided in supplemental Figure 1C-D. The percentage of NKG2C+/NKG2A– NK cells within the g-NK subset ranged from 1.7% to 51 % (mean ± SEM: 26.8% ± 13.9%). Thus, there is phenotypic overlap between g-NK cells and NKG2C+ or NKG2A– NK cells, but they are not identical (NKG2C antibodies were purchased from R&D Systems, Minneapolis, MN; NKG2A antibodies were purchased from Beckman Coulter, Brea, CA).
Because there was insufficient yield of cNK cells from CMV-seronegative donors and preferential expansion of g-NK cells from CMV-seropositive donors using the g-NK expansion method, an alternative method was used to expand cNK cells for the in vitro functional and in vivo studies. This expansion method used K562-mbIL15-41BBL feeder cells and 500 IU/mL IL-210 to expand cNK cells 180-fold ± 89-fold (n = 5 CMV–) over 2 weeks. The proportion of g-NK cells in the 5 CMV– donors (age 38.9 ± 9.8 years; 3 males, 2 females) was 1.5% ± 0.5% before and 1.6% ± 0.4% after expansion.
Comparison of CD38 and SLAMF7 on g-NK, cNK, and MM.1S cells by flow cytometry
In total, 2.0 × 105 NK cells and/or MM.1S cells from patients with MM (American Type Culture Collection [ATCC], Manassas, VA) were aliquoted into flow tubes and stained with anti-CD45, anti-CD38, anti-CD3, anti-SLAMF7, and anti-CD56 antibodies according to the manufacturer recommendations (Miltenyi Biotec) as described in supplemental Table 1. After a 10-minute incubation at 4°C, the cells were washed, and intracellular staining was performed using an anti-FcεRI antibody (Millipore) as described in the “Determination of g-NK frequency by flow cytometry” section. After the staining process was complete, the percentage of CD38- and SLAMF7-expressing g-NK, cNK, and MM.1S cells was assessed by 7-color flow cytometry.
Pre-expansion ADCC assays
g-NK cells are rarely detected in CMV-seronegative donors and are detectable at levels of 3% to 10% of total NK cells in only 25% to 30% of CMV-seropositive donors.7 For the ADCC studies, de-identified frozen PBMC samples were obtained from Bloodworks Northwest (Seattle, WA). CMV-seropositive donors were selected for having high proportions of g-NK cells (30.0% ± 2.1% of total NK cells). We obtained samples from 12 g-NKhigh donors from a pool of 50 CMV-seropositive donors (the g-NK percentage for all 50 CMV-seropositive donors was 10.2% ± 9.3%). The 38 CMV-seropositive donors that were not used had an average g-NK cell proportion of only 3.1% ± 1.6%.
Donors for the ADCC assays were CMV-seropositive (n = 14) and CMV-seronegative (n = 2) (age 38.8 ± 11.4 years; 9 males, 7 females). All donors were pre-screened for the percentage of g-NK cells and categorized as either g-NK donors (n = 12 CMV+) or conventional donors (n = 2 CMV+; n = 2 CMV–) based on the proportion of g-NK cells. The proportion of g-NK cells in the g-NK donors was 30.0% ± 2.1% (% of total NK cells), whereas the proportion of g-NK cells was only 1.5% ± 0.3% (% of total NK cells) in the conventional donors.
By following a proprietary sorting strategy, the proportion of g-NK cells was increased to 84.3% ± 2.4% for g-NK donors. CD3–/CD56+ NK cells were magnetically sorted for the cNK cells. ADCC assays were performed using the MM cell line MM.1S. Myeloma target cells were labeled with 20 μL of anti-CD71 antibody (Thermo Fisher Scientific, Waltham, MA) per 1 × 106 cells. As we described previously,11 NK cells were cocultured with CD71-labeled myeloma target cells at 0.5:1, 1:1, 2.5:1, and 5:1 NK:target cell ratios in a final volume of 2.2 mL of 10% fetal bovine serum–supplemented RPMI-1640 with 1 μg/mL daratumumab (anti-CD38) or 1 μg/mL elotuzumab (anti-CD319). Basal cytotoxicity was also measured without antibody present. Tubes with only target cells were used to control for spontaneous cell death (<10% for all assays). After a 4-hour incubation at 37°C in a CO2 incubator, the cells were washed and stained with anti-CD3 and CD56 (Miltenyi Biotec) antibodies to quantify the number of NK cells in the tube. After a final wash, propidium iodide was added, and the numbers of NK cells, live target cells, and dead target cells were resolved using 4-color flow cytometry.12
Postexpansion ADCC and effector function assays
Ten donors were prescreened for the percentage of g-NK cells, and they were categorized as either g-NK donors (n = 5 CMV+) or conventional donors (n = 5 CMV–) on the basis of the proportion of g-NK cells (age 38.3 ± 10.3 years; 6 males, 4 females). The proportion of g-NK cells in the g-NK donors was 30.3% ± 2.0% before expansion and 84.0% ± 2.5% after expansion, whereas the proportion of g-NK cells in the conventional donors was 1.5% ± 0.5% before and 1.6% ± 0.4% after expansion. The g-NK cells were expanded by using our proprietary expansion method, and the cNK cells were expanded by using K562-mbIL15-41BBL plus 500 IU/mL IL-2.10 After expansion, g-NK and cNK cells were cryopreserved (1 × 107 cells per mL in CS-10). Upon thawing, 104 NK cells were cocultured with MM target cells (AMO1, KMS11, KMS18, KMS34, LP1, and MM.1S) under 3 conditions: 0.5:1 NK:MM ratio (no mAb, 1 μg/mL daratumumab, or 1 μg/mL elotuzumab). Samples were processed and analyzed as described in the “Pre-expansion ADCC assays” section. Tubes with NK cells only were added to quantify the effects of daratumumab- and elotuzumab-induced fratricide (no mAb vs daratumumab vs elotuzumab). The media used for all postexpansion ADCC and effector function assays was 10% fetal bovine serum–supplemented RPMI-1640. The AMO1 and LP1 cell lines were obtained from the DSMZ German Collection of Microorganisms and Cell Cultures. The KMS11, KMS18, and KMS34 cell lines were obtained from the Japanese Cancer Research Resources Bank. The MM.1S cell line was obtained from ATCC.
In addition, 2.0 × 105 NK cells were added to the same MM target cells under 3 conditions: 0.5:1 NK:MM ratio (no mAb, 1 μg/mL daratumumab, or 1 μg/mL elotuzumab). Then, 2 µL of VioGreen-conjugated anti-CD107a was added to the coculture and incubated for 1 hour at 37°C in a CO2 incubator. After the 1-hour incubation, 0.67 µL/mL of monensin (BD GolgiStop) was added to each tube for the degranulation assay (tube 1), and 1 µL/mL of brefeldin A (BD GolgiPlug) was added to each tube for the cytokine expression assay (tube 2) (the antibody panel is listed in supplemental Table 2). After adding monensin and brefeldin A, the cells were incubated for an additional 5 hours at 37°C in a CO2 incubator. After incubation, the cells were harvested, washed, and stained with anti-CD45, anti-CD3, and anti-CD56 antibodies according to the manufacturer’s instructions (Miltenyi Biotec). The cells were then fixed and permeabilized using the Inside Stain Kit from Miltenyi Biotec according to the manufacturer’s instructions. The cells were then stained with anti-FcεRIγ, anti-perforin, anti-granzyme B, anti-interferon-γ (IFN-γ), and anti-tumor necrosis factor-α (TNF-α) antibodies as appropriate (supplemental Table 2). After a final wash, the cells were resolved using 8-color flow cytometry.
g-NK persistence study
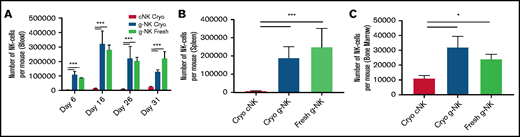
All experiments were approved by the University of Houston Animal Care and Use Committee. Mice were housed under pathogen-free conditions. After 1 week of acclimation, a single dose of 1.0 × 107 expanded g-NK (freshly expanded or cryopreserved) or cNK cells (cryopreserved) was injected intravenously through the tail vein into female NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ (NSG) mice (6-8 weeks old; The Jackson Laboratory, Bar Harbor, ME) with recombinant human IL-15 support (2 µg per mouse intraperitoneally [IP] every 3 days [Peprotech]) (supplemental Table 3). cNK cells were expanded for 2 weeks using the transgenic leukemia cell line K562-mb15-41BBL and IL-2 as previously described.10 All cells were expanded from cryopreserved NK cells and cryopreserved feeder cells. The freeze media for the cryopreserved cells was CS-10 (1 × 107 cells per mL). Cryopreserved cell products were thawed rapidly in a hot water bath before being administered to the mice (37°C). For blood collection, 50-µL blood draws were obtained using EDTA vacutainers at days 6, 16, 26, and 31 postinfusion for immediate flow cytometry analysis. At day 31, all mice were euthanized, and bone marrow and spleen were harvested for immediate flow cytometry analysis.
In vivo efficacy in a disseminated orthotopic xenograft MM.1S model of MM
NSG mice (6-8 weeks old) were purchased from The Jackson Laboratory. All experiments were approved by the University of Houston Animal Care and Use Committee. Mice were housed under pathogen-free conditions. After 1 week of acclimation, 5 × 105 luciferase-labeled MM.1S human myeloma cells were injected intravenously (IV) into the tail veins of female NSG mice in 100 μL of PBS and allowed to grow for 14 days (control group, n = 8; g-NK group, n = 7; cNK group, n = 7). Then, 6.0 × 106 expanded g-NK or cNK cells in 200 μL of PBS were administered IV to mice in combination with daratumumab administered IP (10 μg per mouse; Janssen, Beerse, Belgium) once per week for 5 weeks (supplemental Table 4). cNK cells were expanded for 2 weeks by using the transgenic leukemia cell line K562-mb15-41BBL and IL-2, as previously described.10 All cells were expanded from cryopreserved NK cells and cryopreserved feeder cells. IL-15 (PeproTech) support was provided over the course of the entire study (2 µg per mouse, IP once every 3 days starting 2 weeks after tumor inoculation). Bioluminescence imaging (BLI; IVIS Lumina XRMS imager, PerkinElmer, Waltham, MA) was performed twice per week to monitor tumor burden beginning 1 week after tumor inoculation. Mice were imaged after 15 minutes of subcutaneous injection of D-luciferin (150 mg/kg; Gold Biotechnology, St Louis, MO). Total flux (photons/second) for the entire mouse was quantified using Living Image software (PerkinElmer). Mice were checked once per day for signs of discomfort and tolerability. Body weight was measured twice per week. Tumor-bearing mice were euthanized per University of Houston animal welfare protocol when they developed myeloma symptoms, such as hind limb paralysis and/or lethargy. Time to euthanasia was used as a proxy for survival. All mice were observed until they were euthanized to obtain survival curves. X-ray images were acquired by using an IVIS Imager for mice before they were euthanized. All surviving mice were euthanized 57 days after the initial tumor inoculation for tissue collection. At study completion, whole blood, bone marrow, and spleen samples were harvested for flow cytometry analysis to quantify the number of g-NK, cNK, and MM.1S cells (CD138+CD45–) (VioBlue-conjugated anti-CD45 and phycoerythrin-conjugated anti-CD138 antibodies were purchased from Miltenyi Biotec).
Patient sample cytotoxicity assay
Fresh de-identified primary MM patient bone marrow samples were obtained from the University of California at San Francisco Hematologic Malignancies Tissue Bank under a protocol approved by an institutional review board and with informed consent consistent with Declaration of Helsinki. Red blood cells were lysed, and the remaining primary cells were resuspended in media (RPMI-1640, 10% fetal bovine serum,10 U/mL IL-2) at a density of 1 × 106 cells per mL. Myeloma cells were incubated with daratumumab (1 μg/mL) or elotuzumab (1 μg/mL) for 30 minutes at 37°C and were then washed and resuspended in media at a density of 1 × 106 cells per mL. For each condition (daratumumab or elotuzumab), 2 × 106 isolated mononuclear cells were co-incubated with NK cells (g-NK or cNK) at ratios of 0:1 (no NK:control); 2.5:1 and 20:1 (NK:primary) in a final volume of 1 mL media and then incubated for 4 hours at 37°C in 5% CO2. Cryopreserved expanded g-NK and cNK cells were used as the effector cells for this assay. Samples were plated in a 96-well U-bottom plate and stained with BV421-conjugated anti-CD138 (BD Biosciences, East Rutherford, NJ), fluorescein isothiocyanate–conjugated anti-CD38 (BD Biosciences), phycoerythrin-conjugated anti-SLAMF7 (BioLegend, San Diego, CA), and allophycocyanin-conjugated anti-CD56 antibodies (BioLegend). Resuspended cells were characterized with a Beckman Cytoflex Cytometer B4R3V4, and data were analyzed using FlowJo software (v10). Loss of CD138+ plasma cells under each condition vs the no-NK cell control was used to calculate cytotoxicity.
Statistical analysis
Data were statistically analyzed using Predictive Analytics SoftWare (PASW 22.0) unless otherwise noted. To compare cytotoxicity against MM cells between unexpanded g-NK and cNK cells, a maximum likelihood linear mixed model was built that included main effects for NK cell category (g-NK vs cNK), antibody category (daratumumab or elotuzumab or no mAb), and effector:target (E:T) ratio as well as interaction effects of NK cell category × antibody category and NK cell category × E:T ratio. To compare cytotoxicity between expanded g-NK cells, g-NK cells plus antibody, cNK cells, and cNK cells plus antibody, a one-way analysis of variance (ANOVA) was performed with Bonferroni post hoc testing to determine differences between individual categories. To compare in vivo persistence between g-NK and cNK cells, a one-way ANOVA was performed with Bonferroni post hoc testing to determine differences between fresh or cryopreserved g-NK and cNK cells. To determine the differences in CD38 and SLAMF7 expression between unexpanded g-NK, cNK, and MM.1S cells, a one-way ANOVA was performed with Bonferoni post hoc testing to determine differences between individual cell types. Independent samples Student t tests were used to determine the differences between g-NK and cNK cells before and after expansion. To examine the effect of g-NK cells on tumor burden and body weight in a murine model of MM, a one-way ANOVA was performed for each day to determine differences between treatment groups. For tumor burden and body weight comparisons when only 2 groups remained, independent samples Student t tests were used. To compare perforin and granzyme B expression between g-NK and cNK cells, an independent sample Student t test was used. To compare effector functions against MM cells between expanded g-NK cells, g-NK cells plus antibody, cNK cells, and cNK cells plus antibody, a one-way ANOVA was performed with Bonferroni post hoc testing to determine differences between individual categories. For all linear mixed models, Bonferroni post hoc testing was used to determine precise locations of significant effects. Survival analysis was conducted using Kaplan-Meier model with log-rank test (GraphPad Prism 8.0.2). P < .05 indicated statistical significance.
Results
g-NK cells have greater antibody-dependent cytotoxicity against MM than cNK cells
To initially investigate the potential of g-NK cells to enhance mAb efficacy in myeloma, we used an in vitro coculture cytotoxicity model. We first isolated g-NK cells from 12 independent CMV-seropositive donors. We found that when compared with cNK cells (98% to 99% FcεRIγ+), g-NK cells have markedly elevated ADCC against MM.1S cells when combined with either daratumumab (Figure 1A) or elotuzumab (Figure 1B) at 3 different E:T ratios (1:1, 2.5:1, and 5:1) (P < .001). Notably, there was no difference between the cytotoxicity of g-NK and cNK cells against MM.1S cells when mAb was absent (P = .3) (Figure 1C).
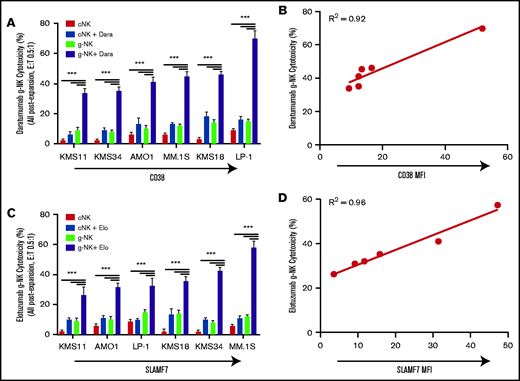
Considering the potential for therapeutic applications of this relatively rare NK cell subtype, we developed a proprietary method to preferentially expand g-NK cells from donor PBMCs. Consistent with our MM.1S results with nonexpanded cells, expanded g-NK cells demonstrated significantly higher cytotoxicity than expanded cNK cells against an extended panel of 6 MM cell lines (AMO1, KMS11, KMS18, KMS34, LP1, and MM.1S) when combined with daratumumab (P < .001) (Figure 2A) or elotuzumab (P < .001) (Figure 2C). Notably, across this panel of MM cell lines, the magnitude of g-NK ADCC was strongly correlated with the expression of target antigen for both daratumumab (R2 = 0.92) (Figure 2B) and elotuzumab (R2 = 0.96) (Figure 2D). Furthermore, similar cytotoxicity assays against primary myeloma tumor cells from a relapsed/refractory patient were also consistent with significantly enhanced lysis by g-NK cells relative to cNK cells after mAb incubation (supplemental Figure 2). Taken together, these in vitro results demonstrate that g-NK cells can potently enhance mAb efficacy in MM and show increased activity vs conventional FcεRIγ+ NK cells.
Expanded g-NK cells have enhanced in vitro ADCC activity proportionate to MM antigen expression. Comparison of the cytotoxicity of expanded g-NK and cNK cells (E:T, 0.5:1) against 6 MM cell lines with increasing CD38 (A-B) and SLAMF7 (C-D) expression in the presence or absence of 1 μg/mL daratumumab (anti-CD38) and 1 μg/mL elotuzumab (anti-SLAMF7), respectively (n = 5). The ADCC of g-NK cells when combined with daratumumab or elotuzumab is proportionate to myeloma CD38 (B) or SLAMF7 (D) expression, respectively. To compare cytotoxicity against MM cells between expanded g-NK, g-NK plus antibody, cNK, and cNK plus antibody, a one-way ANOVA was performed with Bonferroni post hoc testing to determine differences between individual categories. Pearson R2 values were used to assess the correlation between ADCC and antigen expression. Values are mean ± SEM. ***P < .001. MFI, mean fluorescence intensity.
Expanded g-NK cells have enhanced in vitro ADCC activity proportionate to MM antigen expression. Comparison of the cytotoxicity of expanded g-NK and cNK cells (E:T, 0.5:1) against 6 MM cell lines with increasing CD38 (A-B) and SLAMF7 (C-D) expression in the presence or absence of 1 μg/mL daratumumab (anti-CD38) and 1 μg/mL elotuzumab (anti-SLAMF7), respectively (n = 5). The ADCC of g-NK cells when combined with daratumumab or elotuzumab is proportionate to myeloma CD38 (B) or SLAMF7 (D) expression, respectively. To compare cytotoxicity against MM cells between expanded g-NK, g-NK plus antibody, cNK, and cNK plus antibody, a one-way ANOVA was performed with Bonferroni post hoc testing to determine differences between individual categories. Pearson R2 values were used to assess the correlation between ADCC and antigen expression. Values are mean ± SEM. ***P < .001. MFI, mean fluorescence intensity.
g-NK cells show improved persistence compared with cNK cells in NSG mice
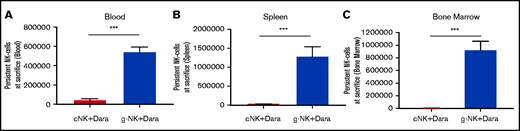
In addition to their superior ADCC functions, g-NK cells were reported to have elevated expression of antiapoptotic proteins including Bcl-2,7 which suggests that they may be able to persist longer than cNK cells in vivo. To test this hypothesis, we injected 9 female NSG mice with a single intravenous dose of 1 × 107 expanded NK cells (fresh g-NK, cryopreserved g-NK, and cryopreserved cNK cells). As used in other preclinical and clinical studies of NK cell therapies,13 we provided cytokine support with intraperitoneal IL-15 every 3 days. Persistence of cryopreserved g-NK cells was >10 times higher than that seen with cryopreserved cNK cells in peripheral blood at multiple time points (P < .001) (Figure 3A) and in spleen when the mice were euthanized at day 31(P < .001) (Figure 3B). Persistence of g-NK cells was higher than that of cNK cells in bone marrow as well (P < .05) (Figure 3C). Furthermore, cryopreserved g-NK cells persisted at levels comparable to those in fresh g-NK cells for at least 26 days posttransfer. This significantly improved persistence emphasizes the potential utility of fresh or cryopreserved g-NK as an off-the-shelf cellular therapy to enhance mAb ADCC.
Expanded g-NK cells demonstrate robust persistence in vivo. Comparison of the number of human NK cells present in whole blood at days 6, 16, 26, and 31 postinjection (A), spleen at day 31 (B), and bone marrow (C) at day 31 between NSG mice infused with 1 × 107 g-NK or cNK cells (n = 3 for each arm) as measured by flow cytometry. To compare in vivo persistence between g-NK and cNK cells, a one-way ANOVA was performed with Bonferroni post hoc testing to determine differences between fresh or cryopreserved (Cryo) g-NK and cNK cells. Values are mean ± SEM. *P < .05; ***P < .001.
Expanded g-NK cells demonstrate robust persistence in vivo. Comparison of the number of human NK cells present in whole blood at days 6, 16, 26, and 31 postinjection (A), spleen at day 31 (B), and bone marrow (C) at day 31 between NSG mice infused with 1 × 107 g-NK or cNK cells (n = 3 for each arm) as measured by flow cytometry. To compare in vivo persistence between g-NK and cNK cells, a one-way ANOVA was performed with Bonferroni post hoc testing to determine differences between fresh or cryopreserved (Cryo) g-NK and cNK cells. Values are mean ± SEM. *P < .05; ***P < .001.
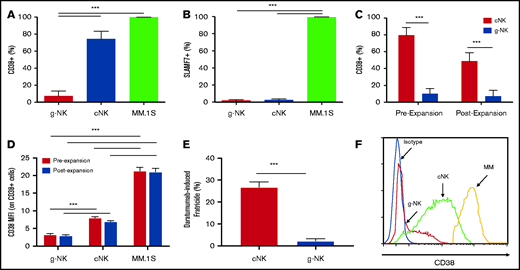
g-NK cells have minimal expression of the daratumumab target CD38 and the elotuzumab target SLAMF7
If NK cells express an mAb target, it may result in fratricide, whereby ADCC activity leads to elimination of NKs in addition to tumor. Importantly, we found that the percentage of CD38+ cells was markedly lower on donor-isolated g-NK cells than on both cNK and MM.1S cells (P < .001 for both) (Figure 4A). This cNK finding is consistent with previous results, which suggests that >90% of CD38high NK cells are rapidly depleted after patients are treated with daratumumab.3 Furthermore, we found that both g-NK and cNK cells express equally low levels of SLAMF7 (P = .8), far lower than on MM.1S cells (P < .001 for both) (Figure 4B). Notably, a decreased percentage of CD38+ NK cells was also seen on ex vivo–expanded g-NK cells when compared with expanded cNK cells (P < .001) (Figure 4C). Furthermore, mean fluorescence intensity of CD38 expression was reduced on CD38+ g-NK cells relative to CD38+ cNK and MM.1S cells (P < .001) (Figure 4D). This lower CD38 expression led to markedly reduced daratumumab-induced fratricide by g-NK cells relative to that seen in cNK cells (P < .001) (Figure 4E). Fratricide was not detected for g-NK or cNK cells treated with elotuzumab (data not shown). A representative histogram depicting the reduced CD38 expression of g-NK cells relative to cNK and MM.1S cells is provided in Figure 4F. Overall, g-NK cells are expected to confer enhanced mAb antitumor activity in MM without suffering from fratricide-related depletion.
g-NK cells express less of the daratumumab target CD38 and are more resistant to daratumumab-induced fratricide than cNK cells. Comparison of the percentage of CD38+ (A) and SLAMF7+ (B) unexpanded g-NK, cNK, and MM.1S cells (n = 4). Comparison of CD38 expression shown as (C) percentage of NK cells and (D) MFI for CD38+ NK cells before and after expansion between g-NK and cNK cells (n = 4). (E) Comparison of daratumumab-induced fratricide by expanded g-NK and cNK cells. (F) Representative histogram depicting the reduced CD38 expression of g-NK cells relative to cNK and MM.1S cells. To determine the differences in CD38 and SLAMF7 expression between unexpanded g-NK, cNK, and MM.1S cells, a one-way ANOVA was performed with Bonferoni post hoc testing to determine differences between individual cell types. Independent samples Student t tests were used to determine the differences in CD38 expression between g-NK and cNK cells before and after expansion. Independent samples Student t tests were also used to determine the differences in fratricide by expanded g-NK and cNK cells. Values are mean ± SEM. ***P < .001.
g-NK cells express less of the daratumumab target CD38 and are more resistant to daratumumab-induced fratricide than cNK cells. Comparison of the percentage of CD38+ (A) and SLAMF7+ (B) unexpanded g-NK, cNK, and MM.1S cells (n = 4). Comparison of CD38 expression shown as (C) percentage of NK cells and (D) MFI for CD38+ NK cells before and after expansion between g-NK and cNK cells (n = 4). (E) Comparison of daratumumab-induced fratricide by expanded g-NK and cNK cells. (F) Representative histogram depicting the reduced CD38 expression of g-NK cells relative to cNK and MM.1S cells. To determine the differences in CD38 and SLAMF7 expression between unexpanded g-NK, cNK, and MM.1S cells, a one-way ANOVA was performed with Bonferoni post hoc testing to determine differences between individual cell types. Independent samples Student t tests were used to determine the differences in CD38 expression between g-NK and cNK cells before and after expansion. Independent samples Student t tests were also used to determine the differences in fratricide by expanded g-NK and cNK cells. Values are mean ± SEM. ***P < .001.
Expanded g-NK cells express increased levels of cytotoxic enzymes and show more robust effector functions than cNK cells
We next investigated the mechanisms by which g-NK cells confer their enhanced ADCC activity. By using flow cytometry, we found that ex vivo–expanded g-NK cells have a higher number of perforin-positive cells and higher overall levels of perforin expression (P < .01) as well as increased granzyme B–positive cells and higher granzyme B expression (P < .001) than expanded cNK cells (supplemental Figure 3). In addition to enhanced ADCC (Figure 1), g-NK cells degranulated more (CD107a+) and expressed more IFN-γ and TNF-α in response to MM cells than cNK cells when combined with daratumumab or elotuzumab (P < .001) (supplemental Figures 4-6). Underscoring specificity, and in line with our ADCC findings, the magnitude of these g-NK effector functions was directly proportionate to the expression of target antigen across a panel of 6 MM cell lines (AM01, KMS11, KMS18, KMS34, LP1, and MM.1S) (supplemental Figures 4-6). These marked increases in key cytolytic enzymes, as well as more robust activation phenotypes, are consistent with previous findings in non-expanded g-NK cells,6,7 and underpin the enhanced capacity of expanded g-NK cells to induce apoptosis of tumor target cells when engaged with mAb via CD16 crosslinking.
Expanded g-NK cells have markedly enhanced ADCC activity when combined with daratumumab in vivo. (A) Effect of treatment with g-NK cells and daratumumab (n = 7) on MM.1S tumor burden (BLI) in NSG mice relative to a cNK cell control plus daratumumab (n = 7) and mice treated with vehicle (n = 8). The cell and antibody combination therapies were given once per week for 5 weeks with IL-15 support (2 µg per mouse IP every 3 days). One mouse (labeled as &) in the g-NK plus daratumumab group missed the first week of treatment and received the treatment from weeks 2 to 6. (B) All remaining mice from the g-NK plus daratumumab group were euthanized on day 57 after tumor inoculation (day 43 after initial therapy). The quantitative BLI (photons per second [p/s]) values for vehicle control and mice treated with cNK plus daratumumab or g-NK plus daratumumab. (C) Effect of treatment with g-NK plus daratumumab on survival relative to treatment with cNK plus daratumumab or vehicle. (D) Comparison of body weight (g) in mice treated with vehicle, cNK plus daratumumab, or g-NK plus daratumumab. (E) Comparison of MM.1S tumor burden (number of CD45–/CD138+ cells) in bone marrow of NSG mice treated with cNK plus daratumumab or g-NK plus daratumumab. (F) Representative flow cytometry dot plots depicting tumor burden and persistence of NK cells in bone marrow for mice treated with vehicle, cNK plus daratumumab, or g-NK plus daratumumab. One-way ANOVA tests were used to determine the differences in BLI and body weight between different groups at each measurement day. For tumor burden and body weight comparisons when only 2 groups remained, independent samples Student t tests were used. Log-rank tests were used to determine differences between survival curves (P < .0001). Independent samples Student t tests were used to determine the differences in bone marrow tumor burden between mice treated with g-NK plus daratumumab or cNK plus daratumumab when the mice were euthanized. Values are mean ± SD for the BLI (B) and body weight (D) graphs and mean ± SEM for the tumor burden graph (E). *P < .05 (one-way ANOVA); #P < .001 (Student t test).
Expanded g-NK cells have markedly enhanced ADCC activity when combined with daratumumab in vivo. (A) Effect of treatment with g-NK cells and daratumumab (n = 7) on MM.1S tumor burden (BLI) in NSG mice relative to a cNK cell control plus daratumumab (n = 7) and mice treated with vehicle (n = 8). The cell and antibody combination therapies were given once per week for 5 weeks with IL-15 support (2 µg per mouse IP every 3 days). One mouse (labeled as &) in the g-NK plus daratumumab group missed the first week of treatment and received the treatment from weeks 2 to 6. (B) All remaining mice from the g-NK plus daratumumab group were euthanized on day 57 after tumor inoculation (day 43 after initial therapy). The quantitative BLI (photons per second [p/s]) values for vehicle control and mice treated with cNK plus daratumumab or g-NK plus daratumumab. (C) Effect of treatment with g-NK plus daratumumab on survival relative to treatment with cNK plus daratumumab or vehicle. (D) Comparison of body weight (g) in mice treated with vehicle, cNK plus daratumumab, or g-NK plus daratumumab. (E) Comparison of MM.1S tumor burden (number of CD45–/CD138+ cells) in bone marrow of NSG mice treated with cNK plus daratumumab or g-NK plus daratumumab. (F) Representative flow cytometry dot plots depicting tumor burden and persistence of NK cells in bone marrow for mice treated with vehicle, cNK plus daratumumab, or g-NK plus daratumumab. One-way ANOVA tests were used to determine the differences in BLI and body weight between different groups at each measurement day. For tumor burden and body weight comparisons when only 2 groups remained, independent samples Student t tests were used. Log-rank tests were used to determine differences between survival curves (P < .0001). Independent samples Student t tests were used to determine the differences in bone marrow tumor burden between mice treated with g-NK plus daratumumab or cNK plus daratumumab when the mice were euthanized. Values are mean ± SD for the BLI (B) and body weight (D) graphs and mean ± SEM for the tumor burden graph (E). *P < .05 (one-way ANOVA); #P < .001 (Student t test).
Expanded g-NK cells have markedly enhanced persistence when combined with daratumumab in vivo. When mice were euthanized, we compared the number of g-NK and cNK cells in the blood (A), spleen (B), and bone marrow (C) of NSG mice treated with daratumumab (n = 7 for g-NK and cNK arms; n = 8 for vehicle arm). To compare persistence of g-NK and cNK cells in a murine model of MM (with daratumumab), an independent samples Student t test was performed when the mice were euthanized. Values are mean ± SEM. ***P < .001.
Expanded g-NK cells have markedly enhanced persistence when combined with daratumumab in vivo. When mice were euthanized, we compared the number of g-NK and cNK cells in the blood (A), spleen (B), and bone marrow (C) of NSG mice treated with daratumumab (n = 7 for g-NK and cNK arms; n = 8 for vehicle arm). To compare persistence of g-NK and cNK cells in a murine model of MM (with daratumumab), an independent samples Student t test was performed when the mice were euthanized. Values are mean ± SEM. ***P < .001.
g-NK cells enhance in vivo efficacy of daratumumab in a disseminated orthotopic xenograft MM.1S model of MM
We next evaluated the in vivo activity of g-NK cells in a mouse model of MM. We designed this study to compare in vivo efficacy of a therapeutic dose of g-NK cells administered in combination with a therapeutic mAb (daratumumab) to the same dose of cNK cells and mAb. In this setting, we found that g-NK cells plus daratumumab eliminated myeloma tumor burden in 5 of 7 mice evidenced by BLI after 5 weeks of treatment (Figure 5A). Quantitative BLI analysis showed that g-NK cells plus daratumumab induced sustained and statistically significant tumor regression (Figure 5B). The Kaplan-Meier survival analysis showed that the overall survival probability of the mice treated with g-NK plus daratumumab was significantly better than that for mice treated with vehicle or with cNK and daratumumab (P < .0001) (Figure 5C). All mice dosed with g-NK cells were energetic with no weight loss or toxicities observed at the conclusion of the study, whereas all control mice or mice treated with cNK cells and daratumumab had severe weight loss and succumbed to myeloma before the study ended (Figure 5D). Interestingly, 1 of the mice treated with g-NK cells was not dosed until day 21 after tumor inoculation because of anesthesia-induced suffocation of 1 of the mice, and this mouse had no detectable tumor BLI at the end of the study despite having the highest peak BLI of the g-NK mice (Figure 5A, mouse labeled as &). Of the 7 mice who were dosed with g-NK cells, only 2 had a minimally detectable amount of residual tumor BLI. Flow cytometry analysis of the bone marrow confirmed that the 5 mice treated with g-NK cells with no detectable tumor BLI were in fact tumor free (no CD138+ cells in bone marrow). Average tumor burden for all 7 mice treated with g-NK was reduced >99.9% relative to mice treated with cNK and daratumumab (P < .001) (Figure 5E). Representative flow cytometry dot plots depicting tumor burden and persistent NK cells in bone marrow are shown in Figure 5F. All of the BLI images taken over the course of the study are provided in supplemental Figure 7. X-ray images were obtained for all of the mice before they were euthanized. Control mice or mice treated with cNK cells and daratumumab had fractures and malformations of the hind limb bones, but none of the mice treated with g-NK cells and daratumumab had any bone deformities (supplemental Figure 8). Taken together, our results underscore the superiority of g-NK cells vs cNK cells for enhancing mAb effects in vivo and suggest that g-NK cells given in combination with daratumumab could be potentially curative for MM.
Furthermore, we observed a large increase in the persistence of g-NK cells relative to that of cNK cells in mice treated with daratumumab (Figure 6A-C). Notably, g-NK cell numbers were >10 times higher than cNK cells in blood, >20 times higher in spleen, and >100 times higher in bone marrow (P < .001). Collectively, these results support a scenario in which enhanced survival and resistance to fratricide result in superior anti-tumor effects and persistence of g-NK cells.
Discussion
In this study, we proposed that co-administration of an allogeneic off-the-shelf, non-engineered NK cellular therapy with endogenously enhanced ADCC activity and low CD38 expression could dramatically increase the efficacy of therapeutic mAbs in MM. Specifically, we described the potential applications of a recently discovered subset of human NK cells that lack expression of the FcεRIγ adapter protein (g-NK cells) and have low CD38 expression.6,7 We found that both non-expanded and ex vivo–expanded g-NK cells are highly cytotoxic relative to FcεRIγ+ cNK cells. When combined with daratumumab, expanded g-NK cells demonstrated markedly superior in vivo efficacy (>99.9% improvement) and persistence relative to cNK cells. Remarkably, g-NK cells were potentially curative when combined with daratumumab for treating MM (g-NK cells completely eliminated tumor in 5 of 7 mice). Conversely, all mice treated with cNK cells and daratumumab succumbed to myeloma before the conclusion of the study. Different expansion methods were used for g-NK and cNK cells in our murine studies. Standard NK expansion protocols10 did not provide adequate expansion of g-NK cells, but we observed superior ADCC of g-NK cells relative to cNK cells whether we used unexpanded cells (Figure 1) or expanded cells (Figure 2). This result indicates that expansion method was not a primary driver of superior g-NK activity. Mechanistically, we found that expanded g-NK cells expressed greater levels of cytotoxic enzymes and effector cytokines when engaged with mAbs, leading to their greater ADCC efficacy.
Daratumumab treatment can lead to fratricidal elimination of NK cells and downregulation of CD38 by myeloma cells, which results in most patients eventually becoming refractory to daratumumab.1 In terms of clinical utility, daratumumab reduces the number of CD38+ NK cells by >90% for up to 6 months after treatment,3 but CD38low NK cells remain and show no decline in anti-MM cytotoxicity ex vivo.5 This observation suggests that adoptive transfer of CD38low NK cells could enhance the efficacy of daratumumab. Importantly, we report here that g-NK cells are endogenously CD38 deficient when compared with cNK cells. We also report that g-NK cells have markedly lower daratumumab-induced fratricide than cNK cells and persist at substantially higher levels in mice treated with daratumumab. This suggests that our g-NK cell product could be optimal for daratumumab-refractory patients because expanded g-NK cells are resistant to daratumumab-induced fratricide, and they enhance daratumumab-specific cell cytotoxicity against even dimly CD38-expressing myeloma cells.
Previous NK cell therapy efforts have been motivated by the observation that adoptive transfer of NK cells in humans does not result in severe GVHD, which allows for use of allogeneic products.9 However, clinical utility has been hampered by limited NK persistence. In addition, we note that others are also developing NK cell products to enhance mAb efficacy. For example, CD38-knockout NK cell lines have been created to avoid daratumumab fratricide14,15 and NK cell lines with noncleavable CD16 have been developed to enhance antitumor ADCC.16 However, potential drawbacks for clinical use include the need for genetic engineering and irradiation of immortalized cell lines. Similarly, CD19 chimeric antigen receptor NK cells have been shown to persist in vivo and mediate strong antileukemia and lymphoma effects,17,18 but they also require genetic engineering and chimeric antigen receptor NK cells must be individually tailored for each kind of cancer. Our method cannot avoid some potential complications of NK cell immunotherapy, including inhibition of antiviral T-cell immunity,19,20 off-target cytotoxic effects, and GVHD risk associated with using NK cells expanded from allogeneic CMV-seropositive donors.21 However, we do not expect our g-NK cells to have greater toxicologic risk than cNK cells because their cytotoxic superiority and enhanced expression of pro-inflammatory cytokines is antibody dependent, thus significantly reducing the risk of off-target cytotoxic effects.
Our data suggest that g-NK cells are ideal for use in combination with daratumumab or elotuzumab because they persist a long time and are superior mediators of ADCC that naturally express low levels of CD38 and SLAMF7 without the need for genetic manipulation. It is noteworthy that the combination of g-NK cells with daratumumab was able to completely eliminate tumor in a disseminated orthotopic xenograft MM.1S model of MM. We are currently focusing on larger-scale expansion and donor standardization to create a clinical-grade product for evaluation in human trials. In conclusion, we propose that this novel NK cell therapy could be administered in an off-the-shelf manner to supercharge mAb efficacy in MM and eventually other malignancies as well.
Acknowledgments
The authors thank the staff of the University of California at San Francisco (UCSF) Helen Diller Family Cancer Comprehensive Cancer Center Preclinical Therapeutic Core Facility, managed by B.H. and supported by a grant from the National Cancer Institute (NCI), National Institutes of Health (NIH) (P30 CA082103).
This study was supported by funding from Indapta Therapeutics (A.B.B.), the UCSF Stephen and Nancy Grand Multiple Myeloma Translational Initiative (N.S. and A.P.W.), the Cancer Prevention and Research Institute of Texas (CPRIT RP150656) (X.L.), the University of Texas M.D. Anderson Cancer Center Duncan Family Institute (X.L.), and in part by grants from the NCI, NIH (R43CA235786 [G.D. and A.P.W.] and R15CA182769, P20CA221731, and P20CA221696 [X.L.]).
Authorship
Contribution: A.B.B., G.D., R.M., B.H., N.S., A.P.W., and X.L. contributed to design of the study; A.B.B., S.S., N.H.A., B.P.-E., and M.H. performed in vitro experiments, manufactured cells for in vivo experiments, and analyzed the data; X.L. performed and oversaw in vivo experiments; A.B.B., S.B., S.T., M.H.M., L.D., S.M., and X.L. performed in vivo experiments and analyzed the data; and A.B.B., G.D., N.S., A.P.W., and X.L. wrote the manuscript with input from all authors.
Conflict-of-interest disclosure: A.B.B., G.D., and R.M. are employees and shareholders at Indapta Therapeutics. A.P.W. is a member of the scientific advisory board and a shareholder at Indapta Therapeutics. S.S. is an employee of Indapta Therapeutics. N.S. received research funding from Celgene/Bristol Myers Squibb, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, and Nektar and served in an advisory role for GlaxoSmithKline, Amgen, Indapta Therapeutics, Sanofi, CareDx, Kite, Karyopharm Therapeutics, Oncopeptides, and CSL Behring. The remaining authors declare no competing financial interests.
Correspondence: Austin B. Bigley, Indapta Therapeutics, 5000 Gulf Freeway, Building 5, Houston, TX 77023; e-mail: austin@indapta.com; Arun P. Wiita, Department of Laboratory Medicine, University of California at San Francisco, 185 Berry St, San Francisco, CA 94143; e-mail: arun.wiita@ucsf.edu; Xinli Liu, Department of Pharmacological and Pharmaceutical Sciences, University of Houston, 4849 Calhoun Rd, Houston, TX 77204; e-mail xliu65@central.uh.edu.
References
Author notes
For any data queries, please contact Austin B. Bigley via e-mail at austin@indapta.com.
The full-text version of this article contains a data supplement.

![Expanded g-NK cells have markedly enhanced ADCC activity when combined with daratumumab in vivo. (A) Effect of treatment with g-NK cells and daratumumab (n = 7) on MM.1S tumor burden (BLI) in NSG mice relative to a cNK cell control plus daratumumab (n = 7) and mice treated with vehicle (n = 8). The cell and antibody combination therapies were given once per week for 5 weeks with IL-15 support (2 µg per mouse IP every 3 days). One mouse (labeled as &) in the g-NK plus daratumumab group missed the first week of treatment and received the treatment from weeks 2 to 6. (B) All remaining mice from the g-NK plus daratumumab group were euthanized on day 57 after tumor inoculation (day 43 after initial therapy). The quantitative BLI (photons per second [p/s]) values for vehicle control and mice treated with cNK plus daratumumab or g-NK plus daratumumab. (C) Effect of treatment with g-NK plus daratumumab on survival relative to treatment with cNK plus daratumumab or vehicle. (D) Comparison of body weight (g) in mice treated with vehicle, cNK plus daratumumab, or g-NK plus daratumumab. (E) Comparison of MM.1S tumor burden (number of CD45–/CD138+ cells) in bone marrow of NSG mice treated with cNK plus daratumumab or g-NK plus daratumumab. (F) Representative flow cytometry dot plots depicting tumor burden and persistence of NK cells in bone marrow for mice treated with vehicle, cNK plus daratumumab, or g-NK plus daratumumab. One-way ANOVA tests were used to determine the differences in BLI and body weight between different groups at each measurement day. For tumor burden and body weight comparisons when only 2 groups remained, independent samples Student t tests were used. Log-rank tests were used to determine differences between survival curves (P < .0001). Independent samples Student t tests were used to determine the differences in bone marrow tumor burden between mice treated with g-NK plus daratumumab or cNK plus daratumumab when the mice were euthanized. Values are mean ± SD for the BLI (B) and body weight (D) graphs and mean ± SEM for the tumor burden graph (E). *P < .05 (one-way ANOVA); #P < .001 (Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/15/10.1182_bloodadvances.2020002440/3/m_advancesadv2020002440f5.png?Expires=1769084994&Signature=t04VVsOMtgMFk5M2F~30~JxTjc710KYNQr5aZ7VVGjuWiK3XX-8FuRphmoy5CNoNWyZ4aKPcrQd6l6lXvxipJJCKtUhJfM7hNOlbhj0vEXpQdLkdixNnTsnuLejuDiMeKe13MWmWultvqLNW~HI9hW4WcV02gjK5fHyBUqXJE4xqKQPvjLxBuCaHV7-9zX0xhaxeGl1qR1vjvWAvT6sBMxGeauMEdFM7EciqWy9uO-gzuqE-j1i4UOPglLlsAY6rIRUE5ioADhsEMvWsk0wA-MxO4Sb-NOrOScRExrHx3noAKu6rlEKVp1KD0198eqjPLkEmocgNQtQ69upMhHyPTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)