Key Points
The long-term phase 2 data indicated attenuated linear growth rates during nilotinib treatment in pediatric patients with CML.
Safety was consistent with prior reports, and no new safety signals were observed despite long-term treatment.
Abstract
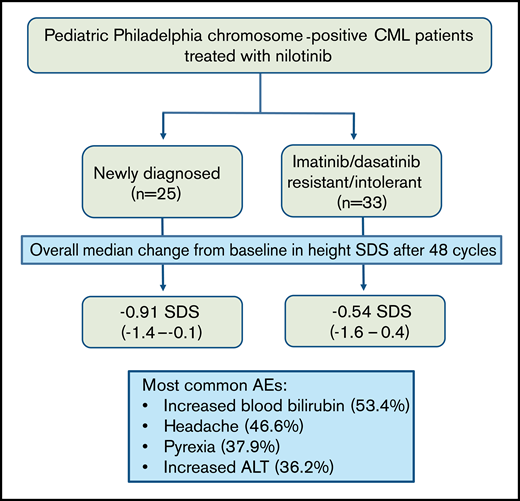
The phase 2, open-label study (DIALOG) of nilotinib in pediatric patients with Philadelphia chromosome-positive chronic myelogenous leukemia (CML) met its coprimary end points, showing sustained nilotinib efficacy in patients with newly diagnosed (ND) or imatinib/dasatinib resistant/intolerant (R/I) CML. This update assessed growth and safety profiles in patients who had completed ≥48, 28-day treatment cycles of nilotinib 230 mg/m2 twice daily, or previously discontinued the study. Height was assessed regularly and reported using standard deviation scores (SDSs) based on World Health Organization growth charts. All data were summarized descriptively (cutoff, 6 March 2019). Overall, 33 patients in the R/I cohort and 25 patients in the ND cohort received nilotinib. Each cohort showed a negative slope in height SDS over the course of the study, indicating attenuated growth rates during nilotinib treatment: overall median change from baseline in height SDS after 48 cycles was −0.54 SDS (range, − 1.6 to 0.4) and −0.91 SDS (−1.4 to −0.1) in R/I and ND cohorts, respectively. Patients in the R/I cohort were shorter at baseline than those in the ND cohort, and remained so throughout the study. The most common all-cause adverse events were increased blood bilirubin (53.4%), headache (46.6%), pyrexia (37.9%), and increased alanine transferase (36.2%). Apart from the impact on growth, the safety profile of nilotinib was generally consistent with previous reports. This study was registered on www.clinicaltrials.gov at #NCT01844765.
Introduction
Chronic myeloid leukemia (CML) accounts for ∼2% to 3% of leukemias in children aged <15 years, and this proportion increases to 9% in adolescents aged 15 to 19.1 CML in children and adolescents differs from CML in adults, with young patients typically having more aggressive clinical features.2 Nonetheless, the tyrosine kinase inhibitors (TKIs) imatinib, dasatinib, and nilotinib have all been approved for the treatment of pediatric patients with CML,3‐5 and the molecular response rates reported with second-generation TKIs in pediatric patients appear to be comparable to those in adults.6,7
Another consideration when treating children and adolescents with CML is that they may be exposed to TKIs for a much longer time than adult patients, including during times of active growth and the onset of puberty. This raises the possibility that treatment could have long-term, off-target endocrinological consequences affecting growth rate, bone metabolism, puberty, and fertility as well as other as yet unknown side effects.2,8,9 Treatment with imatinib has improved clinical outcomes in pediatric patients with CML,10‐12 but retrospective analyses have shown that treatment is associated with growth deceleration in prepubertal and adolescent patients.13‐16 Fewer data have been published on the effect of dasatinib on growth, although a retrospective analysis of data from an open-label, nonrandomized phase 2 trial in pediatric patients also showed some growth deceleration in newly diagnosed (ND) prepubertal patients.17 These reports with imatinib and dasatinib highlight the importance of monitoring growth in this population during prolonged TKI treatment.
Nilotinib has been investigated in pediatric patients in a phase 1 study (www.clinicaltrials.gov #NCT01077544), in which a dose of 230 mg/m2 twice daily was determined to be the recommended dose for further studies in children and adolescents.18 DIALOG is a phase 2, open-label study of nilotinib in pediatric patients with ND CML or with resistance to or intolerance of (R/I) imatinib or dasatinib.7 The study met its coprimary end points, demonstrating efficacy in this patient population. In the ND cohort, cumulative major molecular response (BCR-ABL1IS ≤ 0.1%) by 12, 28-day, treatment cycles and complete cytogenetic response at 12 cycles were each achieved by 16 of 25 patients (64%). In the R/I cohort, 13 of 33 patients (39.4%) achieved major molecular response at 6 cycles.7 Analyses at 12, 24, and 36 cycles showed that nilotinib had a manageable safety profile that was generally consistent with that observed in adults.7,19 However, analysis of height revealed a trend toward growth deceleration over time after ≥36 treatment cycles.19 In the interest of following up this observation further, we report a formal evaluation of growth in study participants, alongside longer term safety data, after patients had received ≥48 cycles of nilotinib treatment or discontinued the study.
Methods
Study design and patient population
The study design and coprimary end points have been described elsewhere.7 In brief, eligible patients aged 1 to <18 years were enrolled in 1 of 3 cohorts:
- 1.
Philadelphia chromosome–positive (Ph+) CML in chronic phase (CML-CP) R/I imatinib or dasatinib.
- 2.
Ph+ CML in accelerated phase (CML-AP) R/I imatinib or dasatinib (no patients were enrolled in this cohort).
- 3.
ND Ph+ CML-CP.
Nilotinib was administered orally at a dose of 230 mg/m2 twice daily (rounded to the nearest 50 mg), up to a maximum dose of 400 mg twice daily. The 230 mg/m2 twice-daily dose was selected for this study based on the results of the phase 1 study.18 It was considered appropriate for the patients R/I to prior TKIs as it was equivalent to the adult dose of 400 mg, which is indicated for this population. It was also considered appropriate for newly diagnosed pediatric patients, because pediatric CML tends to present with more aggressive features than adult CML.2 Furthermore, the 400 mg twice-daily dose has shown overlapping PK results with the 300 mg twice-daily dose, as well as an acceptable safety profile.18 Treatment discontinuation was permitted at any time based on patient or investigator decision, unacceptable toxicity, disease progression, protocol deviations resulting in a significant risk to the patient’s safety, use of prohibited treatments, or pregnancy.
Height was measured at baseline, at every treatment cycle up to cycle 12, then at every 3 treatment cycles up to cycle 24, and then at every 6 treatment cycles. Tanner staging was assessed at baseline and every 6 treatment cycles. Safety assessments included adverse events (AEs), which were recorded by preferred term (PT) according to Medical Dictionary for Regulatory Activities (MedDRA), version 21.1 (https://www.MedDRA.org). The severity of the AEs was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_ 5x7.pdf). AEs of special interest (AESIs) were defined based on groups of PTs. Standard clinical laboratory assessments were undertaken. In addition, thyroid function was assessed by measuring serum levels of free thyroxine (FT4) and thyroid-stimulating hormone (TSH). Bone mineral density (BMD) was measured by dual-energy X-ray absorptiometry scans of the lumbar spine and of the whole body, except for the head. Sexual maturation was monitored by Tanner staging.20,21
Statistical analysis
Descriptive statistics were used in all analyses. Time to onset was determined for AESIs, and the rate of AESIs per year was also quantified. Height, body mass index (BMI), and BMD were reported using standard deviation scores (SDSs, also known as z scores) that were computed at predefined time points. Height and BMI for age and sex were based on World Health Organization Child Growth Standards.22,23 Height was evaluated in a linear mixed-effects model, to assess changes in the slope parameter; height SDS was used as the response variable and time (based on 6-month intervals) as the explanatory variable. The analysis was performed separately for each cohort of patients (ND and R/I), and a random patient effect and an autoregressive covariance structure were implemented to account for repeated measures within each patient.
Further model-based analysis was performed to assess changes in slope, considering covariates (baseline pubertal stage, pubertal status over time, and sex) and their interaction with time. For the purposes of the mixed-model analysis, the distinction between prepubertal and pubertal was defined as attainment of Tanner stage 2 for breast or genitalia in girls and boys, respectively. Shift tables based on normal ranges were produced for TSH and FT4.
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki and local laws and regulations. Prior written informed consent was provided by the patients’ parents or caregivers and by patients able to provide a signature. The study protocol and all amendments were reviewed by the independent ethics committee and/or institutional review board for each study center.
Results
Patient disposition and baseline characteristics
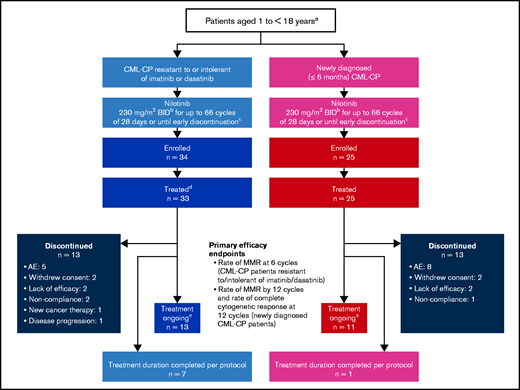
At the data cutoff (6 March 2019), all patients had completed ≥48 cycles of treatment (28 days each) or had discontinued the study treatment (Figure 1). Of the 59 patients who were recruited into the 2 cohorts, all but 1 received the study treatment. Seven patients in the R/I cohort and 1 in the ND cohort had completed the study treatment (66 cycles), per protocol, at the data cutoff. All analyses were performed using the safety set (n = 58), which included 33 patients in the R/I cohort and 25 in the ND cohort. Baseline patient data are presented in Table 1.
Study design and patient disposition. aThe study design includes a third arm that includes patients with CML-AP R/I imatinib or dasatinib. No patients enrolled in this arm. bNilotinib was administered at a dose of 230 mg/m2 twice daily (rounded to the nearest 50 mg) based on the recommended dose for adults of 400 mg twice daily, scaled to body surface area. cAt any time, discontinuation was allowed due to patient/investigator decision or due to unacceptable toxicities, disease progression, protocol deviations resulting in a significant risk to the patient’s safety, use of prohibited treatments, or pregnancy. dOne enrolled patient did not receive any study medication. eAt data cutoff (6 March 2019).
Study design and patient disposition. aThe study design includes a third arm that includes patients with CML-AP R/I imatinib or dasatinib. No patients enrolled in this arm. bNilotinib was administered at a dose of 230 mg/m2 twice daily (rounded to the nearest 50 mg) based on the recommended dose for adults of 400 mg twice daily, scaled to body surface area. cAt any time, discontinuation was allowed due to patient/investigator decision or due to unacceptable toxicities, disease progression, protocol deviations resulting in a significant risk to the patient’s safety, use of prohibited treatments, or pregnancy. dOne enrolled patient did not receive any study medication. eAt data cutoff (6 March 2019).
Patient demographics and baseline characteristics
| . | R/I patients* (n = 33) . | ND patients (n = 25) . |
|---|---|---|
| Median age (range), y | 13 (2-17) | 13 (10-16) |
| Patients aged 1 to <12 y, n (%) | 12 (36) | 6 (24) |
| Patients aged 12 to <18 y, n (%) | 21 (64) | 19 (76) |
| Female, n (%) | 12 (36) | 12 (48) |
| Median height SDS (range) | −0.56 (−4.3 to 1.2) | 0.06 (−0.9 to 1.7) |
| Pubertal status | ||
| Prepubertal, n (%) | 9 (27) | 4 (16) |
| Pubertal, n (%) | 23 (70) | 21 (84) |
| Missing, n (%) | 1 (3) | 0 |
| Prior antineoplastic TKI therapies, n (%) | ||
| Imatinib | 31 (93.9) | NA |
| Dasatinib | 2 (6.1) | NA |
| Intolerant of imatinib/dasatinib, n (%)† | 6 (18)/0 | NA |
| Resistant to imatinib/dasatinib, n (%)† | 28 (85)/2 (6) | NA |
| . | R/I patients* (n = 33) . | ND patients (n = 25) . |
|---|---|---|
| Median age (range), y | 13 (2-17) | 13 (10-16) |
| Patients aged 1 to <12 y, n (%) | 12 (36) | 6 (24) |
| Patients aged 12 to <18 y, n (%) | 21 (64) | 19 (76) |
| Female, n (%) | 12 (36) | 12 (48) |
| Median height SDS (range) | −0.56 (−4.3 to 1.2) | 0.06 (−0.9 to 1.7) |
| Pubertal status | ||
| Prepubertal, n (%) | 9 (27) | 4 (16) |
| Pubertal, n (%) | 23 (70) | 21 (84) |
| Missing, n (%) | 1 (3) | 0 |
| Prior antineoplastic TKI therapies, n (%) | ||
| Imatinib | 31 (93.9) | NA |
| Dasatinib | 2 (6.1) | NA |
| Intolerant of imatinib/dasatinib, n (%)† | 6 (18)/0 | NA |
| Resistant to imatinib/dasatinib, n (%)† | 28 (85)/2 (6) | NA |
NA, not applicable; SDS, standard deviation score; TKI, tyrosine kinase inhibitor.
Patients were R/I to 1 prior TKI, either imatinib or dasatinib.
Three patients were both intolerant of and resistant to imatinib and are counted once as resistant and once as intolerant.
Analysis of growth
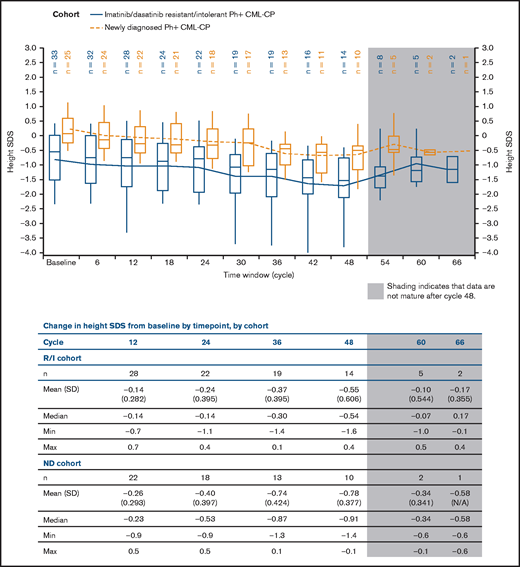
A significant negative slope in height SDS was shown in both cohorts, indicating growth deceleration over time, compared with baseline (Figure 2). Consistent with reports from patients treated with other TKIs,13,17 patients in the R/I cohort were shorter than those in the ND cohort at baseline, and remained so throughout the study. Overall median change from baseline in height SDS after 48 cycles was −0.54 (range, −1.6 to 0.4) and −0.91 (−1.4 to −0.1) in the R/I and ND cohorts, respectively.
Height SDS over time by cohort. Plot shows boxes (25th to 75th percentile) with a horizonal line representing the median. The dots in the boxes and joining lines represent mean values. Whiskers extend to the 10th and 90th percentiles. Values outside this range are not displayed.
Height SDS over time by cohort. Plot shows boxes (25th to 75th percentile) with a horizonal line representing the median. The dots in the boxes and joining lines represent mean values. Whiskers extend to the 10th and 90th percentiles. Values outside this range are not displayed.
A linear mixed-effects model with time as the explanatory variable confirmed the trend: the estimated average loss of height SDS per 6 months after baseline was 5% in the R/I cohort and 8% in the ND cohort (P < .0001 for both; supplemental Table 1).
Further model-based analysis evaluated changes in height, considering the covariates (supplemental Table 3). Pubertal status was analyzed at baseline and as a time-dependent variable. There was no difference in estimated average loss of height SDS by pubertal status (prepubertal vs pubertal) at baseline (P-value for interaction: R/I, .25; ND, .37). When puberty was analyzed as a time-dependent variable, loss of height SDS was higher before puberty (P-value for interaction: R/I, .03; ND, .006). However, this finding should be interpreted with caution, as only 4 patients in the ND cohort were prepubertal at baseline. There was no observed difference in growth deceleration according to sex, and no trend in BMI SDS (supplemental Figure 1) was observed in either cohort.
Despite the growth data showing clear deceleration in growth over the course of this study, only 3 growth-related AEs (described using MedDRA PTs) were reported: 1 patient had grade 2 growth retardation (ND cohort); another patient had growth hormone deficiency and height below normal (both grade 1 and determined to be a serious adverse event [SAE]; R/I cohort); and the third patient had grade 1 growth retardation (R/I cohort). All AEs were suspected to be related to nilotinib, but none was associated with nilotinib dose reduction, interruption, or discontinuation. Median time to onset of these AEs was 21.3 months (range, 9.2-33.4) in the R/I cohort and 30.5 months in the ND cohort.
Thyroid function
One patient in the ND cohort had an AE of autoimmune thyroiditis, which was not categorized as serious and was not thought to be related to nilotinib, although the study drug was permanently discontinued. One patient in the R/I cohort had an AE of decreased blood TSH, but it was not thought to be related to nilotinib, and the study treatment was not interrupted. No other changes in TSH or FT4 concentrations were reported that were considered to be clinically significant (supplemental Text).
Bone density
There was no observed change in BMD SDS over time (supplemental Figure 2), and the SDS for BMD was comparable to baseline in both cohorts.
Safety
All patients in both cohorts reported ≥1 AE. The most common all-cause AEs (≥30% of patients) were increased blood bilirubin (53.4%), headache (46.6%), pyrexia (37.9%), and increase in alanine aminotransferase (ALT; 36.2%). Grade 3/4 all-cause AEs were reported in 19 (57.6%) patients in the R/I cohort and 18 (72%) patients in the ND cohort.
AEs of special interest
AEs of special interest (AESIs) are presented in Table 2, and MedDRA PTs reported in ≥1 patient within each group term are shown in supplemental Table 2.
AESIs
| AE group, n (%) . | R/I patients* (n = 33) . | ND patients (n = 25) . | All patients (n = 58) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Elevated blood cholesterol† | 3 (9.1) | 0 | 2 (8) | 1 (4) | 5 (8.6) | 1 (1.7) |
| Elevated blood glucose‡ | 2 (6.1) | 0 | 0 | 0 | 2 (3.4) | 0 |
| Fluid retention | 2 (6.1) | 0 | 4 (16) | 1 (4) | 6 (10.3) | 1 (1.7) |
| Edema and other fluid retentions | 2 (6.1) | 0 | 4 (16) | 1 (4) | 6 (10.3) | 1 (1.7) |
| Growth retardation | 2 (6.1) | 0 | 1 (4) | 0 | 3 (5.2) | 0 |
| Hepatotoxicity | 19 (57.6) | 9 (27.3) | 17 (68) | 7 (28) | 36 (62.1) | 15 (25.9) |
| Drug-induced liver injury | 1 (3) | 1 (3) | 0 | 0 | 1 (1.7) | 1 (1.7) |
| Hepatic transaminase/bilirubin elevations | 19 (57.6) | 8 (24.2) | 17 (68) | 7 (28) | 36 (62.1) | 15 (25.9) |
| Thrombocytopenia | 1 (3) | 0 | 8 (32) | 3 (12) | 9 (15.5) | 3 (5.2) |
| QT prolongation§ | 5 (15.2) | 0 | 3 (12) | 0 | 8 (13.8) | 0 |
| Rash | 16 (48.5) | 5 (15.2) | 15 (60) | 3 (12) | 31 (53.4) | 8 (13.8) |
| AE group, n (%) . | R/I patients* (n = 33) . | ND patients (n = 25) . | All patients (n = 58) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Elevated blood cholesterol† | 3 (9.1) | 0 | 2 (8) | 1 (4) | 5 (8.6) | 1 (1.7) |
| Elevated blood glucose‡ | 2 (6.1) | 0 | 0 | 0 | 2 (3.4) | 0 |
| Fluid retention | 2 (6.1) | 0 | 4 (16) | 1 (4) | 6 (10.3) | 1 (1.7) |
| Edema and other fluid retentions | 2 (6.1) | 0 | 4 (16) | 1 (4) | 6 (10.3) | 1 (1.7) |
| Growth retardation | 2 (6.1) | 0 | 1 (4) | 0 | 3 (5.2) | 0 |
| Hepatotoxicity | 19 (57.6) | 9 (27.3) | 17 (68) | 7 (28) | 36 (62.1) | 15 (25.9) |
| Drug-induced liver injury | 1 (3) | 1 (3) | 0 | 0 | 1 (1.7) | 1 (1.7) |
| Hepatic transaminase/bilirubin elevations | 19 (57.6) | 8 (24.2) | 17 (68) | 7 (28) | 36 (62.1) | 15 (25.9) |
| Thrombocytopenia | 1 (3) | 0 | 8 (32) | 3 (12) | 9 (15.5) | 3 (5.2) |
| QT prolongation§ | 5 (15.2) | 0 | 3 (12) | 0 | 8 (13.8) | 0 |
| Rash | 16 (48.5) | 5 (15.2) | 15 (60) | 3 (12) | 31 (53.4) | 8 (13.8) |
The MedDRA PTs reported in ≥1 patient within each AESI group term listed in this table are shown in supplemental Table 1. A patient with multiple severity grades for an AE was counted only under the maximum grade. No events were reported for the following AESI group terms: “cardiovascular event” (including ischemic cerebrovascular events, ischemic heart disease, peripheral arterial occlusive disease, and other cardiovascular events); “cardiac failure”; “pancreatitis, lipase, and amylase elevations”; “renal events”; or “significant bleeding” (including central nervous system or gastrointestinal hemorrhage).
Patients were R/I to 1 prior TKI, either imatinib or dasatinib.
Specific group term is “increased blood cholesterol.”
Specific group term is “increased blood glucose.”
MedDRA PT included syncope for 1 patient and QT prolonged for 7 patients (reported in Hijiya et al7).
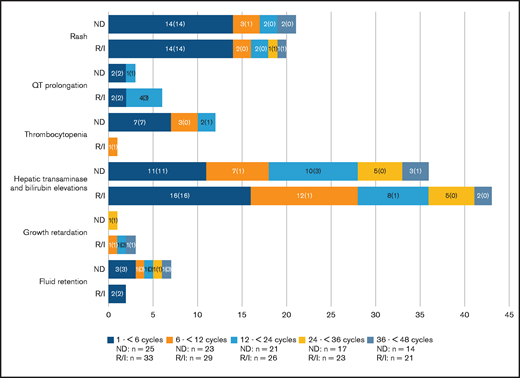
The number of patients with selected AESIs by period and the number of patients with a first event in each period, are shown in Figure 3. The large majority of AESIs occurred during the first 6 months of study treatment.
Number of selected AESIs by period. Bars show the number of patients with each AESI occurring in the first 6 cycles and newly occurring in the second 6 cycles, in years 2 and 3, and so on. Numbers in brackets show the number of patients with a first event. Denominators are the number of patients on treatment during the indicated time window. (The incidence of certain AESIs appear higher in later time windows than in previous time windows, owing to a lower number of patients receiving treatment).
Number of selected AESIs by period. Bars show the number of patients with each AESI occurring in the first 6 cycles and newly occurring in the second 6 cycles, in years 2 and 3, and so on. Numbers in brackets show the number of patients with a first event. Denominators are the number of patients on treatment during the indicated time window. (The incidence of certain AESIs appear higher in later time windows than in previous time windows, owing to a lower number of patients receiving treatment).
Hepatotoxicity
Across both cohorts, an AE corresponding to hepatotoxicity (group term) was reported in 36 (62%) patients. One patient in the R/I cohort had a grade 3/4 drug-induced liver injury, with onset at 13.8 months; there were no signs of severe or progressive liver dysfunction. AEs corresponding to hepatic transaminase and bilirubin elevations were reported in 19 (58%) patients in the R/I cohort and 17 (68%) patients in the ND cohort. Median time to first occurrence was 0.3 months (range, 0.1-44.2) in the R/I cohort and 0.8 months (range, 0-49.7) in the ND cohort. Of those AEs, grade 3/4 events were reported in 15 (26%) patients. Grade 3/4 ALT elevation was reported in 12% of patients in each cohort, and 1 patient in each cohort (3% and 4% of patients in the R/I and ND cohorts, respectively) had a grade 3/4 aspartate aminotransferase (AST) elevation. Grade 3/4 increased bilirubin was reported in 9.1% and 16% of patients in the R/I and ND cohorts, respectively.
In total, 24 patients required nilotinib dose reduction and 7 discontinued nilotinib treatment owing to an AESI corresponding to hepatotoxicity: 3 in the R/I cohort (median time to discontinuation, 7.4 months) and 4 in the ND cohort (median time to discontinuation, 20.7 months). The reported increases in hepatic enzymes and/or bilirubin that led to treatment discontinuation resolved within 20 days for all patients except 1, in whom it was ongoing at the end of the 30-day follow-up after treatment discontinuation. Three patients in each cohort had a concomitant increase in total bilirubin >2 times the upper limit of normal (ULN), in transaminase >3 times ULN, and in alkaline phosphatase ≤2 ULN during the study treatment, but could be managed and were not thought to have drug toxicity, according to Hy’s law.24 There were 2 reported SAEs, 1 in each cohort. In the R/I cohort, 1 patient had grade 3 hepatomegaly in tandem with a confirmed mycoplasma infection on day 824, which resolved within 8 days without any change in nilotinib treatment. This patient had ALT and bilirubin levels within the normal limits and grade 1 elevated AST on day 824; these remained the same until the hepatomegaly resolved. In the ND cohort, 1 patient had hyperbilirubinemia (grade 3), and the event resolved 2 days after nilotinib was permanently discontinued.
Rash
Overall, 31 patients experienced an AE of rash (group term). Median time to onset was 0.3 months (range, 0-35.6) in the R/I cohort and 0.2 months (range, 0-5.9) in the ND cohort.
Dose reduction of nilotinib because of a rash was required in 8 (14%) patients. One patient in the R/I cohort discontinued nilotinib after 7.4 months of treatment and 2 in the ND cohort discontinued nilotinib after a median of 6.4 months of treatment. One of the cases of rash, in the ND cohort, was determined to be an SAE because it was associated with hospitalization, as it occurred in parallel with abdominal pain, headache, and diarrhea requiring intravenous rehydration (all grade 1) and grade 2 syncope.
Thrombocytopenia
Overall, 9 (16%) patients experienced thrombocytopenia, 8 of whom were in the ND cohort. Of those, 3 required nilotinib dose reduction, and 1 discontinued nilotinib after 14 months of treatment. There were no reported SAEs of thrombocytopenia. Time to onset of thrombocytopenia was 10.1 months in the single patient in the R/I cohort, and median time to onset was 1.8 months (range, 0.3-21.9) in the ND cohort.
QT prolongation
QT prolongation (group term) was reported in 8 (14%) patients across both cohorts, including 1 patient with syncope and 7 with prolonged QT interval. The median time to onset of QT prolongation was 16.7 months (range, 0-21.9) in the R/I cohort and 0.3 months (range, 0.3-13.9) in the ND cohort. In 2 patients in the ND cohort, the investigator considered these AEs (grade 2 syncope and grade 1 prolonged QT interval) to be serious. The latter of these events was suspected to be related to nilotinib and required temporary nilotinib interruption. Dose reduction was required in 6 patients, including the patient with syncope. There were no nilotinib discontinuations because of QT prolongation.
Fluid retention
In the ND cohort, 4 patients had an AE of fluid retention (group term), including 1 patient who had a grade 3 AE of increased weight. Median time to onset was 4.2 months (range, 0.1-25.4 months). In the R/I cohort, there were 2 events of fluid retention (group term): 1 patient had increased weight, and another had an AE of lip swelling. Median time to onset was 2.2 months (range, 1-3.5 months). No dose reductions or discontinuations were required in either cohort, and no events of severe fluid retention, such as pericardial or pleural effusion, were reported.
Other AESIs
Elevated blood cholesterol was reported in 5 patients across both cohorts (median time to onset: 9.3 months [range, 1.0-11.2] in the R/I cohort and 11.8 months [range, 4.6-18.9] in the ND cohort). One patient in the ND cohort had grade 3/4 elevated blood cholesterol that required nilotinib dose reduction.
Grade 1/2 elevated blood glucose was reported in 2 patients in the R/I cohort (median time to onset, 8.3 months [range, <7.4-9.3]) and did not require any change in nilotinib treatment.
AEs suspected to be related to nilotinib
A total of 87.9% of patients in the R/I cohort and 92% of patients in the ND cohort experienced an AE that was suspected to be study drug related. Of those, increased blood bilirubin (48.5%), increased ALT (30.3%), increased AST (24.2%), and headache (21.2%) were most frequently reported (≥20% of patients) in the R/I cohort. In the ND cohort, the most frequently reported events were increased blood bilirubin (56%), increased ALT (40%), headache, increased AST (each 32%), rash (28%), and fatigue, nausea, and vomiting (each 20%).
All-grade musculoskeletal and connective tissue disorders that were suspected to be related to nilotinib were reported in 9 patients (27.3%) in the R/I cohort and in 8 patients (32%) in the ND cohort. Of those, the most common was pain in an extremity, which occurred in 6 patients overall. One patient experienced 2 episodes of musculoskeletal pain, 1 each of grade 1 and grade 2 severity. At the time of writing, there have been no new SAEs suspected to be study drug related since those previously reported.7
Nilotinib exposure, dose reductions, and discontinuations
Median duration of exposure to nilotinib was 44 (range, 1-61) months in the R/I cohort and 44 (range, 1-60) months in the ND cohort. Median relative dose intensity was 95% (range, 43-107) in the R/I cohort and 82% (range, 32-102) in the ND cohort.
The number of patients requiring ≥1 dose reduction during the study was 21 (64%) in the R/I cohort and 16 (64%) in the ND cohort. Of those, 16 (49%) and 12 (48%) patients in the R/I and ND cohorts, respectively, required ≥1 dose reduction because of an AE. Other reasons for dose reduction were dosing error (11 [33%] in the R/I cohort and 2 [8%] in the ND cohort); scheduling conflict (3 [9%] in the R/I cohort and 1 [4%] in the ND cohort); and concomitant medication that affected drug exposure (1 patient in each cohort). Information on the reason for dose reduction was missing for 2 patients in each cohort.
The number of patients requiring ≥1 treatment interruption during the study was 21 (64%) in the R/I cohort and 20 (80%) in the ND cohort. Of those, 21 (64%) and 17 (68%) patients in the R/I and ND cohorts, respectively, required ≥1 treatment interruption because of an AE. Other reasons for dose interruption were dosing error (4 [12%] in the R/I cohort and 3 [12%] in the ND cohort); 1 patient in each cohort required a dose interruption because of a scheduling conflict, and 1 patient in the ND cohort had concomitant medication that affected drug exposure. Information on the reason for dose reduction was missing for 1 patient in the ND cohort.
The most frequent AEs requiring dose reduction or interruption of nilotinib treatment (≥10% of patients) were increased blood bilirubin (24.2%), increased ALT (12.1%), and rash (12.1%) in the R/I cohort, and increased blood bilirubin (40%), increased AST (16%), increased ALT (16%), and vomiting (12%) in the ND cohort.
The median duration of nilotinib interruption was 17 days (range, 1-108) in the R/I cohort and 27 days (range, 1-92) in the ND cohort.
AEs leading to permanent discontinuation of nilotinib were reported in 5 (15%) and 8 (32%) patients in the R/I and ND cohorts, respectively. The most common AEs associated with a permanent discontinuation of nilotinib are listed in Table 3.
AEs leading to discontinuation
| . | R/I patients* (n = 23) . | ND patients (n = 25) . | All patients (n = 58) . |
|---|---|---|---|
| Patients with ≥1 AE | 5 (15.2) | 8 (32) | 13 (22.4) |
| Increased blood bilirubin | 3 (9.1) | 3 (12) | 6 (10.3) |
| Rash | 1 (3) | 1 (4) | 2 (3.4) |
| ALT increased | 0 | 1 (4) | 1 (1.7) |
| Anemia | 1 (3) | 0 | 1 (1.7) |
| AST increased | 0 | 1 (4) | 1 (1.7) |
| Autoimmune thyroiditis | 0 | 1 (4) | 1 (1.7) |
| Decreased appetite | 1 (3) | 0 | 1 (1.7) |
| Headache | 1 (3) | 0 | 1 (1.7) |
| Hyperamylasemia | 0 | 1 (4) | 1 (1.7) |
| Keratosis pilaris | 1 (3) | 0 | 1 (1.7) |
| Malaise | 1 (3) | 0 | 1 (1.7) |
| Nausea | 1 (3) | 0 | 1 (1.7) |
| Pain in extremity | 1 (3) | 0 | 1 (1.7) |
| Pancreatic enlargement | 0 | 1 (4) | 1 (1.7) |
| Platelet count decreased | 0 | 1 (4) | 1 (1.7) |
| Rash maculopapular | 0 | 1 (4) | 1 (1.7) |
| . | R/I patients* (n = 23) . | ND patients (n = 25) . | All patients (n = 58) . |
|---|---|---|---|
| Patients with ≥1 AE | 5 (15.2) | 8 (32) | 13 (22.4) |
| Increased blood bilirubin | 3 (9.1) | 3 (12) | 6 (10.3) |
| Rash | 1 (3) | 1 (4) | 2 (3.4) |
| ALT increased | 0 | 1 (4) | 1 (1.7) |
| Anemia | 1 (3) | 0 | 1 (1.7) |
| AST increased | 0 | 1 (4) | 1 (1.7) |
| Autoimmune thyroiditis | 0 | 1 (4) | 1 (1.7) |
| Decreased appetite | 1 (3) | 0 | 1 (1.7) |
| Headache | 1 (3) | 0 | 1 (1.7) |
| Hyperamylasemia | 0 | 1 (4) | 1 (1.7) |
| Keratosis pilaris | 1 (3) | 0 | 1 (1.7) |
| Malaise | 1 (3) | 0 | 1 (1.7) |
| Nausea | 1 (3) | 0 | 1 (1.7) |
| Pain in extremity | 1 (3) | 0 | 1 (1.7) |
| Pancreatic enlargement | 0 | 1 (4) | 1 (1.7) |
| Platelet count decreased | 0 | 1 (4) | 1 (1.7) |
| Rash maculopapular | 0 | 1 (4) | 1 (1.7) |
The MedDRA PT is expressed as the number of patients (percentage of group).
MedDRA, Medical Dictionary for Regulatory Activities.
Patients were R/I to 1 prior TKI, either imatinib or dasatinib.
Deaths
By the data cutoff date, no patient had died in the study while on treatment. Two patients died during survival follow-up. One patient in the R/I cohort discontinued the study because of progression to AP/blast crisis and subsequently died of lymphoid blast crisis. The second patient in the ND cohort discontinued the study because of increased ALT and AST levels and rash. This patient received imatinib after study discontinuation and died of posttransplant lymphoma >3 years after discontinuation.
Discussion
The observed safety profile of nilotinib in this longer term follow-up was generally consistent with that reported in a pediatric patient population,7,19 and most of the AEs could be managed through monitoring and dose reduction. A key finding of this study is that these long-term data show clear growth deceleration over time.
Analysis of height data confirmed a trend toward growth deceleration over time that had initially been reported after ≥36 cycles of nilotinib treatment. Available data showed that patients in the R/I cohort were shorter at baseline than those in the ND cohort and remained so throughout the study. These results are consistent with reports of growth deceleration with imatinib and dasatinib in this patient population.13,17 Interestingly, the number of growth-related AEs reported in this study was very low, in direct contrast to the clear trend seen in the growth data. It is likely that reduction in linear growth or growth velocity has been underreported as a specific AE by study sites, as the assessment of growth retardation requires investigators to calculate and compare height velocity over time, and such a calculation is not a routine requirement in oncology clinical trials. Growth velocity is naturally dynamic in prepubertal vs early-pubertal vs late-pubertal children, making the percentage change in height velocity from baseline a difficult tool to use to identify truly aberrant growth. Furthermore, the consistent accumulation of small declines in height velocity over multiple years could affect adult height, but may not be reported as an AE. Other possible reasons for the low proportion of reported growth deceleration–related AEs could be that growth deceleration had already occurred in patients from the R/I cohort at the beginning of the study, whereas the ND cohort had a higher proportion of pubertal patients. It is possible that rapid, early pubertal growth acceleration could be more resistant to the growth-suppressive effects of TKIs. In late puberty, patients have less remaining growth potential, are experiencing normal physiologic growth deceleration, and are less at risk for cumulative growth attenuation.
A better picture of reduced growth velocity is therefore provided by the analysis of growth data comparing height SDS in the full cohort, which indicates that growth deceleration was more common than the number of growth-related AEs suggests. We therefore recommend that a central analysis of growth data be performed, as in this study, in future studies involving patient populations and treatments similar to those in this one. Finally, in the clinic, any effect of TKIs on growth should be considered in the context of the morbidity and mortality (including an effect on growth) that may be associated with allogeneic hematopoietic cell transplantation.25
The underlying mechanism of growth deceleration in patients treated with TKIs remains unclear; possible explanations are their impact on disruption of the growth hormone-insulin–like growth factor axis and on vitamin D metabolism and bone remodeling.2,8 Nevertheless, these parameters were not assessed in this study. The initial evaluation showing no clear change in BMD over the course of this study so far is reassuring, but BMD requires longer follow-up. Apart from thyroid function, no other systematic assessment of endocrine function was performed in this study.
Drug-related AEs affecting bone growth and development have been reported in 4% of pediatric patients with ND and relapsed or refractory CML treated with dasatinib in a phase 2 study.6 A systematic review is currently ongoing to quantify any endometabolic and bone health effects of TKIs in patients with CML.26 Of note, a recent study of pediatric patients with acute lymphoblastic leukemia showed that imatinib can decelerate height growth.27
When puberty was analyzed as a time-dependent variable, loss of height SDS was higher during the prepubertal than during the postpubertal period. Although this finding should be interpreted with caution because the number of patients in the analysis was low (only 4 patients in the ND cohort were prepubertal at baseline), various factors should be considered that may have contributed to this observation. These could have to do with the biology of puberty or with differences in the amount of linear growth at risk. For example, it is possible that a growth spurt associated with puberty somewhat mitigates earlier effects of growth deceleration, which would be consistent with previous reports of the effect of imatinib.15 Another contributing factor may be that patients who were postpubertal at the time of enrollment would be likely to have completed a greater percentage of their overall growth before nilotinib exposure compared with those who were prepubertal. As such, the amount of potential growth that could be affected by an individual’s exposure to TKIs is greater in prepubertal patients than in those who are partway through, or have even completed, puberty.
The safety profile of nilotinib in this study was generally consistent with its known safety profile in adults.7,28‐30 Analysis of AESIs indicate that all-cause AEs related to elevated bilirubin and liver enzymes were more common than might be expected based on data from adult studies.28‐30 However, the frequency of grade 3/4 AEs related to liver function is generally comparable between pediatric and adult populations. In this study, grade 3/4 AST, ALT, and increased blood bilirubin were observed in 3%, 12%, and 9% of patients in the R/I cohort, respectively, and 4%, 12%, and 16% of patients in the ND cohort. For comparison, in the 2-year analysis of the ENESTnd study of adults with ND CML-CP,30 grade 3/4 AST, ALT, and bilirubin increases were reported in 1%, 4%, and 4% of patients in the 300 mg twice-daily arm, respectively, and 3%, 9%, and 8% in 400 mg twice-daily arm. In adults with imatinib resistance or intolerance treated with nilotinib 400 mg twice daily,28 grade 3/4 AST, ALT, and bilirubin increases were observed in 3%, 4%, and 7% of patients, respectively. The differences in the frequency of AEs of transaminase and bilirubin elevations in this pediatric study may be partly explained by its small sample size compared with that in adult studies.29,30
As previously described, QT prolongation (PT) was observed in 7 patients in this study: 5 in the R/I cohort and 2 in the ND cohort. However, there were no episodes of QTcF >500 ms or increases from baseline of >60 ms. In a 2-year follow-up of a study in adults with ND CML, 1 patient receiving nilotinib 400 mg twice daily had an episode of QTcF >480 ms and 3 (2 receiving nilotinib 400 mg twice daily and 1 receiving nilotinib 300 mg twice daily) had QTcF interval increases of >60 ms from baseline.30 In adult patients with imatinib R/I treated with nilotinib 400 mg twice daily, 8 patients had a QTcF interval increase of >60 ms from baseline and 4 had an episode of QTcF >500 ms.29 Although the number of patients in this pediatric study was smaller than those in the adult study populations, when these data are considered together, the effect of nilotinib on QT interval appears to be comparable in adult and pediatric patients. In addition, no cardiovascular events were reported in this study, whereas there have been reports of ischemic cardiac events in adults.31 Of note, low-grade cardiac events have been reported in pediatric patients treated with dasatinib: in a phase 2 study, 3 patients experienced grade 1/2 congestive heart failure or cardiac dysfunction, and 1 patient reported a grade 1/2 cardiac disorder as an AE. One SAE of grade 1/2 left ventricular dysfunction was also reported.6
Apart from its relatively small sample size, a limitation of this study is that, when it was designed, an effect of nilotinib on growth was not anticipated. Consequently, participants were followed up for survival after study discontinuation, but no growth data were collected after that point.
Given the long duration of TKI treatment in this patient sample and the potential for associated side effects, discontinuation of TKI treatment in patients who achieve a sustained deep molecular response may provide additional advantages in pediatric patients.
In summary, in this longer-term safety analysis of pediatric patients, growth data indicate attenuated linear growth rates compared with baseline during nilotinib treatment. Overall, the safety profile of nilotinib in this analysis was consistent with that in previous reports, and side effects were manageable by monitoring and dose reduction.
Acknowledgments
Medical writing and editorial assistance were provided by Christine Elsner and Kyle Lambe (Synergy Medical Communications, London, United Kingdom) and supported by Novartis Pharmaceuticals Corporation.
This study was sponsored and funded by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: N.H. designed the study; K.T. contributed to data analysis; and all authors contributed to data acquisition and interpretation, writing the manuscript, and review and approval of the final manuscript.
Conflict-of-interest disclosure: N.H. reports receiving honoraria from and serving as a consultant to Novartis. H.G. reports receiving lecture fees from and serving on the advisory board of Novartis. Z.K. reports receiving grants from Novartis. C.M.Z. reports serving as a consultant to Novartis. B.P. reports receiving travel expenses from BMS. J.S. reports serving as a consultant to Novartis. P.A., A.A., and K.T. report employment at and ownership of stock in Novartis. The remaining authors declare no competing financial interests.
Correspondence: Nobuko Hijiya, Division of Pediatric Hematology/Oncology/Stem Cell Transplantation, Columbia University Irving Medical Center, New York, NY 10032; e-mail: nh2636@cumc.columbia.edu.
References
Author notes
Novartis is committed to sharing, with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. Trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
The full-text version of this article contains a data supplement.