Key Points
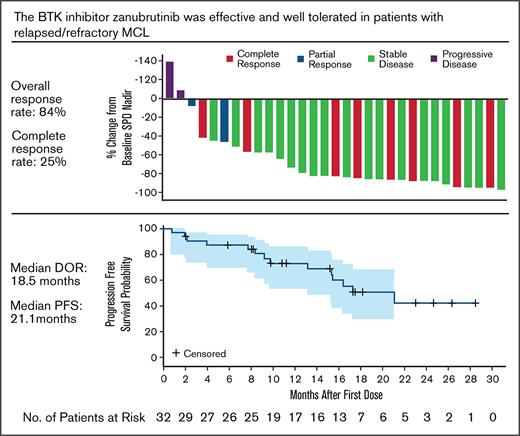
Zanubrutinib provided a high response rate (84%) and extended PFS (median 21 months) in patients with R/R MCL.
Tolerability was favorable, with few adverse events requiring treatment discontinuation or dose reduction.
Abstract
Zanubrutinib, a highly selective Bruton tyrosine kinase inhibitor, was evaluated in a phase 1/2 study in patients with various B-cell malignancies. In the subgroup of patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL), zanubrutinib was administered as 160 mg twice daily (n = 14), 320 mg once daily (n = 18), or ≤160 mg total dose (n = 5). Herein, we report results for patients receiving a total daily dose of 320 mg (N = 32). Median study follow-up was 18.8 months. Eighteen patients discontinued treatment, 10 because of progressive disease and 8 because of adverse events (AEs); 1 AE (peripheral edema) was considered to be related to zanubrutinib treatment. The most common AEs were diarrhea (43.8%), contusion (37.5%), constipation (31.3%), and upper respiratory tract infection (31.3%). Infection was the most commonly reported AE of interest (18.8% of patients experienced grade ≥3 infection). At least 1 AE of grade ≥3 was reported in 59.4% of patients; grade ≥3 AEs that were reported in >2 patients were anemia (12.5%), pneumonia (9.4%), and myalgia (9.4%). Overall response rate was 84%, with 25% achieving a complete response. Median duration of response was 18.5 months. Median progression-free survival (PFS) was 21.1 months. Zanubrutinib was well tolerated and demonstrated activity in patients with R/R MCL. The trial is registered at www.clinicaltrials.gov as #NCT02343120.
Introduction
Mantle cell lymphoma (MCL) is a challenging and incurable subtype of non-Hodgkin lymphoma (NHL), accounting for 2% to 10% of all NHLs.1 Despite enhanced understanding of the biology and the advancement of effective therapeutic strategies resulting in longer survival rates,2 there appears to be a continuous pattern of relapse, with most patients eventually succumbing to MCL or complications of therapy.3 Indications for treatment include disease-related symptoms, disease bulk, and rate of progression, with occasional patients having an indolent phenotype in whom therapy can be delayed for several months.4 The current treatment approach is based on age, with median age at diagnosis being 68 years, and fitness,5 with unfit older patients receiving combination chemoimmunotherapy with/without rituximab maintenance and younger fit patients being treated with intensive combination therapies incorporating cytarabine and rituximab with/without autologous stem cell transplant. Although chemoimmunotherapy can result in high response rates, patients invariably relapse and have poor outcomes,4 with median survival following progression of ∼3 years.6 Fitness, patient preference, previous drug exposure, and response to prior therapy will impact the choice of treatment; stem cell transplant and chimeric antigen receptor T-cell therapy is an option for fitter patients.7,8
Studies have shown the importance of targeting B-cell receptor (BCR) signaling, which is considered central to the survival and proliferation of malignant B cells.9 Bruton tyrosine kinase (BTK) is an integral intermediate of the BCR pathway and is a clinically validated target in MCL. BTK inhibitors have established activity in MCL.10‐13 Pooled data from 3 single-agent studies of ibrutinib in relapsed/refractory (R/R) MCL (PCYC-1104, RAY, and SPARK; N = 370) showed an overall response rate (ORR) of 70% and a complete response (CR) rate of 27% at 3.5 years median follow-up. Median duration of response (DOR), progression-free survival (PFS), and overall survival (OS) were 21.8, 12.5, and 26.7 months, respectively.14 The troublesome common side effects of bruising, fatigue, and diarrhea, as well as uncommon, but serious, toxicities have been associated with ibrutinib. In this pooled data set, grade ≥ 3 treatment-emergent adverse events (AEs) were reported in 296 (80%) patients, including atrial fibrillation (6%).14 Although ibrutinib is effective in R/R MCL, there remains a need for BTK inhibitors that promote deep responses and minimize common side effects, as well as severe or treatment-limiting toxicities. Acalabrutinib is a second-generation BTK inhibitor that showed an improved ORR of 81% and a CR rate of 40%. Common AEs were primarily grade 1 or 2 and consisted of headaches (39%), diarrhea (31%), fatigue (28%), and myalgia (21%). Atrial fibrillation was not observed, and grade ≥ 3 infection and hemorrhage were infrequent.12,15
Zanubrutinib is a novel potent BTK inhibitor. It is more selective for BTK inhibition than ibrutinib in vitro and exhibits less off-target activity against EGFR, TEC, and ITK.16 The median steady-state BTK occupancy was maintained at 100% over 24 hours at a total daily dose of 320 mg in peripheral blood mononuclear cells and at 94% to 100% in lymph nodes following the approved recommended dosage of 320 mg, once daily, or 160 mg, twice daily, in patients with B-cell malignancies.13,16‐20 Clinical studies have shown that zanubrutinib is well-tolerated, with promising antitumor activity in mature B-cell neoplasms, including Waldenström macroglobulinemia (WM), MCL, and chronic lymphocytic leukemia/small lymphocytic lymphoma,16,21,22 and it is approved by the US Food and Drug Administration for adult patients with MCL who have received ≥1 prior therapy. Here, we report the safety and efficacy of zanubrutinib treatment in the subgroup of patients with R/R MCL who are enrolled in an ongoing phase 1/2 study of zanubrutinib in B-cell malignancies.
Methods
Study design and treatment
The phase 1/2 first-in-human multicenter open-label study of zanubrutinib in patients with B-cell malignancies enrolled patients from 24 sites in 6 countries. The study is being conducted in Australia, New Zealand, South Korea, the United States, Italy, and the United Kingdom. Part 1 was a dose-escalation phase to determine the recommended phase 2 dose (RP2D). Part 2 enrolled patients in disease-specific cohorts with R/R or treatment-naive B-cell malignancies, including MCL, chronic lymphocytic leukemia/small lymphocytic lymphoma, and WM. No dose-limiting toxicity was encountered; a maximally tolerated dose was not identified. The RP2D was 160 mg, twice daily, or 320 mg, daily, which are associated with similar pharmacokinetics (Ying C. Ou, Zhiyu Tang, W.N., Aileen Cohen, Kun Wang, Lucy Liu, Yuying Gao, and Srikumar Sahasranaman, manuscript submitted, February 2021). Sixteen treatment-naive MCL patients and 37 R/R MCL patients were enrolled in part 1 and 2. For the R/R MCL patients, 6 were enrolled in part 1 and received total daily doses ≤ 160 mg (n = 5) or total daily doses of 320 mg (n = 1). In part 2, 31 patients with R/R MCL received 160 mg, twice daily (n = 14) or 320 mg, daily (n = 17). Data presented herein are only for R/R MCL patients receiving a total daily dose of 320 mg (N = 32).
All patients provided written informed consent. The study was conducted according to the principles of the Declaration of Helsinki and International Conference on Harmonization guidelines. The protocol was approved by the institutional review boards/independent ethics committees at each site.
Patients
Patients were ≥18 years old; had Eastern Cooperative Oncology Group Performance Status score of 0-2; had adequate hematologic (neutrophil and platelet counts > 1.0 × 109/L and ≥50 × 109/L, respectively), renal (measured or estimated creatine clearance ≥30 mL/min), and liver (transaminase levels ≤3 times the upper limit of normal; total bilirubin ≤1.5 times the upper limit of normal) function at baseline; and had R/R MCL without previous exposure to a BTK inhibitor. Key exclusion criteria were central nervous system involvement, significant cardiac disease, and prior allogeneic stem cell transplantation within 6 months of study entry. Patients requiring concurrent strong CYP3A inhibitors/inducers or QT-prolonging medications were excluded; however, those taking aspirin and other anticoagulants, including warfarin (Coumadin), were not excluded.
Assessments
Zanubrutinib was given orally, once or twice daily in 28-day cycles, until progression or unacceptable toxicity. Bone marrow assessments and imaging evaluations were performed at screening. Bone marrow aspiration and biopsy were performed at screening, postbaseline, and as clinically indicated to confirm CR. Computed tomography (CT) scans were obtained at baseline, every 12 weeks until week 100, and every 24 weeks thereafter. Positron emission tomography (PET) or integrated PET/CT was used at the discretion of the investigator. Gastrointestinal biopsies were performed at the discretion of the treating physician. Responses were assessed by the investigator and an independent review committee (IRC).
Type, frequency, severity, and outcomes of AEs were included in safety assessments. AEs were defined as treatment-emergent AEs occurring from the first day of treatment up to 30 days poststudy treatment discontinuation or initiation of new anticancer therapy. AEs of interest were predefined as those known to be associated with BTK inhibitors and identified using predefined Medical Dictionary for Regulatory Activities (MedDRA; version 20.0) search criteria. MedDRA search terms for AEs of interest included bleeding (including major hemorrhage), atrial fibrillation/flutter, hypertension, second primary malignancies (including skin cancers), tumor lysis syndrome, infections (including opportunistic infections), neutropenia, thrombocytopenia, and anemia. AE severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
Analyses included all patients with R/R MCL who received single-agent zanubrutinib at a total daily dose of 320 mg. The primary efficacy end point was ORR (partial response [PR] or CR, as assessed by the IRC according to the Lugano classification),23 although most patients had CT-based tumor assessment only. Secondary efficacy end points included investigator-assessed ORR, DOR, time to response, IRC-assessed PFS, and OS. Subgroup analyses were performed for those patients achieving an overall response, using prespecified baseline and disease characteristic variables. Response rates (ORR, including CR and PR) were summarized as the percentage of responders for each category with 95% confidence intervals (CIs).24 DOR was defined as the time from the first qualifying response until disease progression or death from any cause. PFS was defined as the time from the first dose of study drug to disease progression or death from any cause. PFS and DOR were censored at the last tumor assessment or before initiation of subsequent anticancer therapy. Median PFS, DOR, and event-free rates at landmark time points were estimated using the Kaplan-Meier method with corresponding 95% CIs.25 The reverse Kaplan-Meier method was used to estimate follow-up times for PFS and DOR. OS was defined as the time from the first dose of study drug until death from any cause. Standard descriptive statistics were used to summarize AE data.
Results
Patients and baseline characteristics
Between 22 September 2014 and 22 March 2018, 37 patients with R/R MCL were enrolled and received zanubrutinib. Thirty-two patients received 320 mg, taken as 160 mg twice daily (n = 14) or 320 mg once daily (n = 18). Five patients received other starting doses: 40 mg once daily (n = 1), 80 mg once daily (n = 2), or 160 mg once daily (n = 2). Baseline characteristics and disease history are summarized in Table 1. The median number of prior therapies was 1, with 30 patients receiving rituximab or a rituximab-containing regimen. Only 1 patient received rituximab as their first and only line of treatment. This patient achieved a PR after 8 cycles of rituximab and received zanubrutinib ∼4 months after last dose of rituximab. This patient achieved a PR and is still on zanubrutinib. The median relative dose intensity was 100% for the once-daily dose (320 mg) and 99.7% for the twice-daily dose (160 mg). Median time on study was 18.8 months, and patients received treatment for a median of 15.4 months. At the time of data cutoff, 14 patients (44%) continued to receive the study drug and 18 patients (56%) had discontinued it. The primary reasons for discontinuing the study drug were progressive disease (PD; 10 patients [31.3%]) and AEs (8 patients [25%], 3 [9.4%] of whom experienced a fatal AE); 1 AE (grade 3 peripheral edema) was considered to be related to zanubrutinib. Twelve patients (38%) had died at the time of data cutoff, 3 from AEs (cerebral infarction, pneumonia, and congestive cardiac failure, all of which were assessed as not being related to the study drug by investigators), 7 from PD, and 2 from other reasons (1 unknown, but PD was reported prior to death, and 1 from septic shock, in a patient who discontinued treatment because of the diagnosis of myelodysplastic syndrome).
Demographics and baseline characteristics per investigator assessment
| . | R/R MCL (N = 32) . |
|---|---|
| Age, median (range), y | 70.5 (42-86) |
| Age category | |
| <65 y | 8 (25.0) |
| ≥65 to <75 y | 12 (37.5) |
| ≥75 y | 12 (37.5) |
| Sex | |
| Male | 22 (68.8) |
| Female | 10 (31.3) |
| Race | |
| White | 30 (81.1) |
| Asian | 3 (8.1) |
| Black or African American | 1 (2.7) |
| Other | 3 (8.1) |
| Baseline ECOG performance score | |
| 0 | 15 (46.9) |
| 1 | 14 (43.8) |
| 2 | 3 (9.4) |
| Stage at study entry | |
| Stage I | 2 (6.3) |
| Stage II | 1 (3.1) |
| Stage III | 1 (3.1) |
| Stage IV | 28 (87.5) |
| MIPI* | |
| Low risk | 9 (28.1) |
| Intermediate risk | 13 (40.6) |
| High risk | 10 (31.3) |
| Blastoid variant | |
| Yes | 2 (6.3) |
| No | 28 (87.5) |
| Missing | 2 (6.3) |
| Bulky disease, cm† | |
| >5 | 7 (21.9) |
| >10 | 3 (9.4) |
| Bone marrow involvement‡ | |
| Yes | 18 (56.3) |
| No | 14 (43.8) |
| Extranodal disease§ | |
| Yes | 25 (78.1) |
| No | 7 (21.9) |
| Refractory disease¶ | 8 (25.0) |
| No. of previous therapies, median (range) | 1 (1-4) |
| Previous therapy | |
| Any chemotherapy | 31 (96.9) |
| Rituximab or rituximab-containing regimen | 30 (93.8) |
| R-CHOP/R-CHOPE/R-CHOPE–like | 19 (59.4) |
| Bendamustine/purine analog | 11 (34.4) |
| Various chemotherapies | 7 (21.9) |
| Hyper-CVAD/hyper-CVAD–like regimen | 7 (21.9) |
| Autologous stem cell transplantation | 5 (15.6) |
| Bortezomib | 2 (6.3) |
| . | R/R MCL (N = 32) . |
|---|---|
| Age, median (range), y | 70.5 (42-86) |
| Age category | |
| <65 y | 8 (25.0) |
| ≥65 to <75 y | 12 (37.5) |
| ≥75 y | 12 (37.5) |
| Sex | |
| Male | 22 (68.8) |
| Female | 10 (31.3) |
| Race | |
| White | 30 (81.1) |
| Asian | 3 (8.1) |
| Black or African American | 1 (2.7) |
| Other | 3 (8.1) |
| Baseline ECOG performance score | |
| 0 | 15 (46.9) |
| 1 | 14 (43.8) |
| 2 | 3 (9.4) |
| Stage at study entry | |
| Stage I | 2 (6.3) |
| Stage II | 1 (3.1) |
| Stage III | 1 (3.1) |
| Stage IV | 28 (87.5) |
| MIPI* | |
| Low risk | 9 (28.1) |
| Intermediate risk | 13 (40.6) |
| High risk | 10 (31.3) |
| Blastoid variant | |
| Yes | 2 (6.3) |
| No | 28 (87.5) |
| Missing | 2 (6.3) |
| Bulky disease, cm† | |
| >5 | 7 (21.9) |
| >10 | 3 (9.4) |
| Bone marrow involvement‡ | |
| Yes | 18 (56.3) |
| No | 14 (43.8) |
| Extranodal disease§ | |
| Yes | 25 (78.1) |
| No | 7 (21.9) |
| Refractory disease¶ | 8 (25.0) |
| No. of previous therapies, median (range) | 1 (1-4) |
| Previous therapy | |
| Any chemotherapy | 31 (96.9) |
| Rituximab or rituximab-containing regimen | 30 (93.8) |
| R-CHOP/R-CHOPE/R-CHOPE–like | 19 (59.4) |
| Bendamustine/purine analog | 11 (34.4) |
| Various chemotherapies | 7 (21.9) |
| Hyper-CVAD/hyper-CVAD–like regimen | 7 (21.9) |
| Autologous stem cell transplantation | 5 (15.6) |
| Bortezomib | 2 (6.3) |
Unless otherwise noted, data are n (%).
ECOG, Eastern Cooperative Oncology Group; hyper-CVAD, cyclophosphamide, vincristine, doxorubicin, cytarabine, methotrexate, and leucovorin; MIPI, MCL International Prognostic Index; R-CHOEP, rituximab cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
Calculated with cutoffs of low (<5.7), intermediate (5.7 to <6.2), and high (≥6.2).
Included 2 R/R patients without baseline target lesion.
Derived from baseline tumor biopsy/aspiration per investigator assessment.
Extranodal disease defined as patients with extranodal baseline target or nontarget lesions or bone marrow involvement by biopsy per investigator assessment.
Refractory disease defined as best overall response of stable disease or PD from last prior anticancer regimen.
Efficacy
Response assessments were based on CT scans and PET imaging, if available. All R/R MCL patients were restaged for CT scans. PET was performed in 8 patients who had baseline and/or postbaseline imaging scans available. When only CT imaging was available, a CT response was determined. When PET was available (n = 8), an integrated CT and PET response was determined. Table 2 shows investigator- and IRC-assessed responses. An objective response by investigator assessment was recorded in 29 of 32 (91%) patients, with 10 patients (31%) achieving a CR. By IRC assessment, 27 patients (84%) had an overall response, with 8 patients (25%) achieving a CR. One patient with investigator-assessed PR was assessed as “unknown” by the IRC because of the lack of informed consent for sharing scan images for independent review. The median time to response (CR+PR) was 2.8 months (range, 1.9-9.8). The median time to CR was 5.5 months (range, 1.9-11.1). There was no discernable difference in response between the 160-mg twice-daily or the 320-mg once-daily dosing regimens (supplemental Table 1); ORRs were 86% and 83% respectively, whereas CR rates were 29% and 22%, respectively.
IRC- and investigator-assessed responses
| Response assessment . | Investigator-assessed response (N = 32) . | IRC-assessed response (N = 32) . |
|---|---|---|
| ORR 95% CI* | 29 (90.6) (75.0-98.0) | 27 (84.4) (67.2-94.7) |
| Best response | ||
| CR | 10 (31.3) | 8 (25.0) |
| PR | 19 (59.4) | 19 (59.4) |
| Stable disease | 1 (3.1) | 2 (6.3) |
| PD | 2 (6.3) | 2 (6.3) |
| Unknown† | 0 | 1 (3.1) |
| Response assessment . | Investigator-assessed response (N = 32) . | IRC-assessed response (N = 32) . |
|---|---|---|
| ORR 95% CI* | 29 (90.6) (75.0-98.0) | 27 (84.4) (67.2-94.7) |
| Best response | ||
| CR | 10 (31.3) | 8 (25.0) |
| PR | 19 (59.4) | 19 (59.4) |
| Stable disease | 1 (3.1) | 2 (6.3) |
| PD | 2 (6.3) | 2 (6.3) |
| Unknown† | 0 | 1 (3.1) |
Unless otherwise noted, data are n (%).
Two-sided Clopper-Pearson 95% CIs.
Patient had discontinued treatment and died before signing an updated informed consent to allow scan collection for IRC review.
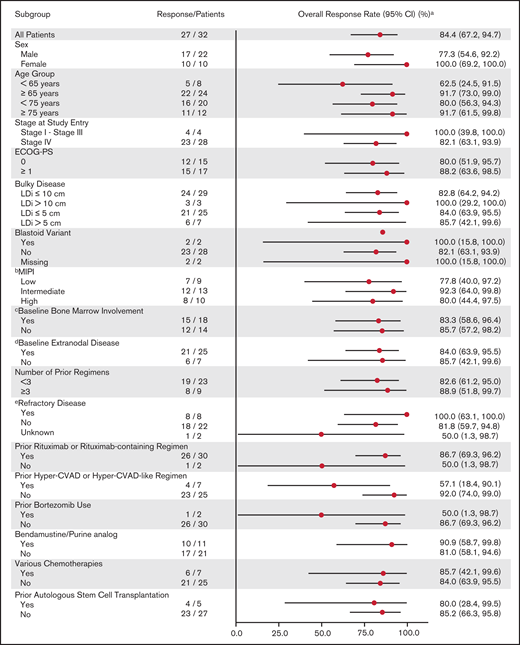
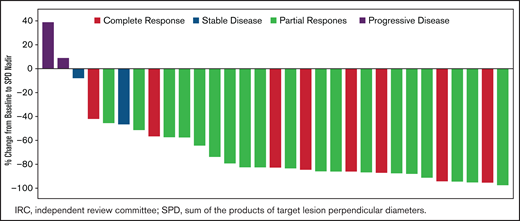
Response to zanubrutinib was independent of baseline characteristics. Figure 1 shows ORR by prespecified subgroups, according to baseline demographic and clinical characteristics. Despite fluctuations in some subgroups with very small sample sizes, treatment benefit was consistent across subgroups, including very elderly patients and those with high MIPI risk scores or refractory disease. Among the 31 patients with baseline lymphadenopathy by CT, 25 (80.6%) had a reduction in the sum of the products of target lesion perpendicular diameters ≥50% (Figure 2).
Forest plot of ORR by IRC assessment (N = 32).a2-sided Clopper-Pearson 95% confidence intervals. bMIPI score was calculated with cutoffs as low (<5.7), intermediate (5.7 to <6.2), and high (≥6.2). cDerived from baseline tumor biopsy/aspiration per investigator assessment. dExtranodal disease is defined as patients with extranodal baseline target or nontarget lesions, or bone marrow involvement by biopsy per investigator assessment. eRefractory disease is defined as best overall response of stable disease or progressive disease from last prior anti-cancer treatment regimen. Hyper-CVAD, cyclophosphamide, vincristine, doxorubicin, cytarabine, methotrexate, and Ara C; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; LDi, longest transverse diameter of a lesion; MIPI, MCL International Prognostic Index.
Forest plot of ORR by IRC assessment (N = 32).a2-sided Clopper-Pearson 95% confidence intervals. bMIPI score was calculated with cutoffs as low (<5.7), intermediate (5.7 to <6.2), and high (≥6.2). cDerived from baseline tumor biopsy/aspiration per investigator assessment. dExtranodal disease is defined as patients with extranodal baseline target or nontarget lesions, or bone marrow involvement by biopsy per investigator assessment. eRefractory disease is defined as best overall response of stable disease or progressive disease from last prior anti-cancer treatment regimen. Hyper-CVAD, cyclophosphamide, vincristine, doxorubicin, cytarabine, methotrexate, and Ara C; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; LDi, longest transverse diameter of a lesion; MIPI, MCL International Prognostic Index.
Best percentage change from baseline in target lesion sum of the products of target lesion perpendicular diameters (SPDs) by overall response assessed by IRC (N = 32).
Best percentage change from baseline in target lesion sum of the products of target lesion perpendicular diameters (SPDs) by overall response assessed by IRC (N = 32).
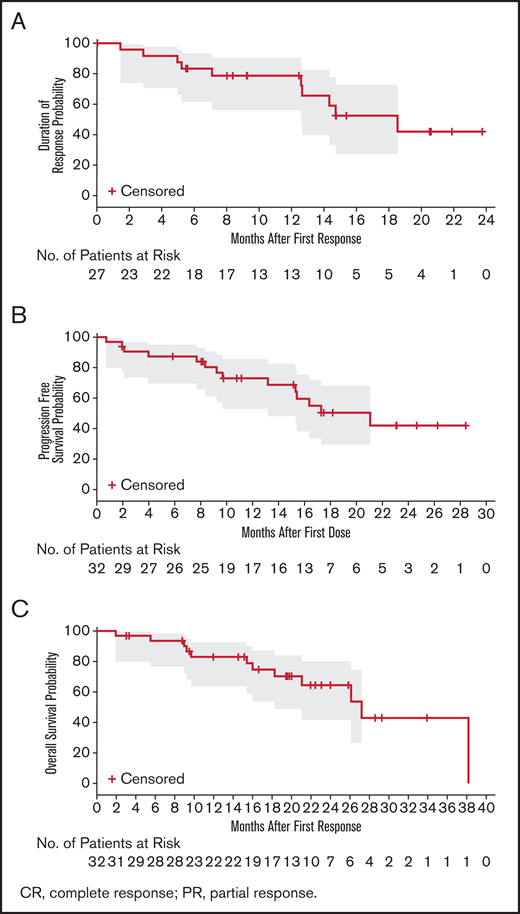
At the time of analysis, the median DOR, as assessed by IRC, was 18.5 months (95% CI, 12.6 to not estimable; Figure 3A). Among the 27 responders (best overall response of PR or CR), 8 patients progressed and 2 died. The percentage of patients still responding to treatment at 6 months and 12 months was 83.3% and 78.7%, respectively. The median PFS (Figure 3B) was 21.1 months (95% CI, 13.2 to not estimable). Fourteen patients (43.8%) had progressed or died, and 18 patients (56.3%) were censored. The event-free rates were 87.3% and 73.0% at 6 and 12 months, respectively. The OS rates at 12 and 24 months were 83% and 64.4%, respectively (Figure 3C).
Duration of response, progression-free survival, and overall survival probability. (A) IRC-assessed DOR in patients with CR or PR (n = 27). PFS (B) and OS (C) in the safety population (N = 32). The gray shaded areas are 95% CI for the KM curve.
Duration of response, progression-free survival, and overall survival probability. (A) IRC-assessed DOR in patients with CR or PR (n = 27). PFS (B) and OS (C) in the safety population (N = 32). The gray shaded areas are 95% CI for the KM curve.
Safety
Eight patients discontinued treatment because of AEs (supplemental Table 2): cerebral infarction (n = 1), pneumonia (n = 2), worsening congestive cardiac failure (n = 1), acute kidney injury with ANCA vasculitis (n = 1), peripheral edema that was considered related to zanubrutinib (n = 1), myelodysplastic syndrome (n = 1), and renal hematoma (n = 1). Sixteen patients (50%) required zanubrutinib dose interruptions, and 1 patient (3.1%) required dose reduction because of AEs. Three patients (9.4%) had AEs leading to death: pneumonia; cerebral infarction in the context of atrial flutter; and cardiac failure in the context of known ischemic heart disease and baseline cardiomyopathy (n = 1 each).
Thirty-one patients (96.9%) reported ≥1 AE of any grade, and 59.4% reported ≥1 AE of grade ≥3 (Table 3). The most commonly reported AEs were diarrhea (43.8%), most of which were grade 1 (28.1%) and 2 (12.5%) (median duration of diarrhea was 12 days), contusion (37.5%), constipation (31.3%), and upper respiratory tract infection (31.3%). Grade ≥3 AEs that were reported in >2 patients included anemia (12.5%), neutropenia (9.4%), pneumonia (9.4%), and myalgia (9.4%).
Any-grade treatment-emergent AEs in ≥15% of patients, grade ≥3 AEs in >2 patients, and all AEs of interest in the safety population (N = 32)
| AE* . | Any-grade AEs . | Grade ≥ 3 AEs . |
|---|---|---|
| Patients with 1 AE | 31 (96.9) | 19 (59.5) |
| Diarrhea | 14 (43.8) | 1 (3.1) |
| Contusion | 12 (37.5) | 0 |
| Constipation | 10 (31.3) | 0 |
| Upper respiratory tract infections | 10 (31.3) | 0 |
| Fatigue | 8 (25.0) | 1 (3.1) |
| Dyspnea | 8 (25.0) | 0 |
| Peripheral edema | 7 (21.9) | 2 (6.3) |
| Back pain | 7 (21.9) | 1 (3.1) |
| Rash | 7 (21.9) | 0 |
| Arthralgia | 6 (18.8) | 0 |
| Cough | 6 (18.8) | 0 |
| Muscle spasms | 5 (15.6) | 0 |
| Pruritis | 5 (15.6) | 0 |
| Localized infection | 5 (15.6) | 0 |
| Urinary tract infection | 5 (15.6) | 0 |
| Pneumonia | 4 (12.5) | 3 (9.4) |
| Myalgia | 3 (9.4) | 3 (9.4) |
| AEs of interest | ||
| Bleeding | 18 (56.3) | 3 (9.4) |
| Major hemorrhage* | 3 (9.4) | 3 (9.4) |
| Atrial fibrillation/flutter | 2 (6.3) grades 2 and 3 | 1 (3.1) |
| Hypertension | 2 (6.3) | 1 (3.1) |
| Second primary malignancies | 6 (18.8) | 1 (3.1) |
| Skin cancers | 5 (15.6) | 1 (3.1) |
| Infections | 22 (68.8) | 6 (18.8) |
| Opportunistic infections | 3 (9.4) | 2 (6.3) |
| Tumor lysis syndrome | 2 (6.3) | 2 (6.3) |
| Anemia | 4 (12.5) | 4 (12.5) |
| Neutropenia† | 4 (12.5) | 3 (9.4) |
| Thrombocytopenia‡ | 4 (12.5) | 2 (6.3) |
| AE* . | Any-grade AEs . | Grade ≥ 3 AEs . |
|---|---|---|
| Patients with 1 AE | 31 (96.9) | 19 (59.5) |
| Diarrhea | 14 (43.8) | 1 (3.1) |
| Contusion | 12 (37.5) | 0 |
| Constipation | 10 (31.3) | 0 |
| Upper respiratory tract infections | 10 (31.3) | 0 |
| Fatigue | 8 (25.0) | 1 (3.1) |
| Dyspnea | 8 (25.0) | 0 |
| Peripheral edema | 7 (21.9) | 2 (6.3) |
| Back pain | 7 (21.9) | 1 (3.1) |
| Rash | 7 (21.9) | 0 |
| Arthralgia | 6 (18.8) | 0 |
| Cough | 6 (18.8) | 0 |
| Muscle spasms | 5 (15.6) | 0 |
| Pruritis | 5 (15.6) | 0 |
| Localized infection | 5 (15.6) | 0 |
| Urinary tract infection | 5 (15.6) | 0 |
| Pneumonia | 4 (12.5) | 3 (9.4) |
| Myalgia | 3 (9.4) | 3 (9.4) |
| AEs of interest | ||
| Bleeding | 18 (56.3) | 3 (9.4) |
| Major hemorrhage* | 3 (9.4) | 3 (9.4) |
| Atrial fibrillation/flutter | 2 (6.3) grades 2 and 3 | 1 (3.1) |
| Hypertension | 2 (6.3) | 1 (3.1) |
| Second primary malignancies | 6 (18.8) | 1 (3.1) |
| Skin cancers | 5 (15.6) | 1 (3.1) |
| Infections | 22 (68.8) | 6 (18.8) |
| Opportunistic infections | 3 (9.4) | 2 (6.3) |
| Tumor lysis syndrome | 2 (6.3) | 2 (6.3) |
| Anemia | 4 (12.5) | 4 (12.5) |
| Neutropenia† | 4 (12.5) | 3 (9.4) |
| Thrombocytopenia‡ | 4 (12.5) | 2 (6.3) |
All data are n (%).
Defined as any serious or grade ≥3 bleed at any site or central nervous system bleed of any grade.
Includes the MedDRA preferred terms neutropenia, neutrophil count decreased, and febrile neutropenia.
Includes the MedDRA preferred terms of thrombocytopenia and platelet count decreased.
The most commonly reported AE of interest was infection. Infections of any grade occurred in 68.8% of patients, with 18.3% of patients experiencing grade ≥3 infection. The most common grade ≥3 infections were pneumonia (n = 3) and cellulitis (n = 2). Fungal infections occurred in 3 patients, including grade 2 anal fungal infection, grade 2 Candida infection, and a patient who had a grade 3 bronchopulmonary aspergillosis for which the study drug was interrupted for 5 days and voriconazole was administered. The study drug was commenced at 160 mg, twice daily, following treatment. At least 1 bruising or bleeding event was reported by 18 patients (56.3%); 15 (83.3%) were grade 1 or 2, and the most commonly reported were contusion (37.5%), hematuria (12.5%), and epistaxis (9.4%). Three were grade 3: GI hemorrhage in the setting of endoscopically proven MCL infiltration (n = 1), tumor hemorrhage (n = 1), and renal hematoma leading to discontinuation of study treatment (n = 1). Atrial fibrillation/flutter was reported in 2 patients (6.3%; grade 2 and grade 3), 1 of whom had a history of paroxysmal atrial fibrillation. Neither patient discontinued the study treatment because of arrhythmia.
Discussion
MCL is a generally aggressive heterogeneous disease with varied clinical presentations. Many patients with relapsed/refractory disease fail to respond to approved treatments. Responses are often not durable, requiring subsequent salvage therapies. Approved agents, such as bortezomib and lenalidomide, have shown modest efficacy but limited DOR.26,27 Consequently, the treatment landscape is now transitioning toward chemotherapy-free treatments, with novel targeted agents, such as ibrutinib and acalabrutinib, showing promise; however, additional phase 3 data are required to fully understand the clinical benefits.
Zanubrutinib is a highly selective, targeted, and orally bioavailable BTK inhibitor. In this study, patients with R/R MCL were treated with zanubrutinib: 320 mg, once-daily, or 160 mg, twice daily. Both regimens were initially studied to understand the pharmacokinetics profile. A maximum tolerated dose was not reached; 160 mg, twice daily, was chosen as the preferred RP2D.13 At a median study follow-up of 18.8 months, an IRC-assessed ORR of 84% and a CR of 25% were achieved. The CR rate was likely affected by the lack of protocol-specified PET imaging, which would be expected to have detected a higher response rate. Responses occurred rapidly, with a median time to response of 2.7 months. Responses were durable, with a median DOR of 18.5 months and a median PFS of 21.1 months.
In addition to durable responses, zanubrutinib demonstrated a favorable safety profile in patients with R/R MCL. Most AEs were mild or moderate and nonserious and did not require dose reduction or treatment discontinuation. Although determination of treatment relatedness of AEs is uncertain, only 1 AE required treatment discontinuation and was considered by the investigator to be treatment related (grade 3 peripheral edema). AEs associated with other BTK inhibitors were also observed with zanubrutinib, including major hemorrhage, atrial fibrillation, second primary malignancy, infection, and cytopenias. Second malignancies were mostly skin cancers. Of note, most patients were enrolled in Australia and New Zealand, where the incidence of UV-induced skin cancers is among the highest in the world.28,29
There was a low frequency of grade ≥3 events in this study, such as diarrhea (3.1%), neutropenia (9.4%), thrombocytopenia (6.3%), and anemia (12.5%). Only 1 grade ≥3 fungal infection was observed (bronchopulmonary aspergillosis); it was assessed as being unrelated to zanubrutinib. Atrial fibrillation was observed in 2 patients (6.3%). This is further supported by the data from a pooled safety analysis of 682 patients with B-cell lymphoproliferative diseases treated with zanubrutinib monotherapy that showed an atrial fibrillation rate of 1.9%.21 In addition, data from the phase 3 randomized study evaluating ibrutinib and zanubrutinib in WM patients showed meaningful differences in AEs, particularly the incidence of atrial fibrillation, which was higher with ibrutinib treatment (14% vs 2% with zanubrutinib; P < .05). Lower rates of major bleeding (5.9% vs 9.2%), diarrhea (20.8% vs 32.7%), and hypertension (10.9% vs 17.3%) were also observed.30
Limitations of our study include the small sample size and single-arm design. However, the ORR, DOR, and PFS are similar to previous results,31 supporting the validity of the current results and the role of zanubrutinib in this setting.
The results presented here add to a now large and growing body of zanubrutinib data, demonstrating its benefits, including potent durable responses and an encouraging safety profile in patients with MCL who have received ≥1 prior therapy. In addition, the results indicate that zanubrutinib can be administered once or twice daily, providing a flexible dosing schedule and potentially improving adherence to therapy and ensuring sustained responses. Viewed in the context of the poor outcomes associated with R/R MCL and currently available treatment options, the benefit-risk profile for zanubrutinib is favorable, suggesting that it offers a promising treatment option for patients with R/R MCL.
Acknowledgments
The authors thank the patients who participated in the study, their supporters, and the investigators and clinical research staff from the study centers.
This work was supported by research funding from BeiGene (Beijing) Co., Ltd. Medical writing and editorial assistance were funded by BeiGene and were provided by Bio Connections LLC (Chicago, IL).
Authorship
Contribution: All of the investigators and their research teams collected data. The sponsor confirmed the accuracy of the data and compiled the data for analysis. All of the authors contributed to data interpretation, reviewed the manuscript and made the decision to submit it for publication, and vouch for the accuracy/completeness of the data and analyses and adherence to the trial protocol. Together with BeiGene authors (S.K.A., R.W., W.N., and J.H.), AU-003 safety monitoring committee members (C.S.T. and S.O.) were responsible for study design and with M.W., D.S., G.C., J.M., T.J.P., W.S.K., S.R., and J.T. further contributed to data interpretation and analysis.
Conflict-of-interest disclosure: C.S.T. receives research funding from Janssen and AbbVie and honoraria from Janssen, AbbVie, BeiGene, Novartis, and Roche. S.O. has acted as a consultant/advisor for AbbVie, Janssen, Gilead, Roche, Mundipharma, Merck, Bristol Myers Squibb, and Celgene; has received research funding from AbbVie, BeiGene, Janssen, Gilead, Roche, Celgene, and Epizyme; and has received honoraria from AbbVie, Janssen, Gilead, Roche, Mundipharma, Merck, Bristol Myers Squibb, and Celgene. D.S. is an employee of and has equity ownership in BeiGene; has received honoraria from AbbVie, Janssen, and Roche; has received research funding from AbbVie, Amgen, BeiGene, Celgene, Roche, MSD, Acerta, Pharmacyclics, Sanofi, and GSK; and has received travel expenses from AbbVie. G.C. has received research funding from BeiGene, Acerta, and Glycomimetics. J.M. has received honoraria from Kyowa and Seattle Genetics; has acted as a consultant/advisor for Pharmacyclics, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, Bristol Myers Squibb, Kyowa, Alexion, BeiGene, Fosunkite, Innovent, and Seattle Genetics; has been a member of the speakers’ bureau for Gilead/Kite Pharma, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech, BeiGene, and Verastem; and has received research funding from Kite Pharma, Celgene, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, and Millennium. T.J.P. has acted as a consultant/advisor for Bayer, Gilead, Seattle Genetics, Genentech, Incyte, and Pharmacyclics and has received research funding from Pharmacyclics and AbbVie. W.S.K. has received research funding from Roche, Takeda, Mundipharma, J&J, Celltrion, Kyowa Kirin, and Donga. S.R. has received research funding from Janssen and has acted as a consultant/advisor for Janssen, Kite, Sunesis, AstraZeneca, and Roche. S.K.A. is an employee of and has equity ownership in BeiGene. R.W. is an employee of and has equity ownership in BeiGene. W.N. is an employee of and has equity ownership in BeiGene. J.H. is an employee of, has a leadership role with, and has equity ownership in BeiGene. M.W. has equity ownership in MORE Health; has received honoraria from Pharmacyclics, Janssen, AstraZeneca, OMI, Targeted Oncology, and OncLive; has acted as a consultant/advisor for Pharmacyclics, Celgene, Janssen, AstraZeneca, MORE Health, Pulse Biosciences, Nobel Insights, Guidepoint Global, Kite Pharma, Juno, Celgene, and Loxo Oncology; has received research funding from Pharmacyclics, Janssen, AstraZeneca, Kite Pharma, Juno, Celgene, Loxo Oncology, VelosBio, and Verastem; and has received travel expenses from Janssen, Pharmacyclics, Celgene, OMI, Kite Pharma, and AstraZeneca. J.T. has received research funding from BeiGene, Roche, Pharmacyclics, Celgene, and Takeda.
ORCID profiles: M.W., 0000-0001-9748-5486; J.T. 0000-0001-8009-4593.
Correspondence: Judith Trotman, Concord Repatriation General Hospital, University of Sydney, Concord, NSW 2137, Australia; e-mail: judith.trotman@health.nsw.gov.au; and Michael Wang, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: miwang@mdanderson.org.
References
Author notes
M.W. and J.T. are joint senior authors.
For original data, please contact Judith Trotman (judith.trotman@health.nsw.gov.au). Individual participant data will not be shared.
The full-text version of this article contains a data supplement.