Key Points
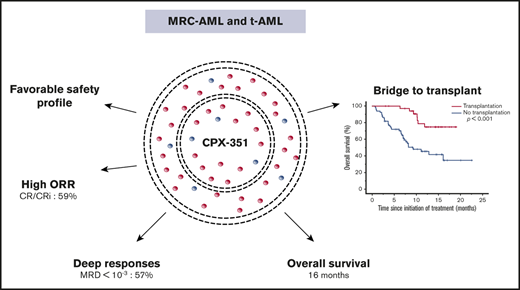
The overall response rate after induction by CPX-351 was 59%, and MRD <10−3 was achieved in 57% of CR/CRi patients.
CPX-351 improves the poor prognosis associated with some unfavorable mutations defined in the 2017 European LeukemiaNet risk stratification.
Abstract
CPX-351 is a liposomal formulation of cytarabine and daunorubicin approved for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (MRC-AML). We retrospectively analyzed the efficacy and safety of CPX-351 in a real-world setting in 103 patients from 12 French centers, including the evaluation of molecular abnormalities at baseline and minimal residual disease (MRD) in responding patients, compared with a historical data set from Bordeaux-Toulouse DATAML registry. A favorable safety profile was observed, with a low frequency of alopecia (11%) and gastrointestinal toxicity (50%). The overall response rate after induction was 59%, and MRD <10−3 was achieved in 57% of complete response (CR)/CR with incomplete hematological recovery (CRi) patients. Only the presence of mutated TP53 (P = .02) or PTPN11 (P = .004) predicted lower response in multivariate analysis. Interestingly, high-risk molecular prognosis subgroups defined by 2017 European LeukemiaNet risk stratification, including ASXL1 and RUNX1 mutations, were not associated with a significantly lower response rate using CPX-351. With a median follow-up of 8.6 months, median overall survival (OS) was 16.1 months. Thirty-six patients underwent allogeneic stem cell transplantation with a significantly longer median OS compared with nontransplanted patients (P < .001). In multivariate analyses, only spliceosome mutations were associated with better OS (P = .04). In comparison with intensive chemotherapy, there was no difference in OS for patients <60 years. These data confirm the efficacy and safety of CPX-351 in high-risk AML (t-AML and MRC-AML) in a real-life setting. CPX-351 is a treatment of choice for patients aged ≥60 years.
Introduction
According to the World Health Organization (WHO) classification,1 acute myeloid leukemia (AML) with myelodysplasia-related changes (MRC-AML) represents 24% to 35% of all AML cases and is defined by the presence of dysplasia in ≥50% of cells in ≥2 hematopoietic cell lineages and/or myelodysplastic syndrome (MDS)–related cytogenetics and/or preexisting MDS or chronic myelomonocytic leukemia (CMML). MRC-AML occurs more frequently in elderly patients. Another subtype of AML, therapy-related AML (t-AML), occurs as a late complication of cytotoxic chemotherapy and/or radiotherapy and accounts for 10% to 20% of all cases. MRC-AML and t-AML are characterized by unfavorable cytogenetics and are generally associated with poorer outcome than de novo AML, independent of older age.2,3
European LeukemiaNet (ELN) 2017 recommendations for the management of AML proposed a new risk stratification into 3 risk groups according to genetic abnormalities and mutational profile. In addition to providing a prognostic framework for genetic abnormalities, the recommendations also underline the importance of minimal residual disease (MRD) status in response assessment.4 Lindsley et al identified a mutation profile associated with secondary AML that may be more selective than the WHO clinical classification to identify AML related to MDS.5
Recently, CPX-351, a liposomal formulation of cytarabine and daunorubicin in a fixed 5:1 molar ratio, has been approved by the US Food and Drug Administration and European Medicines Agency for the treatment of t-AML or MRC-AML. CPX-351 was associated with a superior overall response rate (ORR) and overall survival (OS) compared with classical 7+3 (cytarabine and daunorubicin) intensive chemotherapy (IC). The safety profile was comparable between the 2 arms, except for prolonged myelosuppression in the CPX-351 arm.6-11 Here, we describe the first French experience with CPX-351 in a real-world setting.
Methods
Patients and treatment
We retrospectively collected data from patients in 12 centers in France who received 1 or 2 cycles of induction with CPX-351. All patients were >18 years old and had newly diagnosed, untreated t-AML or MRC-AML.
Patients were treated according to the US Food and Drug Administration/European Medicines Agency–approved dosing and schedule of CPX-351. Allogeneic stem cell transplantation (ASCT) was performed at the discretion of the physician. Moreover, we also compared these outcomes with a historical data set from DATAML registry in France.12 Since 1 January 2000, data from patients with newly diagnosed AML in Toulouse and Bordeaux were collected in this registry. For comparison with patients treated with CPX-351, we selected only patients with MRC-AML or t-AML.
Study oversight
All actions were performed according to MR004 of the Commission Nationale de l’Informatique et des Libertés, and all products were used in the usual way. Every patient received an information sheet and provided oral informed consent. Centre Hospitalier Universitaire de Nice registered this study in Institute National des Données de Santé under reference number MR4809140819.
Baseline and on-study assessments
Clinical, biological, and treatment information was available for all patients. Cytopenias were defined as follows: neutrophil count <1 × 109/L, hemoglobin level <10 g/dL, and platelet count <100 × 109/L. Hyperleukocytosis was defined as white blood cell (WBC) count >50 × 109/L. Mutation screening was performed on 74 patients (72%) at baseline using next-generation sequencing (NGS) (≥19 genes). The prognostic factors of patients were evaluated at diagnosis using 2017 ELN genetic risk stratification.4
Mutations were pooled into functional mutation subgroups of epigenetic modifications, including TET2, DNMT3A, IDH1/2, ASXL1, EZH2, and MLL/KMT2A mutations; spliceosome complex, including SRSF2, SF3B1, U2AF1, and ZRSR2 mutations; the signaling and kinase pathway, including FLT3, KRAS, NRAS, KIT, PTPN11, and NF1 mutations; the cohesin complex, including RAD21, STAG1, STAG2, SMC1A, and SMC3 mutations; and transcription factors, including CEBPA, RUNX1, GATA2, ETV6, and MECOM/EV1. Lindsley’s classifier was used in 80 patients: secondary-type mutations (defined by the presence of a mutation in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, and/or STAG2), TP53-mutated and de novo/pan-AML.5
The ORR was defined by complete response (CR) and CR with incomplete hematological recovery (CRi) using International Working Group 2003 criteria.13 Among the patients in CR or CRi, 28 (46%) were evaluated for MRD. In accordance with the standard MRD methodology used at site, MRD was assessed by flow cytometry, NGS or real-time quantitative polymerase chain reaction (real-time qPCR) in patients with documentation of specific genetic alterations such as NPM1 mutation or gene overexpression (eg, WT1). All patients with MRD <10−3 were considered as negative. Patients were considered to be in relapse if bone marrow blasts were ≥5%, blasts reappeared in the blood, or they developed extramedullary disease after CR/CRi.4
Statistical analysis
Continuous variables are described using medians [interquartile ranges] (minimum; maximum), and qualitative variables are described using counts and percentages. Noncontinuous variables were compared using χ2 test. Mann-Whitney and Kruskal-Wallis tests were used for continuous variables. Adverse events (AEs) were evaluated according to Common Terminology Criteria for Adverse Events classification. The median follow-up time was calculated as the median time from initiation of treatment to last follow-up. OS was calculated from the date of AML diagnosis to the date of death or last follow-up. Survival curves were estimated using the Kaplan-Meier method and compared with the log-rank test.
Univariate and multivariate analyses were performed using Cox model. Variables significantly associated with better OS in univariate analysis were considered as covariates in multivariate analysis. Statistical tests were considered significant when the 2-tailed P value was < .05. Confidence intervals (CIs) were computed with 95% coverage. All statistical analysis were performed using SPSS v.26 software (IBM SPSS Statistics).
Results
Characteristics of the study population
Between April 2018 and November 2019 103 patients treated with CPX-351 were included in this study. The male/female sex ratio was 54/49, and the median age was 67 years (range, 20-83 years). The AML subtypes were MRC-AML (72%), including AML with prior MDS (MDS-AML) (47%) and prior CMML (CMML-AML) (12%), or t-AML (26%). Eighteen patients (18%) had received prior treatment with hypomethylating agents (HMAs) for treatment of MDS and before progression to AML. All patient clinical characteristics are described in Table 1.
Patient characteristics (n = 103)
| . | n . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 67 | 20-83 |
| 18-60 | 21 | 20 |
| >60 | 82 | 80 |
| Sex | ||
| Male | 54 | 52 |
| Female | 49 | 48 |
| AML subtype | ||
| MRC-AML | 74 | 72 |
| MDS-AML | 35 | 47 |
| CMML-AML | 9 | 12 |
| t-AML | 27 | 26 |
| Other* | 2 | 2 |
| WBCs, median (range), × 109/L | 3 | 0-156 |
| Hyperleukocytosis | 9 | 9 |
| Cytopenias | 97 | 94 |
| 1 | 25 | 24 |
| 2 | 41 | 40 |
| 3 | 31 | 30 |
| Karyotype | ||
| Complex | 35 | 34 |
| Monosomal | 28 | 27 |
| Prior HMA | 18 | 18 |
| 2017 ELN genetic risk stratification (n = 102) | ||
| Favorable | 2 | 2 |
| Intermediate | 38 | 37 |
| Adverse | 62 | 61 |
| Lindsley’s classifier (n = 80) | ||
| De novo/pan-AML | 21 | 26 |
| Secondary-type mutations AML | 37 | 46 |
| TP53-mutated AML | 22 | 28 |
| . | n . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 67 | 20-83 |
| 18-60 | 21 | 20 |
| >60 | 82 | 80 |
| Sex | ||
| Male | 54 | 52 |
| Female | 49 | 48 |
| AML subtype | ||
| MRC-AML | 74 | 72 |
| MDS-AML | 35 | 47 |
| CMML-AML | 9 | 12 |
| t-AML | 27 | 26 |
| Other* | 2 | 2 |
| WBCs, median (range), × 109/L | 3 | 0-156 |
| Hyperleukocytosis | 9 | 9 |
| Cytopenias | 97 | 94 |
| 1 | 25 | 24 |
| 2 | 41 | 40 |
| 3 | 31 | 30 |
| Karyotype | ||
| Complex | 35 | 34 |
| Monosomal | 28 | 27 |
| Prior HMA | 18 | 18 |
| 2017 ELN genetic risk stratification (n = 102) | ||
| Favorable | 2 | 2 |
| Intermediate | 38 | 37 |
| Adverse | 62 | 61 |
| Lindsley’s classifier (n = 80) | ||
| De novo/pan-AML | 21 | 26 |
| Secondary-type mutations AML | 37 | 46 |
| TP53-mutated AML | 22 | 28 |
Values represent n (%) of patients unless otherwise indicated.
Two patients were treated after myeloproliferative neoplasm AML (1 with prior essential thrombocythemia and 1 with myelofibrosis secondary to essential thrombocythemia).
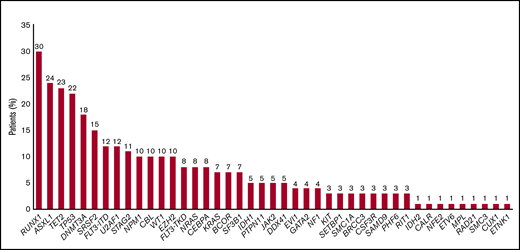
According to the 2017 ELN risk classification, genetic risk was favorable, intermediate, and adverse in 2 patients (2%), 38 patients (37%), and 62 patients (61%), respectively. There were 35 patients (34%) and 28 patients (27%) with complex and monosomal karyotypes, respectively. Among the 74 patients assessed by NGS, the most frequently mutated genes were TP53 (n = 16, 30%), RUNX1 (n = 22, 30%), ASXL1 (n = 18, 24%), TET2 (n = 17, 23%), DNMT3A (n = 13, 18%), SRSF2 (n = 11, 15%), FLT3-ITD (n = 9, 12%), U2AF1 (n = 9, 12%), STAG2 (n = 8, 11%), NPM1 (n = 7, 10%), CBL (n = 7, 10%), WT1 (n = 7, 10%), and EZH2 (n = 7, 10%) (Figures 1 and 2). All of these patients could be classified according to Lindsley’s classifier, and 6 out of 22 patients screened only for TP53 mutations were mutated and thus classified as having TP53-mutated AML. Therefore, 21 patients (26%), 37 patients (46%), and 22 patients (28%) had de novo/pan-AML, secondary-type-mutation AML, and TP53-mutated AML, respectively. Among patients with TP53-mutated AML (n = 22), 9 (41%) had chromosome 17 abnormalities.
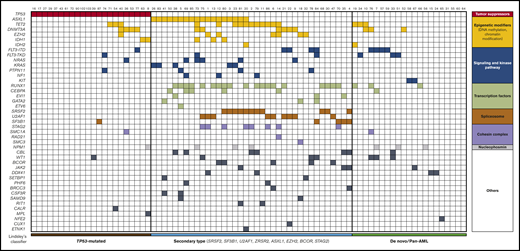
Integrated matrix of the 80 assigned patients and mutations. Mutated genes are grouped by broad biological pathways and according to Lindsley’s classifier.
Integrated matrix of the 80 assigned patients and mutations. Mutated genes are grouped by broad biological pathways and according to Lindsley’s classifier.
Safety
Neutrophil count and platelet count remained >0.5 × 109/L and 20 × 109/L in only 1 patient and 2 patients, respectively. Median time to neutrophil recovery (>0.5 × 109/L) and platelet recovery (>20 × 109/L) after the first induction was 29 days (range, 19-146 days) and 28 days (range, 12-77 days), respectively. Nine patients discontinued treatment due to prolonged hematological toxicity.
The safety profile of CPX-351 is described in Table 2. We reported 12 cases of bleeding, among which only 50% were grade ≥3.
AEs occurring during CPX-351 therapy, regardless of causality
| . | All grades . | Grade ≥3 . |
|---|---|---|
| Sepsis | — | 101 (98) |
| Febrile neutropenia | 94 (91) | 94 (91) |
| Pneumonia | 37 (36) | 30 (30) |
| Bacteremia | — | 25 (24) |
| Invasive pulmonary aspergillosis | — | 10 (10) |
| Bleeding | 12 (12) | 6 (6) |
| Epistaxis | 4 (4) | 0 |
| Oral hemorrhage | 2 (2) | 1 (1) |
| Gastrointestinal hemorrhage | 1 (1) | 1 (1) |
| Intra-alveolar hemorrhage | 1 (1) | 1 (1) |
| Intracranial hemorrhage | 2 (2) | 0 |
| Hematuria | 1 (1) | 1 (1) |
| Ocular hemorrhage | 1 (1) | 0 |
| Hypertensive crisis | 10 (10) | 2 (2) |
| Heart failure | 9 (9) | 7 (7) |
| Gastrointestinal toxicity | 52 (50) | 4 (4) |
| Nausea | 37 (36) | 0 |
| Vomiting | 13 (13) | 1 (1) |
| Diarrhea | 7 (7) | 0 |
| Mucositis | 23 (22) | 3 (3) |
| Rash | 26 (25) | 2 (2) |
| Alopecia | 11 (11) | 0 |
| . | All grades . | Grade ≥3 . |
|---|---|---|
| Sepsis | — | 101 (98) |
| Febrile neutropenia | 94 (91) | 94 (91) |
| Pneumonia | 37 (36) | 30 (30) |
| Bacteremia | — | 25 (24) |
| Invasive pulmonary aspergillosis | — | 10 (10) |
| Bleeding | 12 (12) | 6 (6) |
| Epistaxis | 4 (4) | 0 |
| Oral hemorrhage | 2 (2) | 1 (1) |
| Gastrointestinal hemorrhage | 1 (1) | 1 (1) |
| Intra-alveolar hemorrhage | 1 (1) | 1 (1) |
| Intracranial hemorrhage | 2 (2) | 0 |
| Hematuria | 1 (1) | 1 (1) |
| Ocular hemorrhage | 1 (1) | 0 |
| Hypertensive crisis | 10 (10) | 2 (2) |
| Heart failure | 9 (9) | 7 (7) |
| Gastrointestinal toxicity | 52 (50) | 4 (4) |
| Nausea | 37 (36) | 0 |
| Vomiting | 13 (13) | 1 (1) |
| Diarrhea | 7 (7) | 0 |
| Mucositis | 23 (22) | 3 (3) |
| Rash | 26 (25) | 2 (2) |
| Alopecia | 11 (11) | 0 |
Values represent n (%) of patients.
Hypertensive crisis and acute heart failure occurred in 10 patients (10%) and 9 patients (9%), respectively. Only 4 patients presented with grade 3 gastrointestinal AEs (vomiting in 1 patient and mucositis in 3 patients). Skin rash was observed in 26 patients (25%) and alopecia in only 11 patients (11%).
Grade ≥3 AEs were reported in 101 patients (98%), including 94 patients (91%) with febrile neutropenia. Thirty-seven patients (36%) had pulmonary infection. Other sites of infections are described in Table 3. Ten patients (10%) were treated for invasive pulmonary aspergillosis, including 8 patients (8%) with possible infection, 1 patient (1%) with probable infection, and 1 patient (1%) with proven infection according to European Organization for Research and Treatment of Cancer Mycoses study group criteria.14
Description of infections (grade 3 or higher) occurring during CPX-351 therapy
| Site . | Type of germ . | Grade ≥3 . |
|---|---|---|
| Blood | Gram-positive cocci, gram-negative bacilli | 25 (24) |
| Lung | Aspergillus, gram-positive cocci | 30 (30) |
| Urinary tract | Gram-negative bacilli | 8 (8) |
| Gastrointestinal tract | Gram-negative bacilli, Clostridium | 6 (6) |
| ENT region | — | 4 (5) |
| Catheter | Gram-positive cocci | 4 (2) |
| Skin | Gram-positive cocci | 2 (2) |
| Reproductive tract | — | 1 (1) |
| Liver | Candida | 1 (1) |
| Unknown | Gram-positive cocci, gram-negative bacilli | 44 (43) |
| Site . | Type of germ . | Grade ≥3 . |
|---|---|---|
| Blood | Gram-positive cocci, gram-negative bacilli | 25 (24) |
| Lung | Aspergillus, gram-positive cocci | 30 (30) |
| Urinary tract | Gram-negative bacilli | 8 (8) |
| Gastrointestinal tract | Gram-negative bacilli, Clostridium | 6 (6) |
| ENT region | — | 4 (5) |
| Catheter | Gram-positive cocci | 4 (2) |
| Skin | Gram-positive cocci | 2 (2) |
| Reproductive tract | — | 1 (1) |
| Liver | Candida | 1 (1) |
| Unknown | Gram-positive cocci, gram-negative bacilli | 44 (43) |
Values represent n (%) of patients.
ENT, ear, nose, and throat.
Response
Ninety-eight patients received 1 induction cycle; only 5 patients received 2 induction cycles. After induction, the ORR was 61 out of 103 patients (59%), including 57 CR (55%) and 4 CRi (4%). Only 1 patient needed 2 induction cycles to achieve CR. Moreover, 3 additional patients (3%) achieved a partial response. Early death rates were 6% and 8% at day 30 and day 60, respectively.
Among the 61 patients who achieved CR/CRi, 28 (46%) were evaluable for MRD at the time of the first consolidation cycle and among them 16 (57%) had reached complete molecular response defined as MRD <10−3. Their baseline characteristics are described in supplemental Table 1.
Overall, 44 (43%) patients received ≥1 consolidation cycle. Among them, 36 patients (82%) remained in response (34 CR and 2 CRi) by the end of consolidation treatment.
Twenty-four patients relapsed with a median time to relapse of 5.3 months (1.4-11.4).
Among the 36 transplanted patients, only 5 patients relapsed compared with 19 nontransplanted patients who relapsed.
ORR was significantly different across the AML subtypes (55% for MRC-AML [including 44% for MDS-AML and 22% for CMML-AML] and 70% for t-AML, respectively; P = .01). Prior treatment with an HMA (22% vs 69%, P = .001) and the presence of a monosomal karyotype (39% vs 68%, P = .009) were also identified as poor prognostic factors. Interestingly, the presence of TP53 and EVI1 mutations, belonging to the ELN 2017 adverse risk subgroup, was associated with a lower ORR (P = .04 and P = .03, respectively) whereas ASXL1 and RUNX1 mutations, which also belong to the same adverse risk subgroup, were not associated with a lower ORR (P = .28 and P = .50, respectively). The presence of FLT3 TKD and FLT3 ITD mutations was not associated with a lower ORR (P = .72 and P = .62 in FLT3-ITD and FLT3-TKD mutations, respectively). Moreover, PTPN11 mutations were associated with a lower ORR (P = .007). No prognostic significance was observed with other functional mutation subgroups. Lindsley’s molecular classes were associated with significantly different ORR (86%, 56%, and 41% ORR for de novo/pan-AML vs secondary-type AML and TP53-mutated AML, respectively; P = .009) (Table 4). We observed a significant difference between de novo/pan-AML vs TP53-mutated AML (P = .002) and de novo/pan-AML vs secondary-type AML (P = .03). We failed to observe a significant difference between secondary-type AML vs TP53 AML (P = .27). In multivariate analysis, only the presence of mutated TP53 (P = .02) or PTPN11 (P = .004) predicted lower response.
Best response rates after CPX-351 induction
| . | CR/CRi, n (%) . | P . |
|---|---|---|
| All patients treated | 61 (59) | |
| AML subtype | ||
| t-AML | 19 (70) | .01 |
| MRC-AML | 40 (55) | |
| With/without prior MDS | 15 (44)/46 (68) | .14 |
| With/without CMML | 2 (22)/59 (63) | .03 |
| Hyperleukocytosis | ||
| Presence/absence | 7 (13)/47 (87) | .21 |
| HMA experience | ||
| Prior HMA | 4 (22) | .001 |
| No prior HMA | 56 (69) | |
| Karyotype (presence/absence) | ||
| Complex karyotype | 17 (49)/44 (66) | .09 |
| Monosomal karyotype | 11 (39)/50 (68) | .009 |
| Chromosome 5 abnormalities | 13 (46)/48 (65) | .09 |
| Chromosome 7 abnormalities | 15 (47)/46 (66) | .07 |
| Chromosome 17 abnormalities | 6 (43)/55 (63) | .16 |
| 2017 ELN genetic risk stratification | ||
| Favorable | 2 (100) | .26 |
| Intermediate | 25 (66) | |
| Adverse | 33 (54) | |
| Lindsley’s classifier | ||
| De novo/pan-AML | 18 (86) | .009 |
| Secondary-type-mutation AML | 20 (56) | |
| TP53-mutated AML | 9 (41) | |
| Mutation status (mutated/nonmutated) | ||
| TP53 | 9 (41)/35 (66) | .04 |
| ASXL1 | 9 (53)/37 (67) | .28 |
| RUNX1 | 12 (57)/27 (66) | .50 |
| EVI1 | 1 (17)/50 (63) | .03 |
| FLT3-ITD | 6 (67)/53 (60) | .72 |
| FLT3-TKD | 3 (50)/56 (60) | .62 |
| NPM1 | 4 (57)/55 (59) | .92 |
| Functional group (presence/absence of mutation) | ||
| Epigenetic modifications | 24 (59)/22 (69) | .37 |
| Spliceosome complex | 14 (61)/32 (64) | .80 |
| Signaling and kinase pathway | 16 (55)/30 (68) | .26 |
| Cohesin complex | 6 (60)/40 (63) | .83 |
| Transcription factors | 17 (61)/29 (64) | .75 |
| . | CR/CRi, n (%) . | P . |
|---|---|---|
| All patients treated | 61 (59) | |
| AML subtype | ||
| t-AML | 19 (70) | .01 |
| MRC-AML | 40 (55) | |
| With/without prior MDS | 15 (44)/46 (68) | .14 |
| With/without CMML | 2 (22)/59 (63) | .03 |
| Hyperleukocytosis | ||
| Presence/absence | 7 (13)/47 (87) | .21 |
| HMA experience | ||
| Prior HMA | 4 (22) | .001 |
| No prior HMA | 56 (69) | |
| Karyotype (presence/absence) | ||
| Complex karyotype | 17 (49)/44 (66) | .09 |
| Monosomal karyotype | 11 (39)/50 (68) | .009 |
| Chromosome 5 abnormalities | 13 (46)/48 (65) | .09 |
| Chromosome 7 abnormalities | 15 (47)/46 (66) | .07 |
| Chromosome 17 abnormalities | 6 (43)/55 (63) | .16 |
| 2017 ELN genetic risk stratification | ||
| Favorable | 2 (100) | .26 |
| Intermediate | 25 (66) | |
| Adverse | 33 (54) | |
| Lindsley’s classifier | ||
| De novo/pan-AML | 18 (86) | .009 |
| Secondary-type-mutation AML | 20 (56) | |
| TP53-mutated AML | 9 (41) | |
| Mutation status (mutated/nonmutated) | ||
| TP53 | 9 (41)/35 (66) | .04 |
| ASXL1 | 9 (53)/37 (67) | .28 |
| RUNX1 | 12 (57)/27 (66) | .50 |
| EVI1 | 1 (17)/50 (63) | .03 |
| FLT3-ITD | 6 (67)/53 (60) | .72 |
| FLT3-TKD | 3 (50)/56 (60) | .62 |
| NPM1 | 4 (57)/55 (59) | .92 |
| Functional group (presence/absence of mutation) | ||
| Epigenetic modifications | 24 (59)/22 (69) | .37 |
| Spliceosome complex | 14 (61)/32 (64) | .80 |
| Signaling and kinase pathway | 16 (55)/30 (68) | .26 |
| Cohesin complex | 6 (60)/40 (63) | .83 |
| Transcription factors | 17 (61)/29 (64) | .75 |
OS
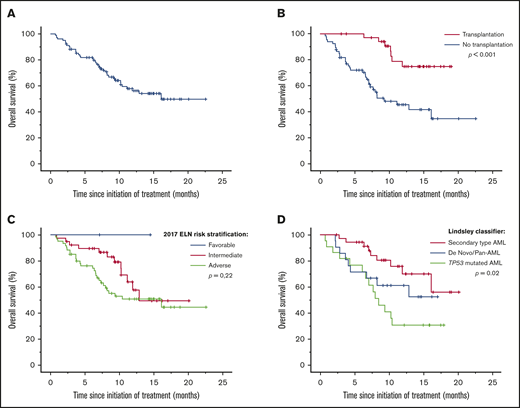
With a median follow-up of 8.6 months (range, 0.7-22.6 months), the median OS was 16.1 months (range, 13.1-16.7 months) (Figure 3A).
Survivals using CPX-351 in a real-life setting. Median OS (A) in the French cohort and subgroup analyses of median OS according to allogeneic hematopoietic cell transplantation (B), 2017 ELN prognostic group (C), and Lindsley’s classifier (D).
Survivals using CPX-351 in a real-life setting. Median OS (A) in the French cohort and subgroup analyses of median OS according to allogeneic hematopoietic cell transplantation (B), 2017 ELN prognostic group (C), and Lindsley’s classifier (D).
The 2017 ELN risk stratification was not able to predict a significant difference in OS (P = .22). Only Lindsley’s classifier was able to identify 3 prognostic subgroups; median OS was not reached in de novo/pan-AML and secondary-type-mutation AML and was 8.5 months (range, 6.1-10.9 months) in TP53-mutated AML (P = .02) (Figure 3C-D).
In univariate analysis, the presence of monosomal karyotype (P = .04), DNMT3A (P = .02), and TP53 (P = .02) mutations were associated with a poorer prognosis, while the presence of spliceosome mutations (P = .02) were associated with better OS. Lindsley’s classifier was also predictive of OS (P = .02). In multivariate analyses, only spliceosome mutations were predictive of better OS (hazard ratio, 0.31; 95% CI, 0.10-0.96; P = .04) (supplemental Table 3).
Comparison with conventional care regimens
We compared OS of our CPX-351 patients to OS from an historical cohort of patients from the Toulouse-Bordeaux DATAML registry.12 This historical comparator included 353 patients with MRC-AML (n = 271) or t-AML (n = 82) treated from January 2015 to December 2018 with either IC (n = 233) or azacitidine (AZA) (n = 120). We observed several significant differences among the 3 arms of treatment, suggesting that 3 specific subgroups of population received CPX-351, IC, or AZA depending of clinical and biological parameters (such as age, number of WBCs, complex and/or monosomal karyotype, 2017 ELN genetic risk, and presence of NPM1 or TP53 mutation) (supplemental Table 4).
Therefore, we analyzed OS according to age and 2017 ELN genetic risk. For the youngest patients (<60 years old), IC and CPX-351 showed no significant differences in OS. CPX-351 confirmed a trend toward better OS in patients aged 60 to 69 years with adverse genetic risk (median OS not reached, 8.9 months, and 5.4 months in patients treated with CPX-351, IC, and AZA, respectively; P = .055) and patients aged >70 years with intermediate genetic risk (median OS not reached, 18.4 months, and 8.9 months in patients treated with CPX-351, IC, and AZA, respectively; P = .058). For the oldest patients (aged >70 years) with adverse cytogenetic risk, we observed no significant differences among the 3 arms (supplemental Figure 2).
ASCT
Thirty-six (35%) patients underwent ASCT. The demographics and clinical characteristics of the patients who underwent ASCT are listed in Table 5. Thirty-three (92%) patients had an engraftment, with median duration to complete chimerism of 30 days (range, 20-212 days). Median time to neutrophil recovery (>0.5 × 109/L) and platelet recovery (>20 × 109/L) after ASCT was 18 days (range, 12-36 days) and 12 days (range, 9-34 days), respectively. No patient experienced hepatic sinusoidal obstruction syndrome. Only 12 patients had acute graft-versus-host disease (grade 1 or 2). Median OS was significantly better in transplanted patients vs nontransplanted patients (median not reached vs 9.3 months [range, 4.4-14.2 months]; P < .001) (Figure 3B). MRD was available only in 14 transplanted patients. So far, we failed to observe significant difference between MRD-negative and MRD-positive patients. Early death rate was 3% at day 100.
Characteristics of ASCT (n = 36)
| . | n . | % . |
|---|---|---|
| Age, median (range), y | 65 | 20-75 |
| Sex | ||
| Male | 14 | 39 |
| Female | 22 | 61 |
| HCT-CI | ||
| 0 | 10 | 37 |
| 1-2 | 5 | 19 |
| ≥3 | 12 | 44 |
| NA | 9 | — |
| AML subtype | ||
| MRC-AML | 26 | 72 |
| MDS-AML | 11 | 31 |
| CMML-AML | 1 | 3 |
| t-AML | 9 | 25 |
| Others | 1 | 3 |
| Disease status at time of ASCT | ||
| CR | 32 | 89 |
| CRi | 1 | 3 |
| PR | 3 | 8 |
| MRD status after CPX-351 induction | ||
| Negative | 8 | 57 |
| Positive | 6 | 43 |
| NA | 22 | — |
| Conditioning regimen | ||
| RIC | 33 | 92 |
| MAC | 3 | 8 |
| Donor | ||
| Matched related donor | 9 | 25 |
| Matched unrelated donor | 16 | 44 |
| Mismatched donor | 5 | 14 |
| Haploidentical donor | 6 | 17 |
| Stem cell source | ||
| Bone marrow | 2 | 6 |
| Peripheral blood | 34 | 94 |
| . | n . | % . |
|---|---|---|
| Age, median (range), y | 65 | 20-75 |
| Sex | ||
| Male | 14 | 39 |
| Female | 22 | 61 |
| HCT-CI | ||
| 0 | 10 | 37 |
| 1-2 | 5 | 19 |
| ≥3 | 12 | 44 |
| NA | 9 | — |
| AML subtype | ||
| MRC-AML | 26 | 72 |
| MDS-AML | 11 | 31 |
| CMML-AML | 1 | 3 |
| t-AML | 9 | 25 |
| Others | 1 | 3 |
| Disease status at time of ASCT | ||
| CR | 32 | 89 |
| CRi | 1 | 3 |
| PR | 3 | 8 |
| MRD status after CPX-351 induction | ||
| Negative | 8 | 57 |
| Positive | 6 | 43 |
| NA | 22 | — |
| Conditioning regimen | ||
| RIC | 33 | 92 |
| MAC | 3 | 8 |
| Donor | ||
| Matched related donor | 9 | 25 |
| Matched unrelated donor | 16 | 44 |
| Mismatched donor | 5 | 14 |
| Haploidentical donor | 6 | 17 |
| Stem cell source | ||
| Bone marrow | 2 | 6 |
| Peripheral blood | 34 | 94 |
HCT-CI, hematopoietic cell transplant comorbidity index; MAC, myeloablative conditioning; NA, not available; PR, partial response; RIC, reduced-intensity conditioning.
Discussion
For several decades, 7+3 has remained the classical induction regimen used for AML in both younger and elderly patients. Over the past 30 years, several improvements have been made, such as the increase in anthracycline dosage,15 the addition of new drugs like gemtuzumab ozogamicin,16 FLT3 inhibitors17 and improvements in ASCT18 and maintenance treatments post-ASCT,19 which have led to an increase in survival rates. However, all of these benefits were obtained in the favorable or intermediate risk subgroups, without upgrade in the adverse risk subgroup. Recently, CPX-351 showed an improvement in OS vs the classical 7+3 regimen in high-risk AML subtypes, specifically MRC-AML and t-AML (9.56 vs 5.95 months; P = .003).8
In our study, we collected data from MRC-AML and t-AML patients treated with CPX-351 in a real-world setting. Baseline patient characteristics were similar to the phase 3 clinical trial with the exception of prior HMA exposure (18% in our cohort vs 40.5% in the CPX-351 arm of the phase 3 study) and inclusion of patients >18 years old vs only the population between 60 and 75 years of age in the phase 3 study. We confirmed the acceptable safety profile already observed by Lancet et al. We observed an increased hematological toxicity but without an increased incidence of infections, including fungal infections (10%).20,21 We also identified a fairly good safety profile regarding AEs not reported in the Lancet et al study, like cutaneous (25%), gastrointestinal (50%), and alopecia (11%) AEs, as compared with 7+3.15 Recently, the safety profile of CPX-351 was compared with 7+3 by pooling safety data from the 5 studies.11 A similar rate of gastrointestinal disorders with a lower incidence of diarrhea was observed in the CPX-351 arms (46% vs 66%). In contrast, the incidence of rash was higher (39% vs 25%).
In our study, the ORR was slightly higher than the one observed in the previous phase 3 study, since we observed a CR/CRi rate of 59%. We confirmed that ASCT was associated with a better outcome as we showed an improved OS (median OS not reached in transplanted patients).
Lindsley et al presented the genetic characteristics and outcomes related to the mutational status from the phase 3 study of CPX-351 vs 7+3 at the ASH 2019 meeting.22 In that study, median OS was longer in the CPX-351 arm vs the 7+3 arm among patients with DNMT3A and TET2 mutations. Median OS was similar among patients with TP53 mutations treated with CPX-351 and 7+3. In our study, only mutations in the spliceosome complex genes (SRSF2, SF3B1, U2AF1, and ZRSR2) were significantly associated with better OS in multivariate analysis. It should be noted that while Lindsley’s classifier included these mutations to define secondary AML, they reported a lower ORR and a shorter median OS in secondary-type AML. In our study, we confirmed the poor prognostic value of TP53-mutated AML, but not of DNMT3A- or TET2-mutated AML. This is likely due to difference in the characteristics of the population and to the smaller size of our cohort compared with the phase 3 population. We also identified unfavorable prognostic factors like AML subtypes, such as prior treatment with HMAs, presence of monosomal karyotype, and TP53 or PTPN11 mutation. Nevertheless only the presence of a TP53 or PTPN11 mutation can predict the response in multivariate analysis.23 Moreover, we confirmed the interesting results already observed in the phase 3 clinical trial with regard to the FLT3-mutated patients. We also observed that CPX-351 overcame the poor response rate typically seen for patients with mutated ASXL1 and RUNX1. Although these mutations belong to the adverse risk subgroup as defined by 2017 ELN risk stratification, we detected no difference in outcome for patients with mutated ASXL1 or RUNX1 vs wild-type in our study. Nevertheless, these results need to be confirmed in a larger cohort. The best prognostic factor identified in our study that predicted ORR and OS was Lindsley’s classifier. Furthermore, longer survival observed in patients with secondary-like AML compared with those with de novo/pan AML should be linked with better prognosis of spliceosome mutations.
Moreover, we observed a significant difference in OS between transplanted and nontransplanted patients. The high rate of CR with low MRD compared favorably to a previous report using 7+3 in elderly unfavorable AML24 and may explain the favorable outcome observed in patients after ASCT, even if it was not detected in our cohort, likely due to a too short follow-up and a small sample size.
Finally, we compared our results to an historical cohort12 in which 233 patients received IC and 120 AZA. Although these comparative data are uncontrolled and not randomized, they suggest that the optimal place of CPX-351 could be in patients aged 60 to 69 years and those with adverse cytogenetic risk, as well as in patients aged >70 years with intermediate cytogenetic risk. Nevertheless, these data obviously need to be confirmed in a larger cohort.
Despite the small number of patients in our cohort, we report for the first time some prognostic factors in patients treated with CPX-351. We showed that CPX-351 can alter the poor prognosis of mutations belonging to the adverse 2017 ELN risk subgroup. We noticed a deeper response that could explain the better median OS observed in transplanted patients in our study and in the previous phase 3 clinical trial. Our data confirmed the benefit of using CPX-351 in high-risk AML and may trigger discussions about future combinations with other agents such as FLT3 inhibitors,17,25-27 APR-246,28,29 or Bcl-2 inhibitors30 to further improve the outcome of this unfavorable subgroup of AML.
Conclusion
Our study confirms the efficacy and safety of CPX-351 in high-risk AML (t-AML and MRC-AML) in a real-life setting. The high rate of CR with low MRD compares favorably with previous reports using 7+3 in elderly unfavorable AML patients and may strengthen the favorable outcome observed in patients after ASCT. Moreover, CPX-351 improves the poor prognosis associated with some unfavorable mutations defined in the 2017 ELN risk stratification. These data need to be confirmed in a larger cohort.
Send data sharing requests via e-mail to the corresponding author, Thomas Cluzeau (cluzeau.t@chu-nice.fr).
Authorship
Contribution: E.C. collected data; E.C., R.R., S.B., P.-Y.D., J.-B.M., F.P., Y.H., P.P., P.C., X.T., M.L., A.G., O.L., M.M., E.R., P.A., A.C., M.J., C.B., G.R.-G., C.L., A.P., N.V., C.R., L.A., and T.C. edited the manuscript; T.C. designed the study; and E.C. and T.C. wrote the manuscript.
Conflict-of-interest disclosure: P.P., A.P., C.R., and T.C. have served as an advisor or consultant for Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Thomas Cluzeau, Centre Hospitalier Universitaire de Nice, Hematology Department, 151 Route Saint Antoine de Ginestière, 06200 Nice, France; e-mail: cluzeau.t@chu-nice.fr.
References
Author notes
The full-text version of this article contains a data supplement.