Key Points
HIGH-2-LOW model identifies high-risk allogeneic HCT patients who may benefit from early thromboprophylaxis after platelet engraftment.
Patients at high risk have a VTE incidence of 10.3% compared with 1.5% for those at low risk between day 30 and 100 post-HCT.
Abstract
Venous thromboembolism (VTE) after allogeneic hematopoietic cell transplantation (HCT) is a significant treatment-associated complication, although optimal timing of thromboprophylaxis remains uncertain when weighing concurrent risks of bleeding. We aimed to derive and internally validate a risk assessment model (RAM) using patients who underwent first allogeneic HCT from 2006 through 2015 (n = 1703). Index date was defined as the 30th day after transplant, at which point we estimated >75% of patients would have achieved platelet engraftment >50 × 109/L. Stepwise logistic regression modeling was used for model development, and internal validation was achieved by fitting a logistic regression model with 1000 bootstrapped resamples to estimate the optimism-corrected c-statistic. The final RAM, “HIGH-2-LOW,” included 7 predictors obtained at 30 days after transplant: History of catheter-related deep venous thrombosis (DVT), Inpatient at day 30, Graft-versus-host disease grade 3 to 4, History of pulmonary embolism or lower-extremity DVT, Lymphoma diagnosis, Obesity with body mass index ≥35 kg/m2, and White blood cell count ≥11 × 109/L. Approximately 16% of patients were stratified as high risk, with incident VTE rate of 10.3% at 100 days compared with 1.5% for those at low risk. VTE odds ratios at 100 days were 5.87 (95% confidence interval [CI], 2.98-11.57) and 2.71 (95% CI, 1.38-5.35) in the high- and intermediate-risk vs low-risk groups, respectively. HIGH-2-LOW model serves as a novel and potentially clinically meaningful tool to identify high-risk allogeneic HCT patients who may benefit from early thromboprophylaxis after platelet engraftment.
Introduction
Venous thromboembolism (VTE) is a relatively common complication after allogeneic hematopoietic cell transplantation (HCT).1 Various factors have been associated with increased risk of VTE in this patient population, including history of VTE, graft-versus-host disease (GVHD), systemic infection, presence of indwelling catheters, prolonged hospital admissions, and underlying hematologic malignancy.2-4 VTE can be particularly difficult to manage given prolonged periods of thrombocytopenia following HCT, making decisions regarding full-dose anticoagulation challenging.5 Furthermore, allogeneic HCT patients are at greater risk for clinically significant bleeding compared with their autologous counterparts, in part due to higher incident acute GVHD in this patient population.6 In addition to risks of bleeding, VTE after HCT can result in increased health care–associated costs and platelet transfusion utilization7 and has independently been associated with increased nonrelapse mortality.8,9
Given the heightened risk of thrombosis and bleeding in allogeneic HCT patients, ongoing uncertainty exists regarding optimal timing and implementation of thromboprophylaxis. A retrospective analysis evaluating time to bleeding and recurrent VTE in patients with hematologic malignancies found that clinically significant bleeding occurred predominantly within the first 30 days after the onset of severe thrombocytopenia, whereas most recurrent VTE occurred after this.7 Because of expected severe thrombocytopenia in the first 30 days posttransplant, pharmacologic thromboprophylaxis is generally not recommended by most consensus guidelines.10 However, the use of low-dose anticoagulation for thromboprophylaxis after 30 days, when the majority of patients achieve platelet engraftment, varies significantly based on institutional practice, clinician judgment, and patient-related risk factors. Based on our institutional experience, the great majority of hospitalized patients never receive thromboprophylaxis at any point during their posttransplant care. Much of this uncertainty relates to the lack of a validated prediction model stratifying patients into risk categories for VTE when thromboprophylaxis becomes feasible after platelet engraftment.
To address these questions regarding optimal timing and patient selection for the use of primary thromboprophylaxis, we conducted a retrospective study using a large cohort of allogeneic HCT patients to evaluate patient-specific factors that may predispose for future VTE. Our objectives for the study include (1) determining the overall cumulative incidence of VTE up to 1-year posttransplant in all patients who underwent first allogeneic HCT, and (2) deriving and internally validating a risk assessment model (RAM) to select allogeneic HCT patients who may benefit from primary thromboprophylaxis after platelet engraftment at 30 days.
Methods
Study design and cohort selection
We performed a single-center retrospective cohort study including all adult patients who underwent first allogeneic HCT at Fred Hutchinson Cancer Research Center (FHCRC) from January 1, 2006 through January 1, 2015. This study was approved by the FHCRC Institutional Review Board. Patients were followed from date of stem cell infusion to 1-year posttransplant, subsequent transplant, or death, whichever occurred first. For patients who underwent multiple HCTs during the study period, only the first allogeneic HCT period was included. For the purpose of risk modeling, the index date (day 0) for the study was defined as the 30th day after allogeneic HCT, at which point we concluded that >75% of patients would have achieved platelet engraftment >50 × 109/L.
Risk factor ascertainment
Pertinent pretransplant patient demographics, mean body mass index (BMI), HCT-specific comorbidity index,11 disease type, donor match, conditioning regimen, initial GVHD prophylaxis regimen, and baseline laboratory results were obtained from the Fred Hutch Gateway database. History and timing of VTE were ascertained through electronic capture and confirmed by chart review. Additional posttransplant characteristics and complications were assessed during the first 30 days following transplant, including the presence of systemic invasive infections, notably bacteremia, invasive aspergillosis, and cytomegalovirus (CMV) reactivation. Severity of acute GVHD was graded by established criteria.12 Anticoagulation usage at day 30, including both prophylactic and therapeutic dosing, was identified. Neutrophil engraftment was defined as the first of 3 consecutive days achieving sustained neutrophil count >500 × 106/L, whereas platelet engraftment was defined separately as platelet count >20 × 109/L and 50 × 109/L independent from platelet transfusion for a least 7 days.13 Duration of hospitalization and pertinent laboratory values at 30 days posttransplant were also included.
Outcome definitions
VTE was defined as pulmonary embolism (PE), lower-extremity deep venous thrombosis (LE-DVT), or catheter-related DVT (CR-DVT) based on radiographic imaging confirmation. We assessed for potential VTE outcomes for up to 365 days after stem cell infusion using a combination of International Classification of Disease (ICD) codes and electronic medical record review; however, because of a large number of patients discharged home after 100 days, we specified day 100 as the primary time of interest for outcome assessment. Specifically, we used ICD-9 codes 12.51, 58.61, 415, 451, and 453 to detect all possible VTE cases, as we have done previously in a similar study setting with autologous HCT patients.14 Confirmation and location of VTE, timing of onset, presence of symptoms, and anticoagulant management were also captured and verified by individual chart review. For cumulative incidence assessment, we counted all new VTE events from −7 days before to +365 days after transplant as relevant outcomes. Because ICD-10 was introduced in October 2015, this outcome assessment method was accurate for all included patients in the current study. For risk predictor modeling (where index day is day 30), we treated all VTE events up to 30 days as historical events and counted all new VTE events from +30 to +100 days after transplant as relevant outcomes.
Statistical methods
Standard descriptive statistics were used to present baseline demographics, with categorical variables presented as frequencies and proportions and numerical variables presented as median and interquartile ranges (IQRs). For overall VTE incidence estimation, we used the unadjusted cumulative incidence competing risk method, where the first new episode of VTE from day −7 to +365 was treated as the outcome and death and subsequent transplant as competing causes. We assessed the cumulative incidence (%) and incidence rate (per 100 patient-year [py]) at both 100 days and 365 days.
For the VTE RAM derivation, a binary outcome variable was defined as whether each patient experienced new VTE from day +30 to +100. Detailed information on inclusion/exclusion as well as parametrization of potential clinically relevant predictors prior to index date is listed in supplemental Table 1. Specifically, we excluded variables that had significant missing data or required complicated derivation. The included covariates had no missing data for complete case analysis. Continuous variables were tested as linear terms as well as prespecified categories using 50th and 95th percentile cutoffs. Interaction terms were not included because of limited sample size.
To build a VTE prediction model, we first employed the backward stepwise logistic regression after locking in history of VTE and acute GVHD variables.15 Detailed model outputs, including odds ratio (OR), 95% confidence interval (CI), and standard error, are shown in supplemental Table 2. The final model included simplified binary covariates that achieved the best fit in the multivariable analysis or were putative predictors of VTE based on prior studies.2,16 To ensure that the stepwise regression did not lead to overfitting, we used the lasso penalized regression as a sensitivity analysis for variable selection.17 The lasso penalty parameter λ was selected through 10-fold cross-validation to minimize the cross-validation mean deviance, and the outputs are reported in supplemental Table 3.
To create a simplified VTE prediction score and avoid overinterpretation of the nonpenalized OR, we assigned an integer score of 1 to all included predictors except history of PE/DVT based on prior knowledge. Final risk categories were formed from a combination of integer scores. The performance and internal validation of this simplified 3-tiered VTE prediction category was then assessed. Discrimination was reported as the optimism-corrected c-statistic, or the area under the curve of the receiver operating curve. The optimism was calculated as the mean difference in c-statistic between the bootstrapped sample and the original sample through 1000 iterations of resampling. The 95% bias-corrected bootstrapped CI was reported. Calibration was assessed using the visualization of the Hosmer-Lemeshow goodness of fit test (HL test) and calibration plot.
We performed several additional sensitivity analyses: the first analyzed the outcome of PE or LE-DVT excluding CR-DVT, and the second excluded patients who had not reached platelet engraftment by day 30. In both analyses, we fit a logistic regression model with the outcome of VTE at 100 days and used the same 3-tiered VTE risk prediction categories to estimate the c-statistic. Finally, to assess if the 3-category RAM derived from logistic regression could be extrapolated over time, we plotted the predicted VTE from the corresponding Cox regression model vs observed VTE from Kaplan-Meier curve as an additional HL test.
Results
Overall cohort characteristics
We identified a total of 1857 patients over the 9-year period who underwent first allogeneic HCT at FHCRC that met study inclusion criteria (Figure 1). Patient characteristics are shown in Tables 1 and 2. Among patients included in the incidence estimation cohort, the median age was 53; 42% were female, and 86% were white. Seventy-seven percent of patients underwent HCT for underlying leukemia; 16% had prior autologous HCT, and 74% had matched donors. Fifty-seven percent underwent myeloablative conditioning, and nearly all patients received calcineurin-based GVHD prophylaxis. Approximately 89% of patients had no history of VTE prior to HCT, and for those who did, 5.4% had a history of PE or LE-DVT and 6.0% had a history of CR-DVT.
Study flow diagram with inclusion and exclusion criteria for the cumulative incidence cohort and the RAM cohort.
Study flow diagram with inclusion and exclusion criteria for the cumulative incidence cohort and the RAM cohort.
Pretransplant characteristics of patients who underwent allogeneic hematopoietic cell transplantation in the overall cumulative incidence estimation cohort (n = 1857)
| . | Values . |
|---|---|
| Patient demographics | |
| Age, y, median (IQR) | 53 (42-61) |
| Female sex, n (%) | 779 (42.0) |
| White race, n (%) | 1598 (86.1) |
| Prior autologous transplant, n (%) | 305 (16.4) |
| BMI in kg/m2, median (IQR) | 27 (24-30) |
| <29.9 (not obese), n (%) | 1376 (74.1) |
| 30 to 34.9 (obese class 1), n (%) | 315 (17.0) |
| 35 to 39.9 (obese class 2), n (%) | 96 (5.2) |
| ≥40.0 (obese class 3), n (%) | 70 (3.8) |
| Type of VTE history, n (%) | |
| None | 1644 (88.5) |
| CR-DVT | 112 (6.0) |
| PE or LE-DVT | 101 (5.4) |
| Timing of VTE history, n (%) | |
| None | 1644 (88.5) |
| VTE >6 mo prior to transplant | 97 (5.2) |
| VTE within 6 mo prior to transplant | 117 (6.3) |
| HCT–comorbidity index score, n (%) | |
| 0 | 268 (14.4) |
| 1 to 2 | 525 (28.3) |
| ≥3 | 905 (48.7) |
| Missing | 159 (8.6) |
| Disease type, n (%) | |
| Leukemia | 1434 (77.2) |
| Lymphoma | 247 (13.3) |
| Myeloma | 111 (6.0) |
| Other | 65 (3.5) |
| Donor match, n (%) | |
| Matched related | 599 (32.3) |
| Matched unrelated | 769 (41.4) |
| Mismatched related | 104 (5.6) |
| Mismatched unrelated | 222 (12.0) |
| Umbilical cord blood | 163 (8.8) |
| Conditioning regimen, n (%) | |
| Nonmyeloablative (reduced intensity) | 808 (43.5) |
| Myeloablative without high-dose TBI | 731 (39.4) |
| Myeloablative with high-dose TBI (≥1200 cGy) | 318 (17.1) |
| GVHD prophylaxis | |
| Tacrolimus | 961 (51.7) |
| Cyclosporine | 752 (40.5) |
| Sirolimus + tacrolimus or cyclosporine | 106 (5.7) |
| Others | 38 (2.1) |
| . | Values . |
|---|---|
| Patient demographics | |
| Age, y, median (IQR) | 53 (42-61) |
| Female sex, n (%) | 779 (42.0) |
| White race, n (%) | 1598 (86.1) |
| Prior autologous transplant, n (%) | 305 (16.4) |
| BMI in kg/m2, median (IQR) | 27 (24-30) |
| <29.9 (not obese), n (%) | 1376 (74.1) |
| 30 to 34.9 (obese class 1), n (%) | 315 (17.0) |
| 35 to 39.9 (obese class 2), n (%) | 96 (5.2) |
| ≥40.0 (obese class 3), n (%) | 70 (3.8) |
| Type of VTE history, n (%) | |
| None | 1644 (88.5) |
| CR-DVT | 112 (6.0) |
| PE or LE-DVT | 101 (5.4) |
| Timing of VTE history, n (%) | |
| None | 1644 (88.5) |
| VTE >6 mo prior to transplant | 97 (5.2) |
| VTE within 6 mo prior to transplant | 117 (6.3) |
| HCT–comorbidity index score, n (%) | |
| 0 | 268 (14.4) |
| 1 to 2 | 525 (28.3) |
| ≥3 | 905 (48.7) |
| Missing | 159 (8.6) |
| Disease type, n (%) | |
| Leukemia | 1434 (77.2) |
| Lymphoma | 247 (13.3) |
| Myeloma | 111 (6.0) |
| Other | 65 (3.5) |
| Donor match, n (%) | |
| Matched related | 599 (32.3) |
| Matched unrelated | 769 (41.4) |
| Mismatched related | 104 (5.6) |
| Mismatched unrelated | 222 (12.0) |
| Umbilical cord blood | 163 (8.8) |
| Conditioning regimen, n (%) | |
| Nonmyeloablative (reduced intensity) | 808 (43.5) |
| Myeloablative without high-dose TBI | 731 (39.4) |
| Myeloablative with high-dose TBI (≥1200 cGy) | 318 (17.1) |
| GVHD prophylaxis | |
| Tacrolimus | 961 (51.7) |
| Cyclosporine | 752 (40.5) |
| Sirolimus + tacrolimus or cyclosporine | 106 (5.7) |
| Others | 38 (2.1) |
Early posttransplant characteristics and complications at 30 days of patients who underwent allogeneic hematopoietic cell transplantation in the overall cumulative incidence estimation cohort (n = 1857)
| . | Values . |
|---|---|
| VTE-related events (%) | |
| New diagnosis of VTE | 33 (1.8) |
| CR-DVT | 21 (1.1) |
| PE or LE-DVT | 12 (0.6) |
| Anticoagulation usage (%) | 105 (5.7) |
| New initiation of prophylactic or therapeutic anticoagulation (excluded from model derivation) | |
| Hospitalization and death | |
| Days of hospitalization, median (IQR) | 15 (2-22) |
| Inpatient admission at 30 d, n (%) | 298 (16.1) |
| Mortality at 30 d, n (%) (excluded from model derivation) | 49 (2.6) |
| Neutrophil engraftment | |
| Days to neutrophil engraftment >0.5 × 109/L, median (IQR) | 17 (14-20) |
| Neutrophil engraftment at 30 d, n (%) | 1812 (97.6) |
| Platelet engraftment >50 | |
| Days to platelet engraftment >50 × 109/L, median (IQR) | 14 (12-24) |
| Platelet recovery >50 at 30 d, n (%)* | 1406 (75.7) |
| Platelet engraftment >20 | |
| Days to platelet engraftment >20 × 109/L, median (IQR) | 13 (10-19) |
| Platelet recovery >20 at 30 d, n (%)* | 1543 (83.1) |
| Acute GVHD (%) | |
| None or grade 1 | 1203 (64.8) |
| Grade 2 | 515 (27.7) |
| Grade 3 to 4 | 139 (7.5) |
| Systemic infections (%) | |
| Bacteremia | 358 (19.3) |
| Invasive aspergillosis infection | 156 (8.4) |
| CMV reactivation | 395 (21.8) |
| Laboratory values at day 30, median (IQR) | |
| White blood cell count, ×109/L | 5.1 (3.2-7.9) |
| Absolute neutrophil count, ×109/L | 3.3 (1.9-5.6) |
| Hemoglobin, g/dL | 10.7 (9.8-11.6) |
| Platelets, ×109/L | 102 (56-153) |
| Creatinine, mg/dL | 1.0 (0.8-1.3) |
| Total bilirubin, mg/dL | 0.7 (0.5-1.1) |
| International normalized ratio | 1.1 (1.1-1.3) (n = 718) |
| Partial thromboplastin time, s | 33 (28-40) (n = 624) |
| D-dimer, μg/mL | 2.8 (1.6-4.6) (n = 124) |
| . | Values . |
|---|---|
| VTE-related events (%) | |
| New diagnosis of VTE | 33 (1.8) |
| CR-DVT | 21 (1.1) |
| PE or LE-DVT | 12 (0.6) |
| Anticoagulation usage (%) | 105 (5.7) |
| New initiation of prophylactic or therapeutic anticoagulation (excluded from model derivation) | |
| Hospitalization and death | |
| Days of hospitalization, median (IQR) | 15 (2-22) |
| Inpatient admission at 30 d, n (%) | 298 (16.1) |
| Mortality at 30 d, n (%) (excluded from model derivation) | 49 (2.6) |
| Neutrophil engraftment | |
| Days to neutrophil engraftment >0.5 × 109/L, median (IQR) | 17 (14-20) |
| Neutrophil engraftment at 30 d, n (%) | 1812 (97.6) |
| Platelet engraftment >50 | |
| Days to platelet engraftment >50 × 109/L, median (IQR) | 14 (12-24) |
| Platelet recovery >50 at 30 d, n (%)* | 1406 (75.7) |
| Platelet engraftment >20 | |
| Days to platelet engraftment >20 × 109/L, median (IQR) | 13 (10-19) |
| Platelet recovery >20 at 30 d, n (%)* | 1543 (83.1) |
| Acute GVHD (%) | |
| None or grade 1 | 1203 (64.8) |
| Grade 2 | 515 (27.7) |
| Grade 3 to 4 | 139 (7.5) |
| Systemic infections (%) | |
| Bacteremia | 358 (19.3) |
| Invasive aspergillosis infection | 156 (8.4) |
| CMV reactivation | 395 (21.8) |
| Laboratory values at day 30, median (IQR) | |
| White blood cell count, ×109/L | 5.1 (3.2-7.9) |
| Absolute neutrophil count, ×109/L | 3.3 (1.9-5.6) |
| Hemoglobin, g/dL | 10.7 (9.8-11.6) |
| Platelets, ×109/L | 102 (56-153) |
| Creatinine, mg/dL | 1.0 (0.8-1.3) |
| Total bilirubin, mg/dL | 0.7 (0.5-1.1) |
| International normalized ratio | 1.1 (1.1-1.3) (n = 718) |
| Partial thromboplastin time, s | 33 (28-40) (n = 624) |
| D-dimer, μg/mL | 2.8 (1.6-4.6) (n = 124) |
Platelet recovery was defined as platelet count >50 or 20 × 109/L without platelet transfusion for at least 7 d.
Within the first 30 days posttransplant, the overall mortality was 3%, and the median duration of hospitalization was 15 days. The median times to neutrophil engraftment and platelet engraftment >50 × 109/L were 17 and 14 days, respectively. Acute GVHD grade 2 or higher occurred in 35% of patients, and systemic bacterial infection, fungal, and CMV reactivation occurred in 19%, 8%, and 22% of patients, respectively.
Cumulative incidence of VTE after allogeneic HCT
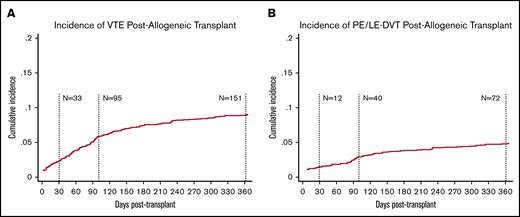
The cumulative incidence of new VTE events measured posttransplant is presented in Figure 2. The median follow-up time was 374 days, and the median time to onset of VTE was 87 days. The overall incidence (and rate) of VTE at 100 and 360 days was 4.9% (18.60 per 100-py, n = 95) and 8.0% (10.26 per 100-py, n = 151), respectively; the corresponding incidence of PE or LE-DVT (excluding CR-DVT) was 2.0% (7.72 per 100-py, n = 40) and 3.9% (4.94 per 100-py, n = 72), respectively. Within the first 30 days, ∼2% of patients developed new VTE (n = 33; 12 PE or LE-DVT, 21 CR-DVT). Six percent of patients (n = 105) were started on anticoagulation at day 30, the majority of whom (n = 95) were on therapeutic anticoagulation, whereas only 10 patients were started on prophylactic anticoagulation.
Incidence of VTE and PE or LE-DVT postallogeneic transplant in unselected cohort. Cumulative incidence was assessed using the competing risk method where death and subsequent transplant were treated as competing risks. The overall cumulative incidence of VTE at day 100 posttransplant was 4.9% (A) compared with 2.0% CI of PE or LE-DVT (B) at day 100.
Incidence of VTE and PE or LE-DVT postallogeneic transplant in unselected cohort. Cumulative incidence was assessed using the competing risk method where death and subsequent transplant were treated as competing risks. The overall cumulative incidence of VTE at day 100 posttransplant was 4.9% (A) compared with 2.0% CI of PE or LE-DVT (B) at day 100.
Derivation of RAM
Patients were further excluded from the cumulative incidence cohort to establish a uniform risk modeling cohort with an index date of day 30 posttransplant to allow adequate time for platelet engraftment. Specifically, patients were excluded if they died (n = 49) or were on anticoagulation for any reason (n = 105) by day 30 (n = 1703; Figure 1). The outcome for the RAM included VTE that occurred between day 30 and day 100 (n = 56). Early VTE events that occurred during the first 30 days were treated as historical events if they were not excluded due to anticoagulant use. The list of 20 candidate predictor variables are shown in supplemental Table 1. Because the index date was set as 30 days posttransplant, both pretransplant variables and those occurring within the first 30 days were included as baseline variables. There were no missing data in any of the selected variables. Stepwise regression selected 7 predictors in the final multivariable model with similar OR. The detailed model specification and outputs, including continuous, categorical, and dichotomized variable transformations, are shown in supplemental Table 2. In our sensitivity analysis, lasso penalized regression selected the same 7 predictors plus creatinine at day 30 (supplemental Table 3). As the inclusion of creatinine did not lead to a better fit in the final model, we elected to use the original model with fewer variables.
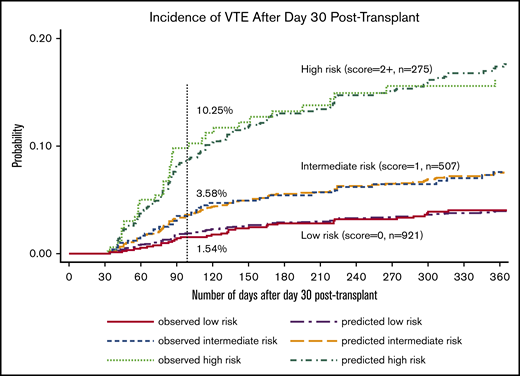
After identifying the key predictors in the multivariable model, we created a simplified prediction score by assigning a higher weight to the history of PE/DVT based on prior knowledge and equal weight to all other variables. As shown in Table 3, variables with their associated weights included: History of CR-DVT (+1), Inpatient at day 30 (+1), GVHD grade 3 to 4 at day 30 (+1), History of PE or LE-DVT (+2), Lymphoma diagnosis (+1), Obesity with BMI ≥35 kg/m2 (+1), and WBC ≥11 × 109/L at day 30 (+1). We named the new RAM “HIGH-2-LOW” and used the sum of the associated integer weights to create 3 unique risk categories. Based on this classification, 921 patients were categorized as low risk with a score of 0; 507 patients were intermediate risk with a score of 1, and 275 patients were high risk with a score of ≥2. As shown in Figure 3, VTE was observed in 10.3% (n = 25/275) of patients in the high-risk group, 3.6% (n = 17/507) of those in intermediate-risk group, and 1.5% (n = 14/921) in the low-risk group between day 30 and day 100.
Derivation of the HIGH-2-LOW VTE RAM
| Proposed risk factor . | OR (95% CI) . | Weight* . | Score distribution . | Risk stratification . |
|---|---|---|---|---|
| History of CR-DVT (n = 81) | 2.10 (0.80-5.53) | 1 | ||
| Inpatient admission (30 d) (n = 280) | 2.02 (1.06-3.86) | 1 | 0 = 921 | |
| GVHD grade 3 to 4 (30 d) (n = 127) | 1.74 (0.77-3.91) | 1 | 1 = 507 | 0 = low risk (n = 921) |
| History of PE or LE-DVT (n = 52) | 2.54 (0.92-7.05) | 2 | 2 = 201 | 1 = intermediate (n = 507) |
| Lymphoma diagnosis (n = 209) | 3.47 (1.89-6.38) | 1 | 3 = 60 | 2+ = high risk (n = 275) |
| Obesity (BMI ≥35 kg/m2) (n = 61) | 2.54 (1.26-5.13) | 1 | 4 = 10 | |
| WBC ≥11 × 109/L (30 d) (n = 202) | 1.95 (0.99-3.84) | 1 | 5 = 4 |
| Proposed risk factor . | OR (95% CI) . | Weight* . | Score distribution . | Risk stratification . |
|---|---|---|---|---|
| History of CR-DVT (n = 81) | 2.10 (0.80-5.53) | 1 | ||
| Inpatient admission (30 d) (n = 280) | 2.02 (1.06-3.86) | 1 | 0 = 921 | |
| GVHD grade 3 to 4 (30 d) (n = 127) | 1.74 (0.77-3.91) | 1 | 1 = 507 | 0 = low risk (n = 921) |
| History of PE or LE-DVT (n = 52) | 2.54 (0.92-7.05) | 2 | 2 = 201 | 1 = intermediate (n = 507) |
| Lymphoma diagnosis (n = 209) | 3.47 (1.89-6.38) | 1 | 3 = 60 | 2+ = high risk (n = 275) |
| Obesity (BMI ≥35 kg/m2) (n = 61) | 2.54 (1.26-5.13) | 1 | 4 = 10 | |
| WBC ≥11 × 109/L (30 d) (n = 202) | 1.95 (0.99-3.84) | 1 | 5 = 4 |
The sum of the covariate weights was used to stratify risk of VTE: low risk = 0, intermediate risk = 1, and high risk ≥2. Baseline risk predictors were assessed at 30 d posttransplant, and binary VTE outcomes were assessed at 100 d posttransplant.
WBC, white blood cell count.
Observed and predicted incidence of VTE based on the HIGH-2-LOW RAM among patients not receiving anticoagulation at 30 days.
Observed and predicted incidence of VTE based on the HIGH-2-LOW RAM among patients not receiving anticoagulation at 30 days.
Model performance, internal validation, and sensitivity analyses
To internally validate the HIGH-2-LOW model, we assessed the discrimination and calibration of the final 3-tiered RAM. As shown in Table 4, the predicted ORs for VTE at 100 days were 5.87 (95% CI, 2.98 to 11.57) and 2.71 (95% CI, 1.38 to 5.35) in the high- and intermediate-risk vs low-risk group, respectively. For discrimination, the optimism-corrected c-statistic was 0.69 (bias-corrected 95% CI, 0.62 to 0.76), where the mean optimism was 0.15%. For calibration, the HL distribution plot and calibration plot both indicated adequate fit (supplemental Figure 1). Furthermore, the observed vs predicted VTE curves overlaid each other in the time-to-event analysis (Figure 3).
Performance and internal validation of the HIGH-2-LOW RAM
| . | Risk assessment category* . | Risk score . | OR for VTE (95% CI) . | VTE incidence 30 to 100 d,† % . | C-statistic‡ (95% CI) . |
|---|---|---|---|---|---|
| Primary analysis Overall VTE as outcome at day 100 | Low (n = 921) | 0 | 1 | 1.54 | 0.69 (0.62-0.76) |
| Intermediate (n = 507) | 1 | 2.71 (1.38-5.35) | 3.58 | ||
| High (n = 275) | 2+ | 5.87 (2.98-11.57) | 10.25 | ||
| Sensitivity analysis 1 PE or LE-DVT (not CR-DVT) as outcome at day 100 | Low (n = 921) | 0 | 1 | 0.66 | 0.71 (0.60-0.81) |
| Intermediate (n = 507) | 1 | 3.01 (1.06-8.50) | 1.51 | ||
| High (n = 275) | 2+ | 7.43 (2.72-20.31) | 5.56 | ||
| Sensitivity analysis 2 Overall VTE at day 100 in patients with platelet recovery >50 × 109/L at day 30 | Low (n = 754) | 0 | 1 | 1.21 | 0.73 (0.65-0.80) |
| Intermediate (n = 363) | 1 | 3.32 (1.42-7.75) | 3.94 | ||
| High (n = 197) | 2+ | 9.35 (4.19-20.89) | 11.05 |
| . | Risk assessment category* . | Risk score . | OR for VTE (95% CI) . | VTE incidence 30 to 100 d,† % . | C-statistic‡ (95% CI) . |
|---|---|---|---|---|---|
| Primary analysis Overall VTE as outcome at day 100 | Low (n = 921) | 0 | 1 | 1.54 | 0.69 (0.62-0.76) |
| Intermediate (n = 507) | 1 | 2.71 (1.38-5.35) | 3.58 | ||
| High (n = 275) | 2+ | 5.87 (2.98-11.57) | 10.25 | ||
| Sensitivity analysis 1 PE or LE-DVT (not CR-DVT) as outcome at day 100 | Low (n = 921) | 0 | 1 | 0.66 | 0.71 (0.60-0.81) |
| Intermediate (n = 507) | 1 | 3.01 (1.06-8.50) | 1.51 | ||
| High (n = 275) | 2+ | 7.43 (2.72-20.31) | 5.56 | ||
| Sensitivity analysis 2 Overall VTE at day 100 in patients with platelet recovery >50 × 109/L at day 30 | Low (n = 754) | 0 | 1 | 1.21 | 0.73 (0.65-0.80) |
| Intermediate (n = 363) | 1 | 3.32 (1.42-7.75) | 3.94 | ||
| High (n = 197) | 2+ | 9.35 (4.19-20.89) | 11.05 |
Score category determined by the sum of the weights of the proposed covariates of interest, with low risk = 0, intermediate risk = 1, and high risk ≥2.
The incidence reported here is an underestimate of the overall incidence of VTE posttransplant, as it represents incident VTE events that occur from day 30 to day 100 (70-d span).
The c-statistic is reported after optimism adjustment, and the bias-corrected CI was derived from bootstrapping the resample 1000 times. The final model calibration and goodness-of-fit are shown in the supplemental figures.
We performed additional sensitivity analyses using stricter outcome definitions and a stricter cohort selection criterion (Table 4). In the first sensitivity analysis, we used PE or LE-DVT as the primary outcome (after excluding CR-DVT); the overall VTE incidence was lower, but the discrimination was similar (c-statistic, 0.71; 95% CI, 0.60 to 0.81). In the second sensitivity analysis, we excluded all patients who did not reach adequate platelet engraftment by 30 days for whom anticoagulation would not be routinely recommended. Both the overall VTE incidence and the discrimination were similar to the primary analysis (c-statistic, 0.73; 95% CI, 0.65 to 0.80).
Discussion
In patients undergoing allogeneic HCT, VTE remains a serious treatment-associated complication that lacks validated risk-stratification models to guide optimal timing and implementation of thromboprophylaxis. In our retrospective analysis of a large cohort of patients undergoing first allogeneic HCT, we derived a new VTE RAM, HIGH-2-LOW, for predicting VTE after platelet engraftment at day 30 to help address this challenging clinical scenario. By identifying patients at highest risk, we hope the HIGH-2-LOW model, if externally validated, will help guide clinical decision making for early implementation of thromboprophylaxis to reduce morbidity and mortality associated with VTE in this patient population.
To our knowledge, no current risk prediction models exist in the literature to help stratify patients into risk categories for VTE in the allogeneic HCT population. Although several have been developed to examine risk in ambulatory patients with solid tumors18 and in patients with multiple myeloma19 and lymphoma,20 clinical utility in the transplant setting is limited. A meta-analysis of 17 studies, including both observational and randomized control trials, evaluating risk of VTE after allogeneic HCT found a significantly elevated risk (47% and 35%) of VTE in patients with acute and chronic GVHD, respectively.21 Although associations between GVHD and other factors for VTE have been described in transplant literature, risk-stratification guidelines are lacking. In the present study, we aimed to fill this highly relevant void by developing a model incorporating risk factors that were statistically significant as well as clinically recognized and easily obtainable.
Similar to prior estimates,1,4,16 incident VTE was detected among 8.0% of patients in our study within 1-year postallogeneic HCT. Most events occurred between day 30 and day 100 with nearly half of all events PE or LE-DVT. Although this estimate is high, understandable hesitancy exists when considering thromboprophylaxis in a fragile patient population at high risk for bleeding. Based on our institutional practice and confirmed through chart review, we found that many hospitalized transplant patients were never started on thromboprophylaxis despite guidelines recommending initiation after initial engraftment without absolute contraindications to therapy.10 In fact, we identified only 10 patients out of nearly 300 patient charts reviewed in our present study who were started on thromboprophylaxis at day 30. Therefore, when deriving our RAM, we intentionally chose a clinically relevant timeline of 30 days following allogeneic HCT as our index date, having estimated that most patients would have achieved sustained platelet engraftment >50 × 109/L (76% in the present study), for which the implementation of thromboprophylaxis would be both safe and feasible. This proposed model demonstrated an incident VTE rate of 10.3% from day 30 to day 100 posttransplant in high-risk patients compared with 1.5% in low-risk patients. We believe that patients falling into the high-risk group, which clinically correlates to 16% of overall patients in our present study, would benefit from thromboprophylaxis. This is especially important for patients with a history of VTE or those with prolonged hospitalization. Although not yet externally validated, model testing has demonstrated internal consistency, further strengthening its use in the clinical setting.
Our novel HIGH-2-LOW model incorporated 7 objective clinical predictors associated with increased risk of VTE in the posttransplant setting. Many of the variables included in our model are well-known risk factors for VTE in allogeneic HCT patients, including history of VTE, acute hospitalization, and acute GVHD.2 Elevated BMI >25 kg/m2 was associated with increased risk of VTE in a select group of patients with non-Hodgkin lymphoma who underwent allogeneic HCT.22 Although underlying hematologic malignancy substantially increases risk of VTE, history of multiple myeloma, rather than lymphoma, has more commonly been associated.2 The discrepancy in our analysis is likely due to the lower proportion of patients with multiple myeloma undergoing allogeneic HCT compared with those with lymphoma and the lack of immunomodulatory drug exposure during HCT. Furthermore, we believe that there is a screen-detection bias, as lymphoma patients posttransplant undergo more routine computed tomographic scans that lead to increased detection of asymptomatic VTE. If we limited our outcomes to symptomatic VTE only, we would likely no longer observe this association with lymphoma. Whether asymptomatic VTE posttransplant should be treated similarly to symptomatic VTE requires additional studies beyond the scope of the current paper. Last, to our knowledge, high WBC count is not an associated risk factor in published transplant literature but may confer an elevated risk of inflammation predisposing to VTE occurrence. The ease of obtaining the variables necessary to compute a risk assessment score using the HIGH-2-LOW model substantially increases its clinical utility. All predictors incorporated in the model are easily obtained through routine blood work and historical inquiry, simplifying its use for practitioners faced with this common clinical challenge.
Beyond assessing risk of VTE in allogeneic HCT patients, management strategies regarding pharmacologic thromboprophylaxis continue to evolve. At this time, low-molecular-weight heparin remains the standard of care for thromboprophylaxis in the postallogeneic HCT setting, primarily because of limited drug-drug interactions and well-studied efficacy in preventing VTE in hospitalized patients.23 Recently, low-dose direct oral anticoagulants (DOACs), specifically apixaban and rivaroxaban, have been shown in randomized control trials to reduce VTE when used as prophylaxis in a selective high-risk group of ambulatory patients with solid tumor malignancy and lymphoma receiving systemic therapy.24,25 A subsequent meta-analysis of the 2 trials showed that in the subgroup of patients with highest risk for VTE (Khorana score 3+) where the 6-month VTE incidence was 11.57% in the placebo group, low-dose DOAC compared with placebo was associated with a relative risk for VTE of 0.47 (95% CI, 0.25-0.89) and a relative risk for major bleeding of 1.60 (95% CI, 0.42-6.01).26 The 6-month incidence of VTE in our high-risk group stratified by the HIGH-2-LOW model was 13.8%, which would place these patients in a similar high-risk category as these previously cited clinical trials. Future studies are warranted to determine the safety and efficacy of DOACs in postallogeneic HCT patients after initial engraftment and discontinuation of antifungal medications. The safety and efficacy of outpatient thromboprophylaxis after engraftment in allogeneic HCT patients at highest risk, particularly those with a history of PE or LE-DVT, also necessitate further investigation.
This retrospective cohort study has several strengths. First, we used a uniform and stringent selection schema to prevent selection bias, with a consistent short-term follow-up duration where all patient outcomes were captured within 100 days. Second, we had little outcome misclassification, as we screened for both historical and incident VTE outcomes and confirmed each event with individual chart review. Third, the cumulative incidence of VTE in our patient population was similar to prior estimates in the allogeneic HCT population, making us confident that our findings are reproducible compared with previously published data. Furthermore, different model selection algorithms led to similar outputs, and model testing with additional sensitivity analyses was concordant with our derived RAM, providing further validation of our stratification system.
There are also inherent limitations to our study. First, we likely underestimated VTE events after patients were discharged from the transplant center given inherent limitations of relying on ICD-9 coding to capture VTE events. To mitigate this concern, we tested our derived model using VTE outcomes at 100 days and did not find any systemic bias. Second, we did not have access to an additional data set for external validation. We performed internal validation via bootstrapping and found that the lower bound c-statistic value of the CI was 0.62, effectively excluding 0.50. Third, we acknowledge that risk factors for the development of CR-DVT may differ from those associated with PE and LE-DVT, and symptomatic VTE may behave differently than asymptomatic VTE. We performed a separate sensitivity analysis of PE/LE-DVT outcomes and found similar discrimination as the primary analysis, thus providing additional confidence that our RAM would be applicable in assessing risk for all VTE outcomes. We also did not collect bleeding complications from this population. This outcome is actively being collected and will likely strengthen the findings of the current study. Finally, certain variables, such as duration of hospitalization, GVHD, and systemic infection, would be best assessed as time-varying covariates. However, it would not be feasible to incorporate time-varying covariates in a simple predictive model applicable at a fixed time point of 30 days posttransplant. Our primary goal of the current study was to create a user-friendly and clinically meaningful predictive model after engraftment rather than an explanatory model for association testing.
In summary, the HIGH-2-LOW model is a simple and effective tool that incorporates readily attainable pre- and posttransplant risk factors to identify allogeneic HCT patients at highest risk for VTE. Further validation of our model is warranted, which we aim to accomplish by testing in large internal and external retrospective cohorts with additional data gathering to delineate rates of inpatient vs ambulatory VTE diagnosis, which may further encourage use of inpatient thromboprophylaxis. It may also be feasible to design a future prospective study at a high-volume bone marrow transplant center to better assess validity. With future validation, we hope this model will clinically inform the use of thromboprophylaxis in high-risk patients after engraftment to prevent ongoing morbidity and mortality related to VTE in the allogeneic HCT population.
The patient level data are protected under study agreement with FHCRC and could not be shared publicly. For detailed questions regarding the modeling specifications, please contact Ang Li at ang.li2@bcm.edu.
Acknowledgments
This work was supported by the ASH Hematology Opportunities for the Next Generation of Research Scientists (HONORS) Award (K.L.M.), the Texas Cancer Prevention and Research Institute of Texas (RR190104), the Hemostasis and Thrombosis Research Society (Mentored Research Award), an independent medical educational grant from Shire, and by the National Hemophilia Foundation Shire Clinical Fellowship Award (A.L.).
This study was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board affiliated with the University of Washington Medical Center.
Authorship
Contribution: K.L.M. and A.L. conceived the study idea; A.L. designed the methodology; K.L.M., C.D., and M.K. collected data; A.L. and W.L.d.C. performed the statistical analysis; K.L.M. and A.L. drafted the manuscript; and C.I.A., S.J.L., N.A.Z., D.A.G., and A.L. critically reviewed and edited the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ang Li, Baylor College of Medicine, One Baylor Plaza, MS:307, Rm 610D, Houston, TX 77030; e-mail: ang.li2@bcm.edu.
References
Author notes
The full-text version of this article contains a data supplement.