Key Points
The half-life of rVIII-SingleChain improved with coadministration of rD′D3-FP.
Sustained pharmacodynamics effects were seen with coadministration of rVIII-SingleChain and rD′D3-FP.
Abstract
A novel mechanism for extending the circulatory half-life of coagulation factor VIII (FVIII) has been established and evaluated preclinically. The FVIII binding domain of von Willebrand factor (D′D3) fused to human albumin (rD′D3-FP) dose dependently improved pharmacokinetics parameters of coadministered FVIII in all animal species tested, from mouse to cynomolgus monkey, after IV injection. At higher doses, the half-life of recombinant FVIII (rVIII-SingleChain) was calculated to be increased 2.6-fold to fivefold compared with rVIII-SingleChain administered alone in rats, rabbits, and cynomolgus monkeys, and it was increased 3.1-fold to 9.1-fold in mice. Sustained pharmacodynamics effects were observed (ie, activated partial thromboplastin time and thrombin generation measured ex vivo). No increased risk of thrombosis was observed with coadministration of rVIII-SingleChain and rD′D3-FP compared with rVIII-SingleChain alone. At concentrations beyond the anticipated therapeutic range, rD′D3-FP reduced the hemostatic efficacy of coadministered rVIII-SingleChain. This finding might be due to scavenging of activated FVIII by the excessive amount of rD′D3-FP which, in turn, might result in a reduced probability of the formation of the tenase complex. This observation underlines the importance of a fine-tuned balance between FVIII and its binding partner, von Willebrand factor, for hemostasis in general.
Introduction
Hemophilia A is an inherited bleeding disorder, resulting from a deficiency in blood coagulation factor VIII (FVIII). It predominantly affects males, with an incidence of 1 to 2 individuals per 10 000; however, female carriers with symptoms like menorrhagia have been described.1 The recommended treatment is prophylaxis by infusion of plasma-derived (pdFVIII) or recombinant FVIII (rFVIII), because early prophylaxis prevents acute bleeding events and the development of hemophilic arthropathy.2,3 Because of its short plasma half-life (∼8-12 hours),4 effective prophylaxis requires IV injections of FVIII concentrates ∼3 times per week to maintain hemostasis. To improve the treatment regimen, there have been significant efforts to develop technologies to increase FVIII half-life, which would reduce administration frequency and maintain higher trough levels. However, established approaches, including conjugation of rFVIII with polyethylene glycol5-7 or polysialic acid8 or fusion to the Fc part of immunoglobulin G9 to allow recycling of FVIII via the FcRn pathway,10 have resulted in only modest FVIII half-life (up to ∼1.6-fold).11
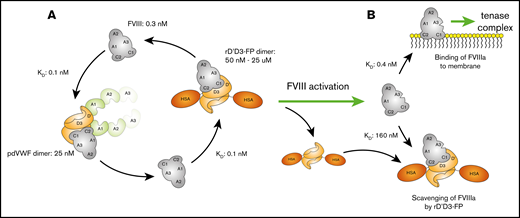
To further extend FVIII half-life, more recent approaches aimed to uncouple FVIII from its carrier protein, von Willebrand factor (VWF), as its short plasma half-life may be a limiting barrier.12 Binding of FVIII to VWF is mediated by the noncovalent interaction between the D′D3 region of VWF and the a3 and C1 domains of FVIII, with additional contributions from A3 and C2 (Figure 1).13-16 Binding of FVIII to VWF stabilizes FVIII in plasma, protecting it from premature elimination.17 Binding of FVIII to VWF monomers occurs at a stoichiometric ratio of 1:1,18 with a total molar excess of VWF over FVIII ∼50:1.19 The abundance of VWF, together with the high affinity of FVIII for VWF,20 results in the majority of FVIII circulating in complex with VWF.21,22 High association and dissociation rate constants ensure that a dynamic equilibrium is maintained.23 In the absence of VWF, such as in von Willebrand disease, FVIII is rapidly cleared from the circulation (plasma half-life, ∼2 hours).12 The importance of VWF for FVIII half-life is further emphasized by the finding that patients with type 2N von Willebrand disease have normal VWF but low FVIII plasma levels as a result of mutations in the VWF D′D3 region, which markedly reduce the binding affinity to FVIII.24
Domain structure of full-length VWF and rD′D3-FP. (A) Domain structure of native full-length VWF residues 1 to 2813, including propeptide D1D2 (blue) and the D′D3 domain (orange). Cysteines responsible for intra- and intermolecular dimerization are shown as yellow stars (C1099 and C1142 in the D3 domain; C2771, C2773, and C2811 in the CK domain). (B) rD′D3-FP monomer consisting of the propeptide domain D1D2 and the D′D3 domains of VWF N-terminally fused to human serum albumin (HSA; purple). This monomer dimerizes via its D3 domain to form the pro-rD′D3-FP dimer, which subsequently is processed by furin-catalyzed cleavage of the propeptide D1D2 to yield the final product, mature dimeric rD′D3-FP, as shown in panel C.
Domain structure of full-length VWF and rD′D3-FP. (A) Domain structure of native full-length VWF residues 1 to 2813, including propeptide D1D2 (blue) and the D′D3 domain (orange). Cysteines responsible for intra- and intermolecular dimerization are shown as yellow stars (C1099 and C1142 in the D3 domain; C2771, C2773, and C2811 in the CK domain). (B) rD′D3-FP monomer consisting of the propeptide domain D1D2 and the D′D3 domains of VWF N-terminally fused to human serum albumin (HSA; purple). This monomer dimerizes via its D3 domain to form the pro-rD′D3-FP dimer, which subsequently is processed by furin-catalyzed cleavage of the propeptide D1D2 to yield the final product, mature dimeric rD′D3-FP, as shown in panel C.
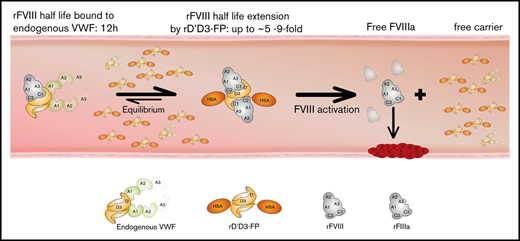
Strategies to extend FVIII half-life via coadministration of a half-life–extended VWF have not been successful. Unlike other recombinant proteins, the high molecular weight of multimeric VWF may prevent its recycling via the neonatal Fc receptor pathway using albumin- or Fc-fusion technologies.25-27 Furthermore, to outcompete endogenous VWF, half-life–extended VWF would need to be given in molar excess, which might be associated with increased thrombotic risk.28,29 One possibility to overcome these challenges and to extend FVIII half-life beyond that currently available is to uncouple FVIII from VWF using a recombinant VWF D′D3 domain. The most recent approach, based on the Fc-mediated dimerization of FVIII-Fc and a monomeric D′D3-Fc combined with XTEN technology,30 resulted in an FVIII half-life of up to 41 hours in phase 1 clinical studies.31 This demonstrates that uncoupling FVIII from endogenous VWF can significantly extend FVIII half-life.
An alternative strategy would be to dose a half-life–extended variant of D′D3, at competitive levels to endogenous VWF, independently of FVIII. To test this approach, we designed a VWF fragment reduced to the FVIII binding domain D′D3 that showed significantly improved pharmacokinetics properties through fusion to albumin (rD′D3-FP). As a result of the missing functional domains, this molecule can be given in competitive doses to endogenous VWF without the risk of increased thrombogenicity. Current data show that, at therapeutic doses, this approach can significantly extend the half-life of coadministered rVIII-SingleChain in all tested animal species without affecting hemostasis in murine hemophilia A models.
Materials and methods
Cloning and expression of rD′D3-FP
The expression cassette for rD′D3-FP, consisting of complementary DNA encoding VWF aa 1 to 1242, a 31–amino acid glycine-serine linker, and the complementary DNA of human albumin, was prepared by custom gene synthesis (Eurofins Genomics, Ebersberg, Germany) and cloned into pIRESneo3 (BD Biosciences, Franklin Lakes, NJ). The expression plasmid was transfected into Chinese Hamster Ovary K1 cells using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA), together with an expression plasmid encoding PACE/furin to maximize propeptide cleavage.32 Cells were grown in serum-free medium (CD-CHO; Invitrogen, Grand Island, NY) in the presence of 500 to 1000 µg/mL Geneticin (Thermo Fisher Scientific). Cell clones were expanded in shake flasks or small-scale fermenters, and rD′D3-FP was purified (supplemental Information). rD′D3-His protein was generated accordingly, with a His tag replacing the albumin domain.
Surface plasmon resonance analysis
Kinetics, affinity, and stoichiometry of rD′D3-FP dimer and monomer binding to rVIII-SingleChain (CSL Behring, Marburg, Germany) were analyzed by surface plasmon resonance (SPR) using a Biacore 4000 docked with a Series S CM5 sensor chip (supplemental Information).
Antigen and activity quantification
Enzyme-linked immunosorbent assays were used to measure human FVIII, human VWF, rD′D3-FP, and human albumin (supplemental Information). A COAMATIC FVIII kit (CHROMOGENIX, Milan, Italy) was used to measure chromogenic FVIII activity of all species, using standard human plasma or Haemate P as standard.
In vitro experiment for activated partial thromboplastin time evaluation
Three batches of standard human plasma were spiked with rD′D3-FP (0.05-25 µM, corresponding to 0.006-3 mg/mL), as well as rD′D3-His (0.05-25 µM, 0.003-1.404 mg/mL). Both rD′D3 proteins were diluted with isotonic saline, which was also used as a control (0 mg/mL). rD′D3-FP, rD′D3-His, or isotonic saline was added to the plasma at a constant volume ratio (1 part rD′D3-FP, rD′D3-His, or vehicle + 4 parts plasma).
Activated partial thromboplastin time (aPTT) was determined using a coagulation analyzer (BCS XP System; Siemens Healthcare Diagnostics, Marburg, Germany). Plasma samples were incubated with Pathromtin SL reagent for 2 minutes, and coagulation was started by the addition of calcium chloride solution (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany).
In vivo experiments
Animal care.
All animals received care in compliance with the European Convention on Animal Care, and the studies were approved by local governmental authorities (supplemental Information).
Pharmacokinetics.
The pharmacokinetics profiles of FVIII after dosing rVIII-SingleChain coadministered with rD′D3-FP, plasma-derived VWF (pdVWF), or Haemate P were determined following a single IV injection to FVIII-knockout (ko) mice, VWF-ko mice, NMRI mice, Crl:CD (SD) rats, Chinchilla Bastard rabbits, or cynomolgus monkeys (Macaca fascicularis). There was no difference whether VWF controls were sourced from Haemate or pdVWF only (data not shown). Test animals were dosed according to chromogenic FVIII units, as labeled. rD′D3-FP dose levels were determined based on albumin enzyme-linked immunosorbent assay for all species. A product standard, defined retrospectively by a mass spectroscopy method, allowed quantification of the rD′D3-FP molecule dose by multiplying the albumin concentration by 2.86. As a result, rD′D3-FP was dosed in a range from 0.1 to 10 mg of albumin per kilogram, corresponding to 0.286 to 28.6 mg/kg rD′D3-FP. Doses of pdVWF were 300 to 500 IU/kg. FVIII-ko mice were dosed with 106 IU/kg Haemate based on FVIII, relating to 290 IU/kg based on VWF. Plasma samples were drawn predose (rabbits and monkeys) and at various time points after treatment up to 72 hours in FVIII-ko mice and rats and up to 168 hours in rabbits and monkeys (all n = 3 per time point). Blood samples were immediately processed to plasma in 10% citrate (9:1 mixing ratio; 3.13% weight-to-volume ratio) for mice and monkeys and in 20% citrate (8:2 mixing ratio; 3.13% weight-to-volume ratio) for rats and rabbits.
Pharmacokinetics analysis/statistics.
A 2-compartmental modeling approach was used for mouse data; a noncompartmental approach was used for rat, rabbit, and monkey data. Parameter estimation was conducted by weighted least squares fitting using the fmincon method in Matlab R2017a. Possible baseline values were subtracted from the measured data. Values below the lower limit of quantification, as well as clotted samples, were excluded from the analysis.
Thrombin generation assay and aPTT (ex vivo).
FVIII-ko mice received 100 IU/kg rVIII-SingleChain, with or without 2.86 mg/kg rD′D3-FP IV, compared with vehicle-treated FVIII-ko mice. Plasma samples were taken at 2, 24, 48, 72, and 96 hours postadministration, and thrombin-generation parameters were determined in platelet-poor plasma (supplemental Information).
Tail-clip model in FVIII-ko mice (in vivo).
FVIII-ko mice received 100 IU/kg rVIII-SingleChain IV, with or without 2.86 mg/kg rD′D3-FP, at 6 or 16 hours prior to tail clip compared with vehicle-treated FVIII-ko mice. NMRI mice received vehicle or high-dose (250 mg/kg) IV rD′D3-FP 15 minutes prior to tail clip. Hemostatic efficacy was determined using a subaquatic tail-tip bleeding model, quantifying time to hemostasis and total blood loss (supplemental Information).
FeCl3-induced arterial occlusion in FVIII-ko mice (in vivo).
FVIII-ko mice received 100 IU/kg rVIII-SingleChain IV, with or without 2.86 mg/kg rD′D3-FP, 1 hour prior to induction of arterial thrombosis compared with vehicle-treated FVIII-ko mice. NMRI mice received vehicle or high-dose (250 mg/kg) IV rD′D3-FP 15 minutes prior to induction of arterial thrombosis. Arterial injury was induced by topical ferric chloride treatment, and blood flow and time to occlusion were monitored by Doppler sonography until arterial occlusion had occurred or until 40 minutes postthrombus induction. Occlusion rate was then calculated.
Results
Expression, purification, and characterization of rD′D3-FP
The structures of full-length FVIII and rD′D3-FP are shown in Figure 1. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot analysis confirmed the absence of covalently bound D1D2 (data not shown). rD′D3-FP was expressed as a mixture of monomer and dimer with a ratio ranging from 1:1 to 1:4, depending on cell clone and media conditions (data not shown). rD′D3-FP dimer was separated from the monomer by anti–human albumin affinity and size-exclusion chromatography to yield highly purified rD′D3-FP dimer. The absence of monomeric rD′D3-FP, as well as cleaved D1D2 propeptide, which binds noncovalently and with high affinity (30.9 nM) to rD′D3-FP,33 was confirmed by subsequent analysis (supplemental Figure 1A-B). SPR showed that dimeric rD′D3-FP bound rVIII-SingleChain with similarly high affinity as pdVWF (82.5 ± 7.9 and 101 ± 9.8 pM, respectively; supplemental Figure 1C; supplemental Table 1).34 Conversely, the binding affinity of monomeric rD′D3-FP to rVIII-SingleChain was reduced substantially (964 ± 147 pM), underlining the importance of rD′D3-FP dimerization for high-affinity binding of FVIII. SPR demonstrated that the binding of rVIII-SingleChain to rD′D3-FP monomer approached a stoichiometry of 1 and a stoichiometry of 2 to the dimer (supplemental Information; supplemental Table 2).
Pharmacokinetics analysis of rD′D3-FP
rD′D3-FP clearance, terminal half-life (t1/2), and mean residence time (MRT) were not affected by the dose; thus, means were calculated over the administered doses. rD′D3-FP was cleared much more slowly than human pdVWF in all species tested, resulting in an extended MRT and t1/2 (Table 1). The average endogenous VWF plasma concentration in 12 untreated rats was determined to be 0.95 U/mL; it was 0.24 U/mL predose in 6 rabbits.
Pharmacokinetics characterization of rD′D3-FP
| Species . | Treatment . | CL, mL/h/kg . | MRT, h . | t1/2, h . |
|---|---|---|---|---|
| FVIII-ko mouse | rD′D3-FP (0.286-28.6 mg/kg, 100 IU/kg FVIII) | 1.2 ± 0.4 | 61.0 ± 15.0 | 47.0 ± 13.0 |
| VWF (Haemate, 106 IU/kg FVIII, 290 IU/kg VWF) | 23.6 | 1.8 | 5.1 | |
| Fold difference | 20* | 34† | 9† | |
| VWF-ko mouse | rD′D3-FP (2.86 mg/kg, 100 IU/kg FVIII) | 1.3 | 49.0 | 34.0 |
| NMRI mouse | rD′D3-FP (2.86-28.6 mg/kg, 100 IU/kg FVIII) | 2.4 ± 0.1 | 33.0 ± 4.0 | 26.0 ± 2.0 |
| Rat | rD′D3-FP (0.14-28.6 mg/kg, 200 IU/kg FVIII); rD′D3-FP (2.86-28.6 mg/kg, 100, 300, 1000 IU/kg FVIII) | 1.2 ± 0.1‡; 1.2 ± 0.1‡ | 55.1 ± 5.7‡; 59.5 ± 5.9‡ | 39.2 ± 3.9‡; 42.7 ± 4.2‡ |
| VWF (pdVWF, 400 IU/kg) | 68.2 | 1.1 | 1.3 | |
| Fold difference | 59*; 57* | 50†; 54* | 30†; 33* | |
| Rabbit | rD′D3-FP (1.4-8.6 mg/kg, 150 IU/kg FVIII) | 0.5 ± 0.1 | 162.0 ± 17.0 | 112.0 ± 12.0 |
| VWF (pdVWF, 300 IU/kg) | 22.2 | 4.0 | 4.2 | |
| Fold difference | 44* | 41† | 27† | |
| Cynomolgus monkey | rD′D3-FP (7.15-28.6 mg/kg) | 0.6-0.7 | 115.0-151.0 | 92.0-116.0 |
| VWF (pdVWF, 500 IU/kg) | 5.21 | 15.5 | 11.3 | |
| Fold difference | 8-9* | 7-10† | 8-10† |
| Species . | Treatment . | CL, mL/h/kg . | MRT, h . | t1/2, h . |
|---|---|---|---|---|
| FVIII-ko mouse | rD′D3-FP (0.286-28.6 mg/kg, 100 IU/kg FVIII) | 1.2 ± 0.4 | 61.0 ± 15.0 | 47.0 ± 13.0 |
| VWF (Haemate, 106 IU/kg FVIII, 290 IU/kg VWF) | 23.6 | 1.8 | 5.1 | |
| Fold difference | 20* | 34† | 9† | |
| VWF-ko mouse | rD′D3-FP (2.86 mg/kg, 100 IU/kg FVIII) | 1.3 | 49.0 | 34.0 |
| NMRI mouse | rD′D3-FP (2.86-28.6 mg/kg, 100 IU/kg FVIII) | 2.4 ± 0.1 | 33.0 ± 4.0 | 26.0 ± 2.0 |
| Rat | rD′D3-FP (0.14-28.6 mg/kg, 200 IU/kg FVIII); rD′D3-FP (2.86-28.6 mg/kg, 100, 300, 1000 IU/kg FVIII) | 1.2 ± 0.1‡; 1.2 ± 0.1‡ | 55.1 ± 5.7‡; 59.5 ± 5.9‡ | 39.2 ± 3.9‡; 42.7 ± 4.2‡ |
| VWF (pdVWF, 400 IU/kg) | 68.2 | 1.1 | 1.3 | |
| Fold difference | 59*; 57* | 50†; 54* | 30†; 33* | |
| Rabbit | rD′D3-FP (1.4-8.6 mg/kg, 150 IU/kg FVIII) | 0.5 ± 0.1 | 162.0 ± 17.0 | 112.0 ± 12.0 |
| VWF (pdVWF, 300 IU/kg) | 22.2 | 4.0 | 4.2 | |
| Fold difference | 44* | 41† | 27† | |
| Cynomolgus monkey | rD′D3-FP (7.15-28.6 mg/kg) | 0.6-0.7 | 115.0-151.0 | 92.0-116.0 |
| VWF (pdVWF, 500 IU/kg) | 5.21 | 15.5 | 11.3 | |
| Fold difference | 8-9* | 7-10† | 8-10† |
Data are mean (± standard deviation) for mice and FVIII-competent rats, rabbits, and cynomolgus monkeys, as well as their improvement over human pdVWF.
CL, clearance.
Fold decrease.
Fold increase over human pdVWF.
Two independent studies.
Pharmacokinetics analysis of rVIII-SingleChain coadministered with rD′D3-FP
rD′D3-FP improved the pharmacokinetics profile of rVIII-SingleChain in all tested animal species in a dose-dependent manner (Table 2). A dose-dependent decrease was seen with regard to clearance, in parallel with an increase in MRT and t1/2 (Table 2). The prolongation of rVIII-SingleChain t1/2 by rD′D3-FP is shown in Figure 2A and B. To calculate the molar ratio of rD′D3-FP over endogenous VWF, the measured VWF concentrations of 0.95 U/mL (in rats) and 0.24 U/mL (in rabbits) were used. Because these values were measured against human standard, they may not reflect the correct molar concentration. However, commercial standards for the species used are not available for activity or for antigen measurements. rD′D3-FP doses used in the rat pharmacokinetics experiment (0.14-28.6 mg/kg) reflected a molar ratio of rD′D3-FP monomer/endogenous VWF monomers of 0.56 to 113, with a dose of 0.25 mg/kg being equimolar to endogenous VWF (both calculated as monomers). Coadministration of 200 IU/kg rVIII-SingleChain with 0.14 to 0.57 mg/kg rD′D3-FP (molar ratio of 0.568-2.3 for rD′D3-FP/endogenous VWF) reduced clearance slightly and prolonged MRT and t1/2 of rVIII-SingleChain (Table 2) compared with rVIII-SingleChain alone. This effect was dose dependent starting at 1.43 mg/kg (molar ratio, 5.6) and was maximal at the highest dose of rD′D3-FP (28.6 mg/kg; molar ratio, 113; Table 2). Figure 2C shows the observed increase in rVIII-SingleChain t1/2 in rat plasma when combined with increasing doses of rD′D3-FP against the respective calculated molar ratio of rD′D3-FP/endogenous VWF.
Pharmacokinetics characterization of rVIII-SingleChain alone or coadministered with rD′D3-FP
| Parameter . | Treatment . | FVIII-ko mouse . | VWF-ko mouse . | NMRI mouse . | Rat . | Rabbit . | Cynomolgus monkey . | |
|---|---|---|---|---|---|---|---|---|
| Measured by: . | FVIII:chr . | FVIII:Ag . | FVIII:Ag . | FVIII:Ag . | FVIII:Ag . | FVIII:Ag . | FVIII:chr . | |
| CL, mL/h/kg | rVIII-SingleChain | 3.6 | 3.4 | n.d. | 25.8 | 8.3 | 3.7 | 2.0 |
| rVIII-SingleChain + rD′D3-FP | 1.3-2.9 | 1.3-3.1 | 1.7 | 3.5 | 1.7-4.9 | 1.0-1.8 | 0.4-0.4 | |
| Fold difference | 1.3-2.8* | 1.1-2.6* | n.a. | 7.4† | 1.7-4.9* | 2.1-3.7* | 4.5-5.5* | |
| MRT, h | rVIII-SingleChain | 10.1 | 14.0 | n.d. | 2.0 | 7.4 | 23.0 | 19.9 |
| rVIII-SingleChain + rD′D3-FP | 11.4-32.7 | 23.0-44.0 | 31.0 | 18.0 | 10.5-29.6 | 33.0-64.0 | 85.3-99.0 | |
| Fold difference | 1.1-3.2† | 1.6-3.1† | n.a. | 8.9* | 1.4-4.0† | 1.4-2.8† | 4.3-5.0† | |
| t1/2, h | rVIII-SingleChain | 7.0 | 10.0 | n.d. | 1.4 | 5.2 | 17.0 | 13.1 |
| rVIII-SingleChain + rD′D3-FP | 7.9-34.3 | 19.0-31.0 | 21 | 13 | 7.1-20.3 | 21.0-44.0 | 56.1-66.1 | |
| Fold difference | 1.1-4.9† | 1.9-3.1† | n.a. | 9.1* | 1.4-3.9† | 1.2-2.6† | 4.3-5.0† | |
| Parameter . | Treatment . | FVIII-ko mouse . | VWF-ko mouse . | NMRI mouse . | Rat . | Rabbit . | Cynomolgus monkey . | |
|---|---|---|---|---|---|---|---|---|
| Measured by: . | FVIII:chr . | FVIII:Ag . | FVIII:Ag . | FVIII:Ag . | FVIII:Ag . | FVIII:Ag . | FVIII:chr . | |
| CL, mL/h/kg | rVIII-SingleChain | 3.6 | 3.4 | n.d. | 25.8 | 8.3 | 3.7 | 2.0 |
| rVIII-SingleChain + rD′D3-FP | 1.3-2.9 | 1.3-3.1 | 1.7 | 3.5 | 1.7-4.9 | 1.0-1.8 | 0.4-0.4 | |
| Fold difference | 1.3-2.8* | 1.1-2.6* | n.a. | 7.4† | 1.7-4.9* | 2.1-3.7* | 4.5-5.5* | |
| MRT, h | rVIII-SingleChain | 10.1 | 14.0 | n.d. | 2.0 | 7.4 | 23.0 | 19.9 |
| rVIII-SingleChain + rD′D3-FP | 11.4-32.7 | 23.0-44.0 | 31.0 | 18.0 | 10.5-29.6 | 33.0-64.0 | 85.3-99.0 | |
| Fold difference | 1.1-3.2† | 1.6-3.1† | n.a. | 8.9* | 1.4-4.0† | 1.4-2.8† | 4.3-5.0† | |
| t1/2, h | rVIII-SingleChain | 7.0 | 10.0 | n.d. | 1.4 | 5.2 | 17.0 | 13.1 |
| rVIII-SingleChain + rD′D3-FP | 7.9-34.3 | 19.0-31.0 | 21 | 13 | 7.1-20.3 | 21.0-44.0 | 56.1-66.1 | |
| Fold difference | 1.1-4.9† | 1.9-3.1† | n.a. | 9.1* | 1.4-3.9† | 1.2-2.6† | 4.3-5.0† | |
Data are shown for mice and for FVIII-competent rats, rabbits, and cynomolgus monkeys, as well as their respective improvement over coadministered human pdVWF.
The following doses were used:
FVIII-ko mice: rVIII-SingleChain, 100 IU/kg and rD′D3-FP, 0.286-28.6 mg/kg
VWF-ko mice: rVIII-SingleChain, 100 IU/kg and rD′D3-FP, 2.86 mg/kg
NMRI mice: rVIII-SingleChain, 100 IU/kg and rD′D3-FP, 2.86-28.6 mg/kg
Rats: rVIII-SingleChain, 200 IU/kg and rD′D3-FP, 0.14-28.6 mg/kg
Rabbits: rVIII-SingleChain, 150 IU/kg and rD′D3-FP, 1.4-8.6 mg/kg
Cynomolgus monkeys: rVIII-SingleChain, 250 IU/kg and rD′D3-FP, 7.2-28.6 mg/kg; FVIII activity was corrected for endogenous levels.
FVIII:Ag, FVIII antigen assay; FVIII:chr, FVIII chromogenic assay; n.a., not applicable; n.d., not done.
Fold decrease.
Fold increase over rVIII-SingleChain.
Time course of rVIII-SingleChain plasma levels after coadministration with rD′D3-FP in rats and rabbits and dependency of FVIII half-life extension on molar excess of rD′D3-FP over endogenous VWF. Plasma levels of rVIII-SingleChain (antigen, dosed at 200 IU/kg in rats [A] and 150 IU/kg in rabbits [B]), alone or coadministered with increasing doses of rD′D3-FP and pdVWF (400 IU/kg in rats and 300 IU/kg in rabbits) as control. The data show the dose-dependent increase in rVIII-SingleChain plasma exposure with increased dosing of rD′D3-FP. The shorter half-life of rVIII-SingleChain coadministered with pdVWF might be explained by a shorter half-life of human VWF in a rat and rabbit model. The horizontal dashed line represents the detection limit of the assay; data are mean ± standard deviation (SD) (n = 2-3). (C) Fold increase in the terminal plasma half-life of rVIII-SingleChain as a function of the molar excess of rD′D3-FP over endogenous VWF for rats (●) and for rabbits (○). The dotted gray line represents a ratio of 1; ie, the terminal plasma half-life of rVIII-SingleChain alone.
Time course of rVIII-SingleChain plasma levels after coadministration with rD′D3-FP in rats and rabbits and dependency of FVIII half-life extension on molar excess of rD′D3-FP over endogenous VWF. Plasma levels of rVIII-SingleChain (antigen, dosed at 200 IU/kg in rats [A] and 150 IU/kg in rabbits [B]), alone or coadministered with increasing doses of rD′D3-FP and pdVWF (400 IU/kg in rats and 300 IU/kg in rabbits) as control. The data show the dose-dependent increase in rVIII-SingleChain plasma exposure with increased dosing of rD′D3-FP. The shorter half-life of rVIII-SingleChain coadministered with pdVWF might be explained by a shorter half-life of human VWF in a rat and rabbit model. The horizontal dashed line represents the detection limit of the assay; data are mean ± standard deviation (SD) (n = 2-3). (C) Fold increase in the terminal plasma half-life of rVIII-SingleChain as a function of the molar excess of rD′D3-FP over endogenous VWF for rats (●) and for rabbits (○). The dotted gray line represents a ratio of 1; ie, the terminal plasma half-life of rVIII-SingleChain alone.
In rabbits, t1/2 prolongation was not as pronounced, but FVIII plasma elimination in the initial phase was delayed, again leading to longer FVIII exposure in the presence of rD′D3-FP (Figure 2B).
The improvement in pharmacokinetics parameters of rVIII-SingleChain is a function of the molar excess of rD′D3-FP over endogenous VWF (Figure 2C). Similar data were obtained for mice and cynomolgus monkeys (Table 2). In rats, rabbits, and cynomolgus monkeys, rVIII-SingleChain coadministered with rD′D3-FP improved MRT, t1/2, and clearance by 2.6- to 5.5-fold compared with rVIII-SingleChain alone, whereas the increase covered a wider range in mice (2.6- to 9.1-fold).
Pharmacodynamics effects of rD′D3-FP
aPTT and thrombin-generation assay.
In FVIII-ko mice, rVIII-SingleChain plus rD′D3-FP (2.86 mg/kg; molar ratio of rD′D3-FP/VWF, 11.3) prolonged pharmacodynamics activity in plasma compared with rVIII-SingleChain alone, as indicated by a more sustained reduction in aPTT (Figure 3A) and longer elevation of thrombin peak levels (Figure 3B). rVIII-SingleChain resulted in an aPTT of 33 seconds, which increased to 43 seconds after 48 hours (Figure 3A). The clotting time went back to 55 seconds at 72 hours postadministration, which is within baseline range for FVIII-ko mice. rVIII-SingleChain coadministered with rD′D3-FP produced a sustained reduction in aPTT at 96 hours postadministration (41 seconds).
Impact of rD′D3-FP coadministration on aPTT and thrombin-generation assays in rVIII-SingleChain–treated FVIII-ko mice. The effect of rVIII-SingleChain alone (red lines) or coadministered with rD′D3-FP (blue lines) was assessed as aPTT (A) or in a thrombin-generation assay (B). A vehicle control (gray lines) was tested as negative control. (A) For aPTT, rVIII-SingleChain combined with rD′D3-FP resulted in a reduction of the clotting time even at 96 hours postadministration, whereas rVIII-SingleChain alone resulted in an aPTT similar to that in vehicle-treated animals after 72 hours. (B) Thrombin generation by rVIII-SingleChain in the presence of rD′D3-FP was increased from 48 to 96 hours compared with rVIII-SingleChain alone. Data are mean ± SD of 3 to 10 animals. Dashed lines represent mean vehicle values and dotted lines represent mean ± SD vehicle values.
Impact of rD′D3-FP coadministration on aPTT and thrombin-generation assays in rVIII-SingleChain–treated FVIII-ko mice. The effect of rVIII-SingleChain alone (red lines) or coadministered with rD′D3-FP (blue lines) was assessed as aPTT (A) or in a thrombin-generation assay (B). A vehicle control (gray lines) was tested as negative control. (A) For aPTT, rVIII-SingleChain combined with rD′D3-FP resulted in a reduction of the clotting time even at 96 hours postadministration, whereas rVIII-SingleChain alone resulted in an aPTT similar to that in vehicle-treated animals after 72 hours. (B) Thrombin generation by rVIII-SingleChain in the presence of rD′D3-FP was increased from 48 to 96 hours compared with rVIII-SingleChain alone. Data are mean ± SD of 3 to 10 animals. Dashed lines represent mean vehicle values and dotted lines represent mean ± SD vehicle values.
In the thrombin-generation assay, peak thrombin levels for rVIII-SingleChain alone continuously declined, reaching the level of vehicle-treated animals at 96 hours postadministration (Figure 3B). When coadministered with rD′D3-FP, peak thrombin levels were 149 nM at 96 hours, which was substantially higher than for rVIII-SingleChain alone (28 nM) or for vehicle-treated animals (17 nM, at 2 hours).
Tail-clip bleeding model.
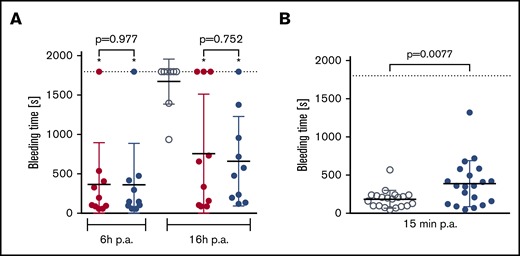
In a mouse subaquatic tail-clip model, bleeding times were similar in animals treated with rVIII-SingleChain alone (100 IU/kg) or cotreated with rD′D3-FP (2.86 mg/kg) (Figure 4A). Under these conditions, cotreatment with rD′D3-FP did not impact hemostatic efficacy of rVIII-SingleChain. Conversely, in wild-type NMRI mice, doses of rD′D3-FP above the pharmacological range (250 mg/kg) prolonged bleeding time slightly (Figure 4B). Nevertheless, this prolonged bleeding time was in the range of that for rVIII-SingleChain–treated FVIII-ko mice.
Effect of rD′D3-FP on tail-clip bleeding in FVIII-ko mice and NMRI mice. (A) The effect of rVIII-SingleChain (100 IU/kg, red circles), alone or coadministered with rD′D3-FP (2.86 mg/kg, blue circles), was assessed in a tail-clip bleeding model to rule out potential negative effects of rD′D3-FP on the hemostasis restored by rVIII-SingleChain (n = 10). A vehicle control (gray circles) was tested at 16 hours postadministration (p.a.) as negative control. There was no significant effect of rD′D3-FP on the rVIII-SingleChain–induced reduction of bleeding time at 6 or 16 hours postadministration, as calculated using variance models to the logarithmically transformed data (pairwise comparisons with Welch's t test). Significant differences as compared to vehicle-treated FVIII-ko mice are marked with an asterisk (*). Graphs show mean ± SD and individual results. (B) Bleeding time in vehicle-treated NMRI mice (saline, gray circles) was compared with NMRI mice treated with high-dose rD′D3-FP (250 mg/kg, blue circles) at 15 minutes after tail clip (n = 20). High-dose rD′D3-FP significantly prolonged bleeding time at 15 minutes postadministration as calculated using an unpaired Student t test. Dotted line represents the observation period.
Effect of rD′D3-FP on tail-clip bleeding in FVIII-ko mice and NMRI mice. (A) The effect of rVIII-SingleChain (100 IU/kg, red circles), alone or coadministered with rD′D3-FP (2.86 mg/kg, blue circles), was assessed in a tail-clip bleeding model to rule out potential negative effects of rD′D3-FP on the hemostasis restored by rVIII-SingleChain (n = 10). A vehicle control (gray circles) was tested at 16 hours postadministration (p.a.) as negative control. There was no significant effect of rD′D3-FP on the rVIII-SingleChain–induced reduction of bleeding time at 6 or 16 hours postadministration, as calculated using variance models to the logarithmically transformed data (pairwise comparisons with Welch's t test). Significant differences as compared to vehicle-treated FVIII-ko mice are marked with an asterisk (*). Graphs show mean ± SD and individual results. (B) Bleeding time in vehicle-treated NMRI mice (saline, gray circles) was compared with NMRI mice treated with high-dose rD′D3-FP (250 mg/kg, blue circles) at 15 minutes after tail clip (n = 20). High-dose rD′D3-FP significantly prolonged bleeding time at 15 minutes postadministration as calculated using an unpaired Student t test. Dotted line represents the observation period.
FeCl3-induced arterial occlusion model.
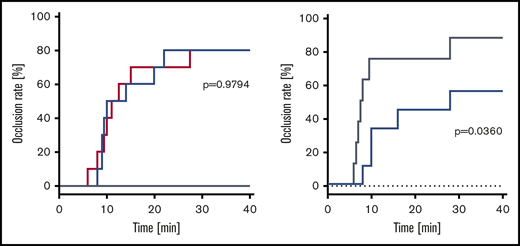
No vehicle-treated animal developed an occlusive thrombosis within the observation period. Occlusion rate was comparable in animals treated with rVIII-SingleChain alone or coadministered with rD′D3-FP (2.86 mg/kg; molar ratio of rD′D3-FP/VWF, 11.3), with 80% of mice (n = 8/10 per group) developing an occlusive thrombosis. Median time to occlusion was not impacted by rD′D3-FP (Figure 5A). To investigate the effect of high doses of rD′D3-FP on the occlusion rate, wild-type (NMRI) mice were treated with 250 mg/kg rD′D3-FP. Although NMRI mice treated with vehicle showed an occlusion rate of 88%, hemostasis was significantly reduced and delayed by high-dose rD′D3-FP (occlusion rate, 56%) (Figure 5B). Median time to occlusion after treatment with vehicle or high-dose rD′D3-FP was 7.8 or 28.0 minutes, respectively.
Effect of rD′D3-FP on FeCl3-induced arterial occlusion in FVIII-ko and NMRI mice. (A) The effect of rVIII-SingleChain (100 IU/kg, red line), alone or coadministered with rD′D3-FP (2.86 mg/kg, blue line), was assessed 1 hour after FeCl3-induced damage of the endothelium in an arterial occlusion model in FVIII-ko mice (n = 10). A vehicle control (gray line) was tested as negative control (n = 5), remaining at 0% occlusion rate. There was no significant effect of rD′D3-FP on the coagulation restored by the coadministered rVIII-SingleChain, suggesting no negative impact of rD′D3-FP on hemostasis. (B) Arterial occlusion in vehicle-treated NMRI mice (saline, gray line) was compared with that after administration of high-dose rD′D3-FP (250 mg/kg, blue line) 15 minutes after FeCl3-induced damage of the endothelium in an arterial occlusion model (n = 8-9). There was a significant effect of high-dose rD′D3-FP on coagulation in NMRI mice. P values were calculated using the log-rank Mantel-Cox test.
Effect of rD′D3-FP on FeCl3-induced arterial occlusion in FVIII-ko and NMRI mice. (A) The effect of rVIII-SingleChain (100 IU/kg, red line), alone or coadministered with rD′D3-FP (2.86 mg/kg, blue line), was assessed 1 hour after FeCl3-induced damage of the endothelium in an arterial occlusion model in FVIII-ko mice (n = 10). A vehicle control (gray line) was tested as negative control (n = 5), remaining at 0% occlusion rate. There was no significant effect of rD′D3-FP on the coagulation restored by the coadministered rVIII-SingleChain, suggesting no negative impact of rD′D3-FP on hemostasis. (B) Arterial occlusion in vehicle-treated NMRI mice (saline, gray line) was compared with that after administration of high-dose rD′D3-FP (250 mg/kg, blue line) 15 minutes after FeCl3-induced damage of the endothelium in an arterial occlusion model (n = 8-9). There was a significant effect of high-dose rD′D3-FP on coagulation in NMRI mice. P values were calculated using the log-rank Mantel-Cox test.
In vitro investigation of rD′D3-FP effects on aPTT.
The data described above indicated reduced hemostasis at a dose well above the pharmacological range (250 mg/kg). To understand the potential mechanism for this effect, rD′D3-FP and rD′D3-His were tested in aPTT assays using standard human plasma spiked with increasing concentrations of both molecules (Figure 6). Both proteins significantly prolonged aPTT, reaching ∼10% to 20% at 25 µM; there was no significant difference between the 2 proteins.
Impact of rD′D3-FP on aPTT in human plasma. The effect of rD′D3-FP (blue line) or rD′D3-His (red line) on aPTT was assessed in human plasma. A vehicle control (saline, gray lines) was tested as negative control (mean [dashed line] ± SD [dotted lines] of n = 12). rD′D3-FP and rD′D3-His prolonged aPTT. Data are mean ± SD of 3 samples.
Impact of rD′D3-FP on aPTT in human plasma. The effect of rD′D3-FP (blue line) or rD′D3-His (red line) on aPTT was assessed in human plasma. A vehicle control (saline, gray lines) was tested as negative control (mean [dashed line] ± SD [dotted lines] of n = 12). rD′D3-FP and rD′D3-His prolonged aPTT. Data are mean ± SD of 3 samples.
Discussion
Current strategies for half-life extension of FVIII have resulted in only modest improvements,12,35 probably as a result of the short half-life of the FVIII carrier protein VWF. In fact, recent data from a phase 1 trial of an Fc-based FVIII/VWF-D′D3 heterodimer30 showed that decoupling of FVIII from endogenous VWF circumvented the half-life ceiling imposed by VWF and extended FVIII half-life to 41 hours.31 The present work followed an alternative strategy, to dose a half-life–extended variant of VWF-D′D3 independently of FVIII. Fusing the FVIII-binding fragment of VWF, D′D3, to human albumin increased the fragment’s half-life. Achieving a robust increase in D′D3 half-life was crucial, as Yee and coworkers have shown that various fragments of VWF seem to have a reduced plasma half-life when compared with full-length VWF.36 In addition to its ability to be recycled via the FcRn pathway, a VWF fragment that is limited to its FVIII-binding functionality should not increase the risk of thrombotic events, as discussed by others.28,29
The recombinant VWF fragment tested here was engineered based only on the VWF propeptide D1D2 and the FVIII binding fragment D′D3. The VWF propeptide is a crucial component as its protein-disulfide-isomerase activity is responsible for the dimerization of rD′D3-FP via Cys1099 and Cys1142. In contrast to monomeric rD′D3-FP, which had a reduced affinity for rVIII-SingleChain, dimeric rD′D3-FP bound rVIII-SingleChain with similar characteristics as full-length pdVWF. Maintaining the high-affinity interaction between D′D3 and FVIII is key to ensuring that FVIII is not preferentially bound by endogenous VWF. This phenomenon was reported by Yee et al, whose D′D3-Fc construct did not prolong FVIII half-life in FVIII-ko mice,36 most likely because the reduced affinity of their construct resulted in FVIII dissociation from the D′D3-Fc and rebinding to endogenous VWF. The reduced affinity (despite being dimerized via the Fc domain) could be due to the absence of the D1D2 domain, which might negatively affect the folding of D′D3 and, therefore, its interaction with FVIII.
The present rD′D3-FP construct is based on human albumin to prolong its plasma half-life, which has resulted in significant increases in half-life for other therapeutic proteins, such as rIX-FP.27 Although this half-life extension strategy has been applied successfully, the genetic fusion of proteins carries an inherent risk for creating neoepitopes, which might result in immune reactions. However, ex vivo immunogenicity studies (ProImmune, Oxford, UK; data not shown) did not suggest a potential increased risk for immunogenicity with rD′D3-FP. Most importantly, the binding affinity of rD′D3-FP was very similar to native VWF. Therefore, rD′D3-FP should be the preferred binding partner for FVIII in circulation if dosed in molar excess over endogenous VWF. Indeed, when dosed in molar excess to endogenous VWF, rD′D3-FP effectively competed for binding to FVIII, thereby significantly increasing rVIII-SingleChain’s half-life in all animal species tested, without a negative impact on hemostasis when dosed at therapeutically relevant concentrations. FVIII-ko mice, rats, and rabbits showed a dose-dependent effect of rD′D3-FP on rVIII-SingleChain clearance, as well as t1/2 and MRT. In rats and rabbits, for which endogenous VWF levels are known, the fold increase in t1/2 of the coadministered rVIII-SingleChain directly correlated with the molar ratio of rD′D3-FP over endogenous VWF. Subequimolar doses of rD′D3-FP had only a minor impact on the half-life of coadministered rVIII-SingleChain, whereas at higher doses, half-life extension was 2.6-fold, 3.9-fold, 5.0-fold, and 3.1- to 9.1-fold that of rVIII-SingleChain alone in rabbit, rat, monkey, and mice, respectively. The lack of control over the molar ratios between D′D3-Fc and endogenous VWF in the experiments reported by Yee et al,36 which were based on hydrodynamics tail vein injection of DNA, might also explain the lack of FVIII half-life extension in FVIII-ko mice.
The prolongation of rVIII-SingleChain’s half-life by rD′D3-FP was paralleled by more sustained pharmacodynamics effects. In aPTT assays using FVIII-ko mice, rVIII-SingleChain shortened aPTT up to 48 hours postdose, reaching baseline values at 72 hours. In contrast, rVIII-SingleChain coadministered with rD′D3-FP produced a sustained reduction in aPTT up to 96 hours postadministration, reflecting the observed improvement in pharmacokinetics parameters. Similar results were obtained in thrombin-generation assays, which showed increased thrombin generation after 96 hours when rVIII-SingleChain was administered with rD′D3-FP. At high concentrations (≥1 µM), rD′D3-His, as well as rD′D3-FP, led to a prolongation of aPTT in human plasma. These concentrations are substantially greater than human VWF monomer concentrations of ∼0.05 µM.
Because VWF plays a role in stabilizing FVIII levels, as well as in targeting FVIII to the site of vessel injury,37,38 it was reasonable to speculate that at competing doses, rD′D3-FP, which harbors the FVIII binding site but lacks other VWF functional elements, could have a negative impact on hemostasis in vivo. However, results from the arterial occlusion model suggest that rD′D3-FP has no obvious negative effect on hemostasis. In agreement with these findings, there was no difference in bleeding time in a tail-clip model, indicating that rD′D3-FP did not have a negative effect on the initiation of coagulation at the site of vessel injury.
Nevertheless, when given at 250 mg/kg, which exceeds a potential clinical dose by ∼100-fold, rD′D3-FP reduced hemostatic efficacy in tail-clip and arterial thrombosis models using NMRI mice. This suggests interference of high concentrations of rD′D3-FP with the FVIII-mediated formation of the tenase complex, which is crucial for hemostasis. This interference could be related to (1) the inability of rD′D3-FP to target FVIII to the site of injury (in contrast to VWF), (2) reduced thrombin-mediated activation of rD′D3-FP–bound FVIII due to sterical hindrance by the fused albumin, or (3) scavenging of activated FVIII (FVIIIa) by excess rD′D3-FP. To understand the potential mechanism of this loss of efficacy at high rD′D3-FP concentrations, the molecule was spiked at increasing concentrations into standard human plasma, and coagulation was assessed by aPTT. Because aPTT assays do not depend on targeting FVIII to a site of injury, any observed loss of efficacy could be attributed to mechanisms independent of this functionality. In addition, to exclude the effect of steric hindrance of albumin fusion on the activation of rD′D3-bound FVIII, rD′D3-FP was tested back-to-back with rD′D3-His in aPTT assays using standard human plasma.
In agreement with in vivo observations, aPTT was prolonged at high concentrations of rD′D3-FP. Furthermore, rD′D3-His prolonged aPTT similarly to rD′D3-FP, with only minor differences at the highest concentration. Together, these results indicate that, in the aPTT assay, the missing targeting capability of the truncated VWF fragment rD′D3 and the steric hindrance of the albumin fusion are not responsible for the observed loss of efficacy at high concentrations. However, one cannot rule out that reduced FVIII binding to endogenous VWF in the presence of high concentrations of rD′D3-FP might also contribute to this effect in vivo. Based on aPTT data, the most likely explanation would be the scavenging of FVIIIa before it can form the membrane-based tenase complex. The affinity of FVIIIa for VWF is not easy to measure as a result of its intrinsic instability. However, using truncated constructs, a 1600-fold reduced affinity of activated FVIII vs nonactivated FVIII for VWF has been estimated.16 In our hands, this suggests an equilibrium constant (KD) ∼160 nM for FVIIIa to rD′D3-FP. Because the concentrations of rD′D3-FP that trigger the reduced efficacy of FVIII are in the micromolar range, it is possible that, despite its low KD, FVIIIa might be re-bound by rD′D3-FP, thereby reducing its concentration for the formation of the tenase complex. Figure 7 summarizes this scavenging hypothesis.
Hypothesis for reduced FVIII efficacy at high concentrations of rD′D3-FP in human plasma. (A) FVIII in human plasma (≈0.3 nM) exists primarily bound to its endogenous carrier protein VWF (≈25 nM dimer) as a result of its low KD (≈0.1 nM). The fast on- and off-rate of this interaction allows switching of FVIII to rD′D3-FP. At micromolar concentrations, most FVIII is expected to be bound to rD′D3-FP. (B) Upon activation of FVIII, its affinity for VWF is drastically reduced, resulting in its dissociation from rD′D3-FP. Some portion of this free FVIIIa is suggested to bind membranes to form the tenase complex. However, at high micromolar concentrations of rD′D3-FP, rebinding of FVIIIa might result in reduced availability of FVIIIa and, in turn, the observed loss of efficacy. HSA, human serum albumin.
Hypothesis for reduced FVIII efficacy at high concentrations of rD′D3-FP in human plasma. (A) FVIII in human plasma (≈0.3 nM) exists primarily bound to its endogenous carrier protein VWF (≈25 nM dimer) as a result of its low KD (≈0.1 nM). The fast on- and off-rate of this interaction allows switching of FVIII to rD′D3-FP. At micromolar concentrations, most FVIII is expected to be bound to rD′D3-FP. (B) Upon activation of FVIII, its affinity for VWF is drastically reduced, resulting in its dissociation from rD′D3-FP. Some portion of this free FVIIIa is suggested to bind membranes to form the tenase complex. However, at high micromolar concentrations of rD′D3-FP, rebinding of FVIIIa might result in reduced availability of FVIIIa and, in turn, the observed loss of efficacy. HSA, human serum albumin.
Using rD′D3-FP as a drug would offer some flexibility compared with approaches, such as FVIIIFc-VWF-XTEN, where D′D3 is linked to FVIII via Fc dimerization.30 An FVIII-independent rD′D3-based drug could extend the half-life of various commercially established FVIII products. Moreover, rD′D3-based therapeutics could be used to extend the half-life of endogenous FVIII in patients with moderate hemophilia A. Nevertheless, the dosing of a half-life–extended rD′D3 variant independently of FVIII would always rely on molar excess to endogenous VWF. Therefore, the reduced efficacy of FVIII at very high concentrations of rD′D3-FP might represent an intrinsic characteristic of this FVIII half-life–extension strategy, even if it was only observed at concentrations way beyond the expected therapeutic dose. The present data also suggest that approaches based on the covalent linkage of FVIII and D′D3, such as FVIIIFc-VWF-XTEN,30,31 would not result in a reduced FVIII efficacy, as the fusion partner D′D3-Fc, once cleaved from activated FVIII, would only be available at negligible concentrations compared with endogenous VWF.
In summary, we show that by coadministering rD′D3-FP with rVIII-SingleChain, the half-life of the latter was extended dose dependently up to fivefold in a broad range of animal species, including a FVIII-ko mouse model, without showing any negative impact on hemostasis at pharmacologically meaningful doses. Therefore, rD′D3-FP may increase low levels of existing FVIII (eg, moderate hemophilia A) or extend the half-life of a coadministered FVIII beyond the level that is currently achieved by existing half-life–extended rFVIII products.
For original data, please contact Sabine Pestel (sabine.pestel@cslbehring.com).
Acknowledgments
The authors thank the Research and Development teams in Marburg and Melbourne for their excellent technical support. Editorial support, which was funded by CSL Behring, was provided by Hannah Johnston and Sarah Angus (Meridian HealthComms).
This work was supported by CSL Behring.
Authorship
Contribution: S.P., S.S., and T.W. designed the research; S.P., H.-W.B., P.C., H.L., and E.R. performed research and collected data; S.P., H.-W.B., P.C., H.L., E.R., A.A., V.T., J.S., and S.S. analyzed and interpreted data; S.P., M.M., and S.K.D. performed statistical analyses; S.P., A.T.-W., P.M.S., and T.W. wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: H.L., V.T., J.S., and M.M. are employees of CSL and CSL Behring. S.P., H.-W.B., P.C., E.R., S.K.D., A.A., A.T.-W., S.S., P.M.S., and T.W. are employees and shareholders of CSL and CSL Behring.
Correspondence: Sabine Pestel, Research, CSL Behring GmbH, Emil-von-Behring Str 76, 35041 Marburg, Germany; e-mail: sabine.pestel@cslbehring.com.
References
Author notes
The full-text version of this article contains a data supplement.


![Time course of rVIII-SingleChain plasma levels after coadministration with rD′D3-FP in rats and rabbits and dependency of FVIII half-life extension on molar excess of rD′D3-FP over endogenous VWF. Plasma levels of rVIII-SingleChain (antigen, dosed at 200 IU/kg in rats [A] and 150 IU/kg in rabbits [B]), alone or coadministered with increasing doses of rD′D3-FP and pdVWF (400 IU/kg in rats and 300 IU/kg in rabbits) as control. The data show the dose-dependent increase in rVIII-SingleChain plasma exposure with increased dosing of rD′D3-FP. The shorter half-life of rVIII-SingleChain coadministered with pdVWF might be explained by a shorter half-life of human VWF in a rat and rabbit model. The horizontal dashed line represents the detection limit of the assay; data are mean ± standard deviation (SD) (n = 2-3). (C) Fold increase in the terminal plasma half-life of rVIII-SingleChain as a function of the molar excess of rD′D3-FP over endogenous VWF for rats (●) and for rabbits (○). The dotted gray line represents a ratio of 1; ie, the terminal plasma half-life of rVIII-SingleChain alone.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/9/10.1182_bloodadvances.2019000999/1/m_advancesadv2019000999f2.png?Expires=1769088803&Signature=cqrto~OlvTyfgtPmdU9hwDrrSv4vL4hAxw8qwtVQIb5IewYrn6Ib2SRcS4ZwRE4PsyHlfa6dsbEmhXhwBRDhvcIl7vbzKSX8Kp-rtQojJbA7WmerL0pd4peH8oYl-WWYuDRHf-WRBqPTv06rjP1wR1R1G3aY1fp9lDqPZ5FMvzt2obMYB~gkPtW9I49Jvf6w0bTQ1LcCQITfmi4r41c63NM~BIBS18In-Lq-fYS7BfHrj8EbH-ECemg6S~yi0kib8jpKu5uw8Vg6I80ImhjGmhSn5Zi3E7qyywazikCfJDo7csOXEdoSg1He~Ml~g9hHu3oLAXvO6nogbbmVasuqtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Impact of rD′D3-FP on aPTT in human plasma. The effect of rD′D3-FP (blue line) or rD′D3-His (red line) on aPTT was assessed in human plasma. A vehicle control (saline, gray lines) was tested as negative control (mean [dashed line] ± SD [dotted lines] of n = 12). rD′D3-FP and rD′D3-His prolonged aPTT. Data are mean ± SD of 3 samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/9/10.1182_bloodadvances.2019000999/1/m_advancesadv2019000999f6.png?Expires=1769088803&Signature=z9Wim1uy47RFduEQTajoBBUglq0i2vZZ7ePudoHOT~I9wdrid~aEg3jfmy9YynF2SkkxP9wgFdSBGoIS7ekKFnDxlMJ8wyY87iFeRZvxTutEreYUKM4JrJEiXALw6W0tCup-dR0BOzhGjLiaysaeXBI4b~OUAou3zy9dR1B3WCvU5BvuYfDX3v13NsJETXoSxBHdll9PQd6~lmpozxlT6TWyS0igixcm7xe3T1qAok0ALUQf22h5jxS~3m2i~vqzH91jZeP32Fi8CKvEutpC1Nmp65O3Q8m5jq7fDwmYwn9SSWdfzTK2FzPF96JH~Af-yQANAezo9-QD~ya4dLgDkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)