Key Points
Postinduction BMB affects responses by Lugano and International Working Group criteria in a minority of FL and DLBCL patients.
Response confirmation by BMB should be reconsidered for future trials in FL, but may still have value in DLBCL.
Abstract
The utility of posttreatment bone marrow biopsy (BMB) histology to confirm complete response (CR) in lymphoma clinical trials is in question. We retrospectively evaluated the impact of BMB on response assessment in immunochemotherapy-treated patients with previously untreated follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) in the phase 3 Study of Obinutuzumab (RO5072759) Plus Chemotherapy in Comparison With Rituximab Plus Chemotherapy Followed by Obinutuzumab or Rituximab Maintenance in Patients With Untreated Advanced Indolent Non-Hodgkin's Lymphoma (GALLIUM; NCT01332968) and A Study of Obinutuzumab in Combination With CHOP Chemotherapy Versus Rituximab With CHOP in Participants With CD20-Positive Diffuse Large B-Cell Lymphoma (GOYA; NCT01287741) trials, respectively. Baseline BMB was performed in all patients, with repeat BMBs in patients with a CR by computed tomography (CT) at end of induction (EOI) and a positive BMB at baseline, to confirm response. Positron emission tomography imaging was also used in some patients to assess EOI response (Lugano 2014 criteria). Among patients with an EOI CR by CT in GALLIUM and GOYA, 2.8% and 4.1%, respectively, had a BMB-altered response. These results suggest that postinduction BMB histology has minimal impact on radiographically (CT)-defined responses in both FL and DLBCL patients. In GALLIUM and GOYA, respectively, 4.7% of FL patients and 7.1% of DLBCL patients had a repeat BMB result that altered response assessment when applying Lugano 2014 criteria, indicating that bone marrow evaluation appears to add little value to response assessment in FL; however, its evaluation may still have merit in DLBCL.
Introduction
Clinical trial response assessments for follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) traditionally mandate bone marrow biopsy (BMB) at baseline, and to confirm complete response (CR).1,2 A key advantage of BMB is the acquisition of histologic material; however, BMBs are expensive and cause discomfort to patients.3
The utility of posttreatment BMBs is being reevaluated, in part because of the high sensitivity of positron emission tomography/computed tomography (PET/CT) imaging to detect bone marrow (BM) involvement in both FL and DLBCL.4-7 There is also evidence that repeat BMBs rarely change radiographic response assessment in FL.8 To investigate the requirement for posttreatment BMBs in a larger, broader patient population, we analyzed the impact of confirmatory biopsies on response assessments in FL and DLBCL patients enrolled in the randomized phase 3 Study of Obinutuzumab (RO5072759) Plus Chemotherapy in Comparison With Rituximab Plus Chemotherapy Followed by Obinutuzumab or Rituximab Maintenance in Patients With Untreated Advanced Indolent Non-Hodgkin's Lymphoma (GALLIUM; NCT01332968) and A Study of Obinutuzumab in Combination With CHOP Chemotherapy Versus Rituximab With CHOP in Participants With CD20-Positive Diffuse Large B-Cell Lymphoma (GOYA; NCT01287741) trials.
Study design
In GALLIUM, patients with previously untreated FL received obinutuzumab (GA101; G) or rituximab (R) plus chemotherapy (cyclophosphamide, vincristine, doxorubicin, and prednisone [CHOP]; cyclophosphamide, vincristine, and prednisone; or bendamustine) as induction therapy, followed by maintenance with the same antibody in responders. In GOYA, patients with previously untreated DLBCL received obinutuzumab-CHOP or rituximab-CHOP as induction therapy only. Trial design, patient selection criteria, and treatment regimens for both studies have been reported previously.9,10 GALLIUM and GOYA were conducted in accordance with the Declaration of Helsinki and approved by the institutional review board or independent ethics committee of each institution. All patients provided written and informed consent.
In both trials, BMB was performed at baseline in all patients in the intent-to-treat populations and assessed by local pathology review. BMB cores were required to be >20 mm to be considered adequate for assessment. Morphology was used to determine lymphoma involvement. If morphology was inconclusive, immunohistochemistry was required. In GALLIUM, PET imaging (where available) was mandatory in the first 170 patients and optional thereafter. PET scans in GOYA were mandatory where a PET scanner was available. 18F-fluorodeoxyglucose (FDG)-PET was used to assess response at end of induction (EOI; by independent review committee [IRC] evaluation) according to Lugano 2014 response criteria.11 Response was also assessed from CT scans at EOI by the investigator according to International Working Group (IWG) 2007 response criteria.3 Repeat BMBs were performed in patients with positive BMBs at baseline and CR by CT, to confirm the response. Repeat BMB to confirm complete metabolic response (CMR) by PET was not required in either trial. The results of radiology studies were not used to guide the site of BMBs.
Results and Discussion
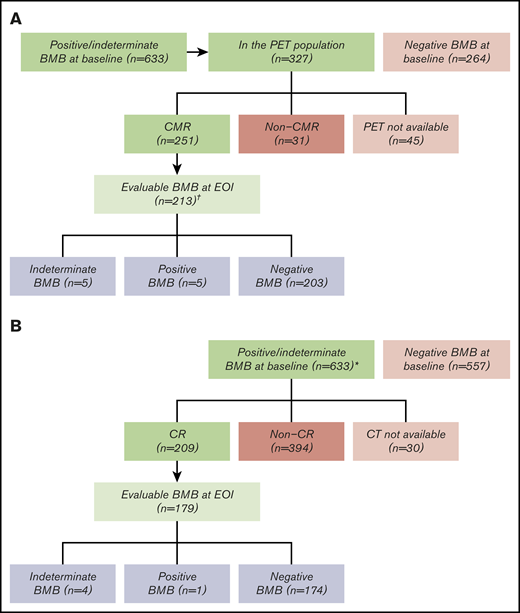
Of the 1202 FL patients in GALLIUM, 633 (52.7%) had a positive (n = 613; 51.0%) or indeterminate (n = 20; 1.7%) baseline BMB, with data missing for 12 (1.0%) (Figure 1). BM involvement was not prognostic for progression-free survival (PFS) in FL patients (hazard ratio, 0.99; 95% confidence interval, 0.80–1.23; supplemental Figure 1). Of the 633 patients with positive/indeterminate baseline BMB, 282 (44.5%) were PET evaluable at EOI. Lugano 2014 criteria were used by the IRC for response assessment in these patients. Overall, 251/282 (89.0%) patients had a CMR, and 213 (84.9%) of these underwent confirmatory BMB at EOI (Figure 1A). BMB results only altered response by Lugano 2014 criteria for 10/213 (4.7%) patients (5 positive, 5 indeterminate; Figure 1A) with a repeat BMB. All patients with a positive BMB at baseline or posttreatment had FL. Of the 5 FL patients with a positive BMB at EOI, 1 progressed 8 months after EOI, and 4 maintained their response between 60 and 84 months of follow-up. Based on CT IWG criteria, 209/633 (33.0%) patients with BM involvement at baseline had a CR at EOI, 179 of whom had a follow-up BMB to confirm response (Figure 1B). Only 5/179 (2.8%) patients (5/1202 patients [0.4%] enrolled in GALLIUM) had a repeat BMB result that altered response assessment.
Bone marrow biopsy at the end of induction to confirm complete responses in the GALLIUM trial. The number of evaluable patients is shown according to BMB result and radiologic response. (A) Postinduction BMB to confirm CMR according to PET. (B) Postinduction BMB to confirm CR according to CT. *Only patients with a CR by CT had a repeat biopsy. †Positive BMB, n = 613 (51.0%); indeterminate BMB, n = 20 (1.7%); data missing, n = 12 (1.0%).
Bone marrow biopsy at the end of induction to confirm complete responses in the GALLIUM trial. The number of evaluable patients is shown according to BMB result and radiologic response. (A) Postinduction BMB to confirm CMR according to PET. (B) Postinduction BMB to confirm CR according to CT. *Only patients with a CR by CT had a repeat biopsy. †Positive BMB, n = 613 (51.0%); indeterminate BMB, n = 20 (1.7%); data missing, n = 12 (1.0%).
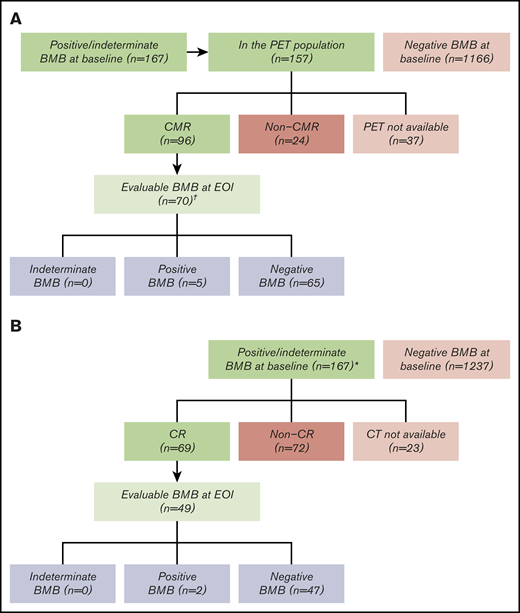
Of the 1418 DLBCL patients in GOYA, 167 (11.8%) had a positive (n = 153; 10.8%) or indeterminate (n = 14; 1.0%) baseline BMB, with data missing for 14 (1.0%) (Figure 2). BM involvement at baseline was prognostic for shorter PFS (hazard ratio, 0.75; 95% confidence interval, 0.56-0.99; 3-year PFS rate 55.3% vs 69.8% for positive vs negative/indeterminate; supplemental Figure 2). Of the 167 patients with positive/indeterminate baseline BMB, 121 (72.5%) were PET evaluable at EOI. Lugano 2014 criteria were used for response assessment by the IRC in these patients. A total of 96/121 (79.3%) patients had a CMR, and 70/96 (72.9%) patients underwent confirmatory BMB (Figure 2A). BMB histology information is not available in GOYA. BMB results altered response assessment for 5/70 (7.1%) patients (5 positive; Figure 2A) with a repeat BMB. Of these 5 patients, 4 progressed between 6 and 36 months after EOI, and 1 maintained a response until study completion. Sixty-nine (41.3%) of the 167 DLBCL patients had a CR on CT according to IWG 2007 criteria and 49 (71.0%) of these had a follow-up BMB (Figure 2B). Similar to the GALLIUM study, just 2/49 (4.1%) DLBCL patients (2/1418 [0.1%] enrolled in GOYA) with a positive/indeterminate baseline BMB and EOI CR by CT had a repeat BMB result that impacted the response assessment.
Bone marrow biopsy at the end of induction to confirm complete responses in the GOYA trial. The number of evaluable patients is shown according to BMB result and radiologic response. (A) Postinduction BMB to confirm CMR according to PET. (B) Postinduction BMB to confirm CR according to CT. *Only patients with a CR by CT had a repeat biopsy. †Positive BMB, n = 153 (10.8%); indeterminate BMB, n = 14 (1.0%); data missing, n = 14 (1.0%).
Bone marrow biopsy at the end of induction to confirm complete responses in the GOYA trial. The number of evaluable patients is shown according to BMB result and radiologic response. (A) Postinduction BMB to confirm CMR according to PET. (B) Postinduction BMB to confirm CR according to CT. *Only patients with a CR by CT had a repeat biopsy. †Positive BMB, n = 153 (10.8%); indeterminate BMB, n = 14 (1.0%); data missing, n = 14 (1.0%).
Our study was limited by PET imaging not being available for response assessment in all study patients, BMB confirmation of CMR not being required, and ∼15% of patients had missing BMB information. Postinduction BMB histology results only altered the response assessment by Lugano 2014 criteria in a minority of patients with previously untreated FL or DLBCL from the GALLIUM and GOYA studies (<8% of patients with an initial positive/indeterminate BMB and EOI CMR by PET who underwent repeat BMB; 0.4%-0.8% of all enrolled patients). Similar results were seen with IWG 2007 criteria (<5% of patients with an initial positive/indeterminate BMB and EOI CR by CT who underwent repeat BMB; 0.1%-0.4% of all enrolled patients). Importantly, our results using IWG 2007 criteria validate an earlier, smaller study in FL that demonstrated a possible response assessment change in 1/99 (1.0%) patients enrolled in studies for which the IWG 2007 criteria was the most commonly used response assessment method.8 Taken together, these data suggest that response confirmation by BMB provides little additional value over radiographic imaging in FL patients. We acknowledge that with emerging prognostic data regarding minimal residual disease (MRD) status in FL, there may be a role for BMB MRD in certain contexts when PET is used.12-15 There is also preliminary evidence that FL patients with negative MRD status and a CMR by PET may have longer PFS compared with either of these findings alone.16 Our results regarding BMB in DLBCL patients undergoing PET are limited because the decision to repeat the BMB was directed by a CT-CR rather than PET-CMR; further research is needed to make definitive conclusions. In addition, investigators should consider incorporating newer strategies, such as peripheral blood MRD and/or cell-free DNA into clinical trials.17-19
When all patients enrolled in GOYA and GALLIUM are included in this analysis, BMB affected response assessment in <1%. Despite the limitations of our study, we believe that the requirement for histologic assessment of BMB to confirm CR by CT should be reconsidered for future trials in FL. Similarly, the value of BMB assessment in FL patients with a CMR may be limited. However, given the conventional use of the Lugano 2014 criteria in response assessment, investigations should continue to evaluate the potential value of BMBs in DLBCL when applying these criteria.
Presented in abstract form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 4 December 2018 (Abstract P-1605).
Acknowledgments
GALLIUM and GOYA were sponsored by F. Hoffmann-La Roche Ltd. Third-party medical writing assistance, under the direction of S.C.R., was provided by Helen Cathro and Zoe Toland of Gardiner-Caldwell Communications, which was funded by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: All authors were involved with the study design, study conduct, and data interpretation; M.H., W.H., L.K., R.M., M.M., L.H.S., M.T., J.T., and U.V. were involved with the recruitment and follow-up of patients; M.H., W.H., L.K., R.M., M.M., L.H.S., M.T., J.T., and U.V. were involved with data collection; S.C.R., T.N., F.M., D.S., G.S., and P.M. were involved with the data analysis; and all authors were involved in the writing of the manuscript, provided their final approval of the manuscript, and are accountable for all aspects of this work.
Conflict-of-interest disclosure: S.C.R. has served as a consultant to AstraZeneca, Celgene, Heron, Juno, Janssen, Karyopharm, Seattle Genetics, and Verastem, and has received research funding from Genentech. J.T. has received research funding from F. Hoffmann-La Roche, Janssen, Celgene, Beigene, and PCYC; support for travelling to a meeting from Roche; and is an unremunerated member of an advisory board for F. Hoffmann-La Roche, Janssen, Celgene, and Takeda. M.H. has received research funding from F. Hoffmann-La Roche and is a member on an entity's Board of Directors or advisory committees for F. Hoffmann-La Roche, Gilead Sciences, Celgene, and Janssen. W.H. has received consultancy fees from F. Hoffmann-La Roche, Gilead Sciences, Janssen, Vector Therapeutics, Celgene; research funding from F. Hoffmann-La Roche, Janssen, and Bayer; and honoraria from F. Hoffmann-La Roche, Gilead Sciences, Janssen, Vector Therapeutics, and Celgene. L.K. has received consultancy from F. Hoffmann-La Roche/Genentech. R.M. has received consultancy at Gilead Sciences and paid advisor, speaker's bureau, and honoraria from F. Hoffmann-La Roche/Genentech. M.M. has received honoraria from F. Hoffmann-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead Sciences, Servier, and is a member on an entity's Board of Directors for Celgene, Janssen, Sandoz, Novartis, and Gilead Sciences. L.H.S. has received honoraria and consultancy fees from F. Hoffmann-La Roche/Genentech, AbbVie, Amgen, Apobiologix, AstraZeneca, Acerta, Celgene, Gilead Sciences, Janssen, Kite, Karyopharm, Lundbeck, Merck, MorphoSys, Seattle Genetics, Teva, Takeda, TG Therapeutics, and Verastem. M.T. has received honoraria at Janssen, Gilead Sciences, Takeda, Bristol-Myers Squibb, Amgen, AbbVie, F. Hoffmann-La Roche, MorphoSys, and Incyte, and consultancy fees from Takeda, Bristol-Myers Squibb, Incyte, AbbVie, Amgen, F. Hoffmann-La Roche, Gilead Sciences, Janssen, Celgene, and MorphoSys. U.V. is a member on an advisory board for Janssen, Celgene, Kite, Juno Therapeutics, and Novartis and speaker’s bureau for Celgene, F. Hoffmann-La Roche, Janssen, Gilead Sciences, AbbVie, and Novartis. D.S., F.M., G.S., and T.N. are employees of F. Hoffmann-La Roche. T.N., D.S., and G.S. have ownership interests in F. Hoffmann-La Roche. P.M. is a consultant for Acerta, Bayer, Celgene, I-MAB, Janssen, Karyopharm, Regeneron, Sandoz. and Teneobio and has received research support from Karyopharm.
Correspondence: Sarah C. Rutherford, Department of Medicine, Weill Cornell Medicine, 1305 York Ave, 7th Floor, New York, NY 10021; e-mail: sar2014@med.cornell.edu.
References
Author notes
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche's criteria for eligible studies are available at https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
The full-text version of this article contains a data supplement.