Key Points
Affinity-matured IgG can promote a robust primary cellular and humoral immune response to an RBC antigen, including memory.
Isolated as an independent variable, IgG subtype alters the immunoregulatory effects of passive IgG.
Abstract
Antibodies are typically thought of as the endpoint of humoral immunity that occur as the result of an adaptive immune response. However, affinity-matured antibodies can be present at the initiation of a new immune response, most commonly because of passive administration as a medical therapy. The current paradigm is that immunoglobulin M (IgM), IgA, and IgE enhance subsequent humoral immunity. In contrast, IgG has a “dual effect” in which it enhances responses to soluble antigens but suppresses responses to antigens on red blood cells (RBCs) (eg, immunoprophylaxis with anti-RhD). Here, we report a system in which passive antibody to an RBC antigen promotes a robust cellular immune response leading to endogenous CD4+ T-cell activation, germinal center formation, antibody secretion, and immunological memory. The mechanism requires ligation of Fcγ receptors on a specific subset of dendritic cells that results in CD4+ T-cell activation and expansion. Moreover, antibodies cross-enhance responses to a third-party antigen, but only if it is expressed on the same RBC as the antigen recognized by the antibody. Importantly, these observations were IgG subtype specific. Thus, these findings demonstrate that antibodies to RBC alloantigens can enhance humoral immunity in an IgG subtype-specific fashion and provide mechanistic elucidation of the enhancing effects.
Introduction
Antibodies are typically considered an effector endpoint of acquired humoral immunity. However, at least as early as the 1890s, the administration of antibodies to naïve animals has been known to regulate how humoral immunity develops.1 Since that time, all antibody isotypes and subclasses have been shown to have the capacity to regulate humoral immunity, with the exception of immunoglobulin D (IgD; a number of recent reviews are available).2-4
The generalization that has been inferred from existing data are that IgM, IgA, and IgE result in enhancement of humoral immunity, whereas IgG has a “dual effect” with the capacity to enhance responses to soluble protein antigens but suppress responses to antigens on the surface of red blood cells (RBCs).3 The term antibody-mediated immune suppression (AMIS) has been applied to this latter effect on RBC antigens. AMIS has been extensively studied in humans through the development anti-RhD (anti-D) as an immune prophylactic in the 1960s.5,6 Moreover, IgG-mediated AMIS has been widely observed with regard to RBC antigens in animal models.7-19 Of particular note is a study by Enriquez-Rincon and Klaus, which supports the dual effect interpretation of IgG by showing that the same anti-hapten IgG causes enhancement or suppression to haptenated soluble protein or RBCs, respectively.20
However, there are empirical contradictions to the paradigm that IgG only suppresses responses to RBCs antigens, with several reports of polyclonal antisera to RBCs enhancing immunity in rabbits or humans.21-23 Moreover, attempts to generate therapeutic monoclonal anti-D in humans demonstrated that whereas some monoclonal antibodies caused suppression, others caused increased numbers of immunized subjects and with increased kinetics of immunization.24-26
Here, we report that the presence of IgG at the time of initial antigen exposure significantly enhances both the kinetics and magnitude of the immune response to a model antigen expressed exclusively on RBCs. This effect is a function of the IgG subtype present. Using this system, a mechanistic elucidation of the resulting immunoregulation is carried out.
Materials and methods
Mice
Immunizations and isolation of anti-HOD (PUMA6) monoclonal antibody
RBCs expressing the human Fya antigen (unpublished animals created by our laboratory and available upon request) were transfused into RBF/DnJ recipients pretreated with polyinosinic:polycytidylic.29 Spleens were fused with a myeloma partner and a new monoclonal antibody (PUMA6) was isolated that binds to an epitope common to Fya and Fyb. The coding regions for both heavy and light chain from PUMA6 were isolated, cloned in frame with expression vectors for IgG switch variants, and transfected into CHO cells. PUMA6 IgG switch variants were purified to homogeneity using protein A/G chromatography.
Blood collection, transfusion, and monitoring or recipient immune response
Donor wild-type B6 and HOD blood was collected and labeled as previously described.30 Mice were infused (as indicated) with PUMA6 or PUMA1 monoclonal antibodies followed by an RBC transfusion consisting of 50 µL packed DiO labeled HOD and 50 µL packed DiI labeled B6 RBCs. Posttransfusion RBC recovery and survival was assessed by flow cytometry as previously described.30 In some experiments, 24 hours before passive immunization, recipient mice were given an adoptive transfer 1 × 106 CD4+ T cells from splenocytes of OTII × B6.Thy1.1 F1 mice. CD4+ T cells were purified by negative selection (Thermo Fisher) and labeled with 10 µM CellTrace-Far Red (Thermo Fisher) before adoptive transfer. Direct antibody tests and flow crossmatch (sera 1:100) were performed as described previously.31 Indirect antibody tests, to detect the HOD antigen, were performed with MIMA-29,32 a monoclonal antibody against Duffy (Fy3) (1:100), followed by goat anti-mouse IgG2a APC. Lymphatic memory was assessed by standard methods (detailed in supplemental Methods).
Statistical analysis
Statistical significance for longitudinal analysis of serum antibodies was determined with a repeated-measures analysis of variance (ANOVA) followed by a Dunnett multiple comparisons test against phosphate-buffered saline (PBS) control. For analysis of multiple groups at 1 time point, appropriate 2-way ANOVA and 1-way ANOVA with a Dunnett multiple comparisons test was used. Significance was set at P ≤ .05, where *P ≤ .05, **P ≤ .01, ***P ≤ .001, and ****P ≤ .0001.
Additional details are given in supplemental Methods.
Results
Individual IgG subtypes have differential effects on RBC clearance and alloantibody production
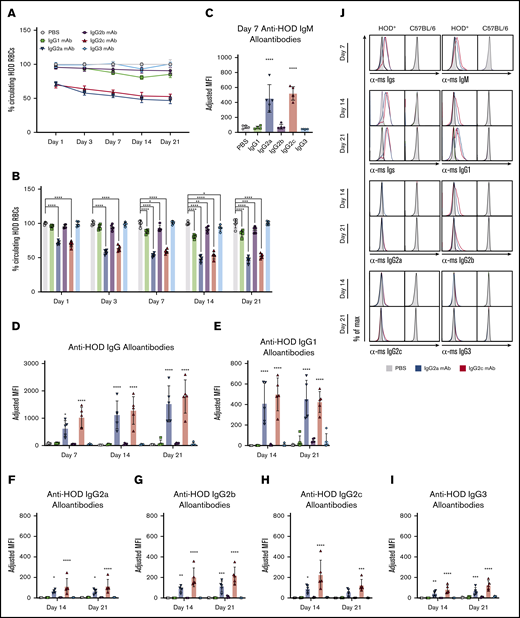
RBC donor mice express a transgenic alloantigen (HOD).27 A novel monoclonal antibody specific for the Duffy portion of the HOD antigen was generated (referred to as anti-HOD monoclonal antibody (anti-HOD mAb) in the rest of this manuscript) and was expressed as a panel of IgG switch variants of the IgG1, IgG2a, IgG2b, IgG2c, and IgG3 subtypes. C57BL/6 (B6) mice were passively immunized with 1 µg of anti-HOD mAb of each IgG subtype or PBS (control) followed by a transfusion of HOD RBCs (experimental design shown in Figure 1). RBC survival and alloantibody production were evaluated over 21 days posttreatment.
Experimental design. Recipient mice received a passive immunization with 1 μg of anti-HOD monoclonal antibody (anti-HOD mAb), nonspecific mAb, or PBS control followed by an RBC transfusion. Each transfusion consisted of 100 μL of packed leukoreduced RBCs at 20% hematocrit, with 50 μL of allogeneic HOD RBCs, and 50 μL syngeneic B6 RBCs, labeled with DiO and DiI lipophilic dyes, respectively. Before transfusion, labeled HOD-DiO and B6-DiI RBCs were mixed at a 1:1 ratio and analyzed on a flow cytometer to determine the pretransfusion ratio. After transfusion, RBCs and sera were collected from experimental mice and used to determine RBC survival and alloantibody production.
Experimental design. Recipient mice received a passive immunization with 1 μg of anti-HOD monoclonal antibody (anti-HOD mAb), nonspecific mAb, or PBS control followed by an RBC transfusion. Each transfusion consisted of 100 μL of packed leukoreduced RBCs at 20% hematocrit, with 50 μL of allogeneic HOD RBCs, and 50 μL syngeneic B6 RBCs, labeled with DiO and DiI lipophilic dyes, respectively. Before transfusion, labeled HOD-DiO and B6-DiI RBCs were mixed at a 1:1 ratio and analyzed on a flow cytometer to determine the pretransfusion ratio. After transfusion, RBCs and sera were collected from experimental mice and used to determine RBC survival and alloantibody production.
Recipient mice passively immunized with anti-HOD mAb IgG2a or IgG2c had significantly reduced survival of HOD RBCs by 24 hours posttransfusion, which persisted throughout the 21-day time course evaluated (Figures 2A-B, P ≤ .0001). In contrast, no significant decrease in HOD RBCs was observed at 24 hours posttransfusion in response to other IgG subtypes. A slight decrease in HOD RBC circulation was seen at later time points in response to IgG1 and IgG2b. The survival kinetic differences observed with individual IgG subtypes was not due to lack of mAb binding or HOD antigen modulation because similar percentages of HOD RBCs had detectable antibodies bound to their surface and similar amounts of HOD antigen were detectable, regardless of treatment (supplemental Figure 1). Together, these data show that the ability of IgG to clear RBCs is a function of IgG subtype and that most clearance occurs by 24 hours, followed by stable circulation of surviving RBCs.
Passive immunization with anti-HOD mAb IgG2a or IgG2c enhances alloantibody production upon HOD RBC transfusion. Recipient mice received a passive immunization of individual anti-HOD mAb subtypes followed by an RBC transfusion. RBCs and sera were collected at multiple time points over 21 days. (A) The survival of allogeneic HOD RBCs, as a function of control B6 RBCs, was calculated. (B) The percentage of circulating HOD RBCs was determined. The percentage of HOD RBCs in PBS-treated animals were normalized to 100% across all time points. Sera were analyzed by flow crossmatch against HOD and control B6 RBCs for IgM (C), Igs (D), IgG1 (E), IgG2a (F), IgG2b (G), IgG2c (H), and IgG3 (I) anti-HOD alloantibodies. (J) Representative flow plots of flow crossmatch data are provided. Each experiment was performed 3 times with 3 to 5 mice per group. Representative data are shown. Data were analyzed with a repeated measures 2-way ANOVA with Dunnett multiple comparisons to PBS-treated animals. *P ≤ .05, **P ≤ .01, ***P ≤ .001, ****P ≤ .0001.
Passive immunization with anti-HOD mAb IgG2a or IgG2c enhances alloantibody production upon HOD RBC transfusion. Recipient mice received a passive immunization of individual anti-HOD mAb subtypes followed by an RBC transfusion. RBCs and sera were collected at multiple time points over 21 days. (A) The survival of allogeneic HOD RBCs, as a function of control B6 RBCs, was calculated. (B) The percentage of circulating HOD RBCs was determined. The percentage of HOD RBCs in PBS-treated animals were normalized to 100% across all time points. Sera were analyzed by flow crossmatch against HOD and control B6 RBCs for IgM (C), Igs (D), IgG1 (E), IgG2a (F), IgG2b (G), IgG2c (H), and IgG3 (I) anti-HOD alloantibodies. (J) Representative flow plots of flow crossmatch data are provided. Each experiment was performed 3 times with 3 to 5 mice per group. Representative data are shown. Data were analyzed with a repeated measures 2-way ANOVA with Dunnett multiple comparisons to PBS-treated animals. *P ≤ .05, **P ≤ .01, ***P ≤ .001, ****P ≤ .0001.
To test the effects of different anti-HOD IgG subtypes upon a subsequent endogenous humoral immune response to HOD, sera were collected on days 7, 14, and 21 following HOD RBC transfusion and assayed for anti-HOD antibodies using a flow cytometry-based crossmatch assay. Consistent with previous reports, transfusion of HOD RBCs into control mice treated with PBS led to low levels of anti-HOD alloantibody production (Figure 2C-D). However, pretreatment with anti-HOD mAb IgG2a or IgG2c significantly enhanced both anti-HOD IgM at 7 days and IgG alloantibodies at later time points (Figure 2C-D). No significant effects on alloimmunization were observed with anti-HOD mAb IgG1, IgG2b, or IgG3 compared with PBS controls.
Subtype specific analysis of the collected sera indicated that IgG1 and IgG2b were the predominate subtypes produced (Figure 2E-J). Low levels of IgG2a, IgG2c, and IgG3 were also observed. Because the increased anti-HOD alloantibodies were mostly of an IgG subtype different than those injected, this rules out the concern that the signal was simply detecting the mAb used for passively immunization. IgG2a and IgG2c are genetic variants that occupy the same loci: B6 mice express IgG2c and not IgG2a. Accordingly, the detection of IgG2a in mice not given anti-HOD IgG2a is likely a cross-reactivity of the anti-IgG2a antisera with IgG2c generated in the mice.
Together, these data demonstrate that preexisting RBC-specific antibodies can modulate adaptive immune responses to RBC antigens. Further, these results show that individual antibody subtypes have differential effects on alloantibody production.
Generation of a new mouse: conditional deletion of the FcR common γ chain, FcRγfl/fl
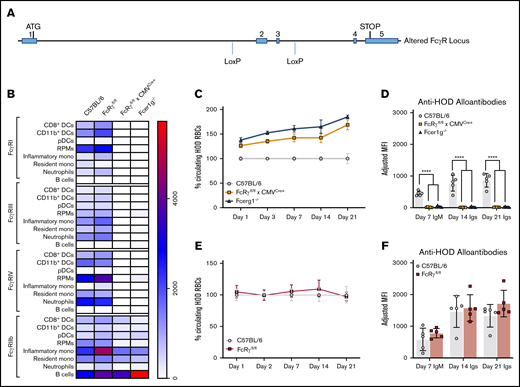
To test the hypothesis that Fc-γ receptors (FcγRs) are involved in immunoregulatory effects of passive antibody, a new mouse was generated in which the common γ-chain allele of the Fc receptor was flanked with LoxP sites (FcRγfl/fl), thereby creating a conditional knockout. This approach was used to allow the removal of activating FcγRs (ie, FcγRI, FcγRIII, and FcγRIV) because each of these requires the common γ-chain for surface expression (Figure 3A). Crossing of FcRγfl/fl mice with mice expressing germline Cre (which will exert its effects in all tissue of progeny) demonstrated deletion of the floxed region by polymerase chain reaction (data not shown). Splenocytes from FcRγfl/fl and FcRγfl/fl x CMVCre+ mice were harvested and stained with antibodies to differentiate FcγR expression on multiple leukocyte populations (Figure 3B; supplemental Figure 2). Commercially available mice with a nonconditional deletion of the common γ chain (Fcer1g−/−) and wild-type B6 mice were used as controls.
Generation of a new FcRγfl/fl mouse. A new mouse was designed to have conditional expression of activating Fc receptors. (A) Fcer1g gene locus showing insertion of LoxP recognition sequences flanking exons 2 and 3. Splenocytes were harvested from B6, FcRγfl/fl, FcRγfl/fl × CMVCre+, and Fcer1g−/− mice and (B) evaluated for expression of FcγRI, FcγRIII, FcγRIV, and FcγRIIb on multiple leukocyte subsets; MFIs of FcγRs are displayed in a heat map. To evaluate whether insertion of LoxP sites interfered with clearance and alloantibody production, B6 and FcRγfl/fl mice were passively immunized with anti-HOD mAb followed by an RBC transfusion. HOD RBC survival (C) and anti-HOD alloantibodies (D) were evaluated over 21 days. In analogous experiments, immune responses in FcRγfl/fl × CMVCre+, and Fcer1g−/− were directly compared and HOD RBC survival (E) and anti-HOD alloantibody production (F) were evaluated. Each experiment was performed 3 times with 3 to 5 mice per group. Representative data are shown. Data were analyzed with a repeated measures 2-way ANOVA with multiple comparisons test to control B6 mice. ****P ≤ .0001.
Generation of a new FcRγfl/fl mouse. A new mouse was designed to have conditional expression of activating Fc receptors. (A) Fcer1g gene locus showing insertion of LoxP recognition sequences flanking exons 2 and 3. Splenocytes were harvested from B6, FcRγfl/fl, FcRγfl/fl × CMVCre+, and Fcer1g−/− mice and (B) evaluated for expression of FcγRI, FcγRIII, FcγRIV, and FcγRIIb on multiple leukocyte subsets; MFIs of FcγRs are displayed in a heat map. To evaluate whether insertion of LoxP sites interfered with clearance and alloantibody production, B6 and FcRγfl/fl mice were passively immunized with anti-HOD mAb followed by an RBC transfusion. HOD RBC survival (C) and anti-HOD alloantibodies (D) were evaluated over 21 days. In analogous experiments, immune responses in FcRγfl/fl × CMVCre+, and Fcer1g−/− were directly compared and HOD RBC survival (E) and anti-HOD alloantibody production (F) were evaluated. Each experiment was performed 3 times with 3 to 5 mice per group. Representative data are shown. Data were analyzed with a repeated measures 2-way ANOVA with multiple comparisons test to control B6 mice. ****P ≤ .0001.
In control B6 and FcRγfl/fl mice, activating FcγRI, FcγRIII, and FcγRIV were detected with similar expression patterns on dendritic cells (DCs), red pulp macrophages (RPMs), monocytes, neutrophils, and B cells, suggesting that the addition of LoxP sites did not significantly alter the expression of FcγRs in FcRγfl/fl mice. In FcRγfl/fl × CMVCre+ mice, little to no expression of activating FcγRs was detectable and patterns were similar to splenocytes from Fcer1g−/− mice. The inhibitory FcγRIIb receptor, which does not require the common γ chain for expression or function, was generally similar between all mice analyzed; however, there was a significant reduction in expression of FcγRIIb in inflammatory monocytes harvested from either FcRγfl/fl × CMVCre+ or Fcer1g−/− mice, when compared with control B6 (supplemental Table 1). Similarly, neutrophils from FcRγfl/fl x CMVCre+ and resident monocytes from Fcer1g−/− also had significant reductions in FcγRIIb expression compared with B6. Direct comparison of FcγR expression between FcRγfl/fl × CMVCre+ and Fcer1g−/− mice revealed significant enhancement of FcγRIIb on B cells harvested from Fcer1g−/− mice (Figure 3B, P ≤ .01). Taken together, FcRγfl/fl mice and control B6 mice have similar activating FcγR phenotypic profiles across multiple leukocyte populations (eg, DCs, monocytes), and when crossed with a ubiquitous cre-expressing mouse, FcRγfl/fl × CMVCre+ mice are phenotypically similar to Fcer1g−/− in that activating FcγRs are undetectable on the majority of leukocyte populations analyzed.
To test the hypothesis that FcγRs are involved antibody-mediated enhancement of humoral immunity, the same experimental design was carried out (Figure 1) using both FcRγfl/fl × CMVCre+ and Fcer1g−/− mice and anti-HOD mAb IgG2c. Both clearance of RBCs and enhancement of alloimmunization by IgG2c were significantly reduced, to similar levels, in FcRγfl/fl × CMVCre+ and Fcer1g−/− recipients, compared with control B6 mice (Figure 3C-D, P ≤ .0001). Importantly, no significant differences in either HOD RBC clearance or magnitude of anti-HOD alloantibody responses in response to IgG2c were observed between FcRγfl/fl (never having encountered Cre) and control B6 mice over 21 days (Figure 3E-F), demonstrating that the altered biology in FcRγfl/fl × CMVCre+ was due to deletion of the γ chain and not an artifact of insertion of the LoxP sites. Together, these data demonstrate that the general mechanism by which IgG2c enhances RBC alloimmunization works through common γ chain-dependent gene product, presumably activating FcγRs.
The FcR γ-chain in CD11c or Zbtb-expressing cells is required for enhanced anti-HOD alloantibody production
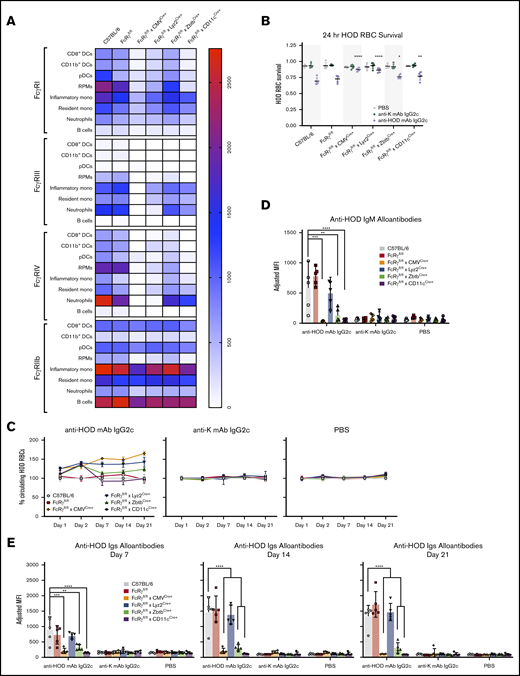
FcRγfl/fl mice were bred with: (1) Lyz2Cre+ promoter, which is expressed by macrophages, monocytes, and neutrophils; (2) ZbtbCre+ promotor, which is reportedly expressed exclusively by conventional DCs (cDCs) (CD8+CD11b− and CD11b+CD8− DCs) with minimal functional consequences to plasmacytoid DCs (pDCs); and (3) CD11cCre+ promotor, which is expressed predominantly by pre-cDC progenitors as well as pDCs and tissue-derived DCs with additional expression on some macrophage populations, but little expression on other myeloid cells.
Initially, we tested the relative cell surface expression of individual FcγRs on individual leukocyte populations for each strain (Figure 4A; supplemental Figure 3). As predicted, there were significant reductions of FcγRI, FcγRIII, and FcγRIV expression in myeloid cells (eg, monocytes) from FcRγfl/fl × Lyz2Cre+ mice (all statistical comparisons are provided in supplemental Table 2); however, DC subsets also had diminished expression of all activating FcγRs. Unexpectedly, in FcRγfl/fl × ZbtbCre+ mice, FcγRI expression was unaltered in cDCs but significantly reduced in pDCs and RPMs. In contrast, FcγRIV expression in cDCs was significantly lower; however, RPM and neutrophil populations had significantly reduced expression of both FcγRIII and FcγRIV. Finally, FcRγfl/fl × CD11cCre+ mice were phenotypically very similar to FcRγfl/fl × CMVCre+ whereby expression of activating FcγRs was reduced to extremely low levels across multiple antigen-presenting cell (APC) subsets; only monocyte and neutrophil populations retained low levels of expression above what was observed in FcRγfl/fl × CMVCre+.
Absence of activating FcγR expression in cells driven by CD11c or Zbtb promoters abrogates RBC alloimmunization. (A) FcRγfl/fl mice were bred with cre-expressing transgenic mice and spleens were harvested and stained with antibodies to delineate cell subsets and FcγR expression and relative MFIs of individual FcγRs are displayed as a heat map. To assess the functional consequence of modulating expression of activating FcγRs, recipients were passively immunized with anti-HOD mAb IgG2c, nonspecific anti-K mAb IgG2c, or control PBS followed by an RBC transfusion (as described in Figure 1). Survival of HOD RBCs was calculated at 24 hours posttransfusion (B) and over a 21-day time course (C). IgM (D) and total Igs (E) anti-HOD alloantibodies were determined for days 7, 14, and 21 by flow crossmatch. Each experiment was performed 3 times with 3 to 5 mice per group. Representative data are shown. Data were analyzed with a 1-way ANOVA with Dunnett multiple comparisons test to control B6 mice. *P ≤ .05, **P ≤ .01, ***P ≤ .001, ****P ≤ .0001.
Absence of activating FcγR expression in cells driven by CD11c or Zbtb promoters abrogates RBC alloimmunization. (A) FcRγfl/fl mice were bred with cre-expressing transgenic mice and spleens were harvested and stained with antibodies to delineate cell subsets and FcγR expression and relative MFIs of individual FcγRs are displayed as a heat map. To assess the functional consequence of modulating expression of activating FcγRs, recipients were passively immunized with anti-HOD mAb IgG2c, nonspecific anti-K mAb IgG2c, or control PBS followed by an RBC transfusion (as described in Figure 1). Survival of HOD RBCs was calculated at 24 hours posttransfusion (B) and over a 21-day time course (C). IgM (D) and total Igs (E) anti-HOD alloantibodies were determined for days 7, 14, and 21 by flow crossmatch. Each experiment was performed 3 times with 3 to 5 mice per group. Representative data are shown. Data were analyzed with a 1-way ANOVA with Dunnett multiple comparisons test to control B6 mice. *P ≤ .05, **P ≤ .01, ***P ≤ .001, ****P ≤ .0001.
Conditional knockouts using each Cre strain were passively immunized with anti-HOD mAb IgG2c followed by transfusion with HOD RBCs (the same experimental design as Figure 1). To control for nonspecific antibody effects that were not a function of epitope binding, anti-K mAb IgG2c (with specificity against human Kell antigen and not expressed in this system) was used as a control. IgG2c-induced clearance of HOD RBCs was significantly decreased in both FcRγfl/fl × CMVCre+ and FcRγfl/fl × Lyz2Cre+ mice, and to a lesser extent in both FcRγfl/fl × ZbtbCre+ and FcRγfl/fl × CD11cCre+. Moreover, early differences in clearance were observed throughout the 21-day time course (Figure 4C, left). The observed clearance patterns were a function of antibody-binding RBCs and neither an artifact of nonspecific antibody effects nor from changes in RBC biology in recipient strains because HOD RBCs had essentially identical clearance across all recipient groups treated with either anti-K mAb IgG2c (Figure 4C, middle) or PBS (Figure 4C, right).
IgG2c enhancement of RBC alloimmunization was significantly blunted (both IgM and total Igs) in FcRγfl/fl × CMVCre+, FcRγfl/fl × ZbtbCre+, and FcRγfl/fl × CD11cCre+ mice (Figure 4D-E). Unexpectedly, despite the observed reduction in clearance of circulating HOD RBCs in FcRγfl/fl × Lyz2Cre+ recipients, the anti-HOD alloantibody response was similar to both B6 and FcRγfl/fl recipients. No significant differences in anti-HOD IgM or total Ig were observed in any group treated with PBS or anti-K mAb IgG2c.
In aggregate, these data demonstrate that modulation of activating FcγR expression on particular cell subsets affects both IgG2c-mediated removal of RBCs from circulation and also enhancement of RBC alloimmunization. These findings were not due to nonspecific effects of antibody injection because injection of antibody against a third-party antigen (not contained in the system) had no effect. In addition to firmly establishing a mechanistic role of FcγRs in the consumption of RBCs by different phagocytic subsets and the enhancement of RBC alloimmunization, these data also demonstrate that the phagocytes responsible for the majority of RBC clearance are separate from the APCs that drive IgG2c-mediated enhancement of RBC alloimmunization.
Anti-HOD mAb IgG2c alters the pattern of erythrophagocytosis by distinct APC subsets
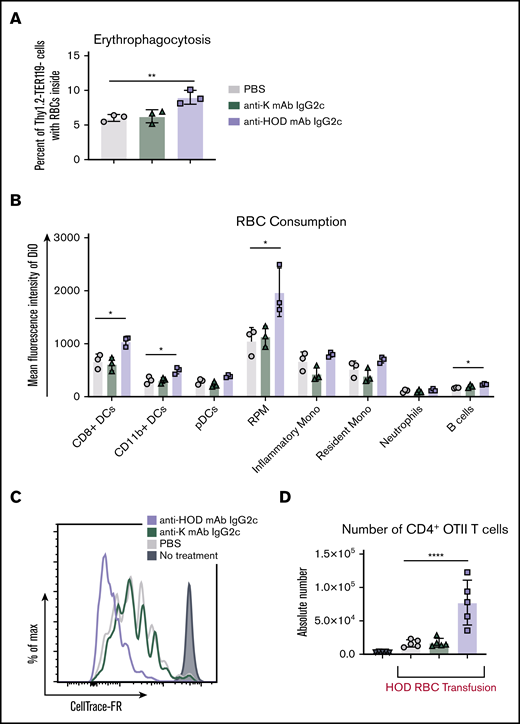
To test the hypothesis that RBC consumption patterns were affected by IgG2c bound to RBCs, B6 mice were passively immunized with anti-HOD mAb IgG2c, non-specific anti-K mAb IgG2c, or PBS followed by an allogeneic HOD RBC transfusion, as described in Figure 1. Spleens were harvested 24 hours later and leukocytes were stained with antibodies to delineate cell subtype. Because the RBCs were labeled with DiO, one can use DiO+ fluorescence by APC subsets as an indirect measure of RBC consumption, as previously described.31,33 Compared with controls, mice passively immunized with anti-HOD mAb IgG2c had a significantly higher percentage of overall leukocytes that participated in erythrophagocytosis (Figure 5A, P ≤ .01). Anti-HOD mAb IgG2c significantly increased RBC consumption by CD8+ DCs, CD11b+ DCs, RPMs, and B cells, as determined by the mean fluorescence intensity (MFI) of DiO, when compared with PBS infusion (Figure 5B, P ≤ .01). No significant differences were observed with pDCs, monocyte populations, or neutrophils.
Anti-HOD mAb IgG2c leads to enhanced T-cell proliferation and increases RBC consumption by DCs. To evaluate RBC consumption, B6 mice were passively immunized followed by a DiO+ HOD RBC transfusion. Spleens were harvested 18 to 24 hours posttransfusion and leukocytes were stained to delineate cell subsets. The percent erythrophagocytosis of total Thy1.2−TER119− leukocytes was determined (A) and the DiO MFI of individual APC subsets was calculated (B). To assess whether passive immunization modulated T-cell responses, B6 mice were adoptively transferred with 1 × 105 purified CD4+ OTII T cells labeled with CellTrace-far-red (FR). The next day, recipient mice were passively immunized with PBS, nonspecific anti-K mAb IgG2c, or anti-HOD mAb IgG2c followed by a HOD RBC transfusion. CellTrace-FR dilution (C) and absolute number (D) was determined for CD4+Va2+Vb5+Thy1.1+ OTII T cells 3 days posttransfusion. Each experiment was performed 3 times with 3 to 5 mice per group. Representative data are shown. Data were analyzed with a 1-way ANOVA with Dunnett’s multiple comparisons test to the control PBS group. *P ≤ .05, **P ≤ .01, ****P ≤ .0001.
Anti-HOD mAb IgG2c leads to enhanced T-cell proliferation and increases RBC consumption by DCs. To evaluate RBC consumption, B6 mice were passively immunized followed by a DiO+ HOD RBC transfusion. Spleens were harvested 18 to 24 hours posttransfusion and leukocytes were stained to delineate cell subsets. The percent erythrophagocytosis of total Thy1.2−TER119− leukocytes was determined (A) and the DiO MFI of individual APC subsets was calculated (B). To assess whether passive immunization modulated T-cell responses, B6 mice were adoptively transferred with 1 × 105 purified CD4+ OTII T cells labeled with CellTrace-far-red (FR). The next day, recipient mice were passively immunized with PBS, nonspecific anti-K mAb IgG2c, or anti-HOD mAb IgG2c followed by a HOD RBC transfusion. CellTrace-FR dilution (C) and absolute number (D) was determined for CD4+Va2+Vb5+Thy1.1+ OTII T cells 3 days posttransfusion. Each experiment was performed 3 times with 3 to 5 mice per group. Representative data are shown. Data were analyzed with a 1-way ANOVA with Dunnett’s multiple comparisons test to the control PBS group. *P ≤ .05, **P ≤ .01, ****P ≤ .0001.
Anti-HOD mAb IgG2c leads to increased T-cell proliferation
Mice were adoptively transferred with 1 × 105 CellTrace-FR labeled CD4+ OTII T cells, which recognize a peptide epitope from OVA323-339 (contained in the HOD antigen) presented by MHCII (I-Ab). Mice were than passively immunized with IgG2c and transfused with HOD RBCs (as described in Figure 1). Absolute numbers of CD4+ OTII T cells were assessed 3 days posttransfusion and proliferation was determined based upon CellTrace-FR dilution.
Recipient mice that received CD4+ OTII T cells followed by a PBS infusion served as a negative control and no CellTrace-FR dilution was observed (Figure 5C). OTII T cells from mice that received PBS and a HOD RBC transfusion exhibited OTII proliferation as determined by diluted CellTrace-FR and increased absolute CD4+ OTII T cell numbers (Figure 5D); the PBS group establishes the background OTII T-cell proliferative response to HOD RBCs and is consistent with previous studies.34 In animals that received anti-HOD mAb IgG2c, CellTrace-FR was further diluted, compared with controls, indicating more robust proliferation, which correlated with a significant increase in OTII T-cell numbers (Figure 5D, P ≤ .0001). No such increase was seen in mice that received a passive immunization with anti-K mAb IgG2c (black), ruling out a nonspecific effect of IgG2c antibodies. These data demonstrate that enhanced CD4+ T-cell proliferation correlates with conditions in which alloimmunization is increased.
Anti-HOD mAb IgG2c promotes memory formation
To test the effects of anti-RBC mAb on memory and germinal center formation, recipient B6 mice were passively immunized followed by a HOD RBC transfusion (as shown in Figure 6A). After 56 days, whereby the initial immune response had formed and contracted, recipient B6 mice were euthanized and their splenocytes were harvested and 25 × 106 total cells were adoptively transferred into B6.IgHa mice, thereby providing a congenic difference to discern antibody origin (the initial B6 mice used are of the IgHb genotype). Following adoptive transfer, the B6.IgHa recipients were given a transfusion of 100 μL of packed HOD RBCs. Alloantibody production was evaluated 7 days posttransfusion by flow crossmatch against HOD and control B6 RBCs. Adoptive transfer of splenocytes from mice that had previously been exposed to anti-HOD mAb IgG2c + HOD RBCs led to the development of IgG1b HOD-specific alloantibodies in response to HOD RBCs (no passive antibody was given in the second mouse); however, no anti-HOD IgG1b was observed in mice receiving the same amount of splenocytes from donors that had received a control third-party antibody or PBS (Figure 6B-D). Because the assay is specific for IgG1b, the antibody detected was due to B cells from the initial animal. Consistent with this, no experimental group made detectable anti-HOD IgG1a (data not shown). However, all groups, independent of prior passive immunization, made IgMa, indicating an early adaptive immune response to HOD transfusion into B6, consistent with previous reports (data not shown). To assess whether germinal center B cells could be detected, splenocytes from B6.IgHa recipients were stained with antibodies against CD19, I-Ab (MHCII), GL7, and PNA. In mice that were primed with anti-HOD mAb IgG2c, GL7+PNA+ germinal center B cells were readily detectable (Figure 6E-F). Thus, these data show that transfusion with HOD RBCs alone does not promote the generation of memory lymphocytes nor memory alloantibodies; however, anti-HOD mAb IgG2c not only enhances the primary humoral immune response but also drives memory lymphocyte generation.
Passive immunization with anti-HOD mAb promotes memory lymphocyte formation. (A) General experimental design to test whether passive immunization can lead to RBC-specific lymphocyte memory formation. (B) One week posttransfusion sera from B6.IgHa recipients were collected assayed for IgG1b alloantibodies by flow crossmatch against HOD and control B6 RBCs. The percent of IgG1b+ HOD RBCs for a representative experiment is shown (C) and the number of mice with detectable memory responses across all experiments is provided (D). In parallel, splenocytes were harvested and stained with antibodies against CD19, I-Ab, GL7, and PNA to identify germinal center B cells. Representative flow plots (E) and percentage of germinal center B cells (F) are shown. Flow plots are gated on CD19+I-Ab+Thy1.1−Thy1.2− leukocytes. Each experiment was performed 3 times with 3 to 5 mice per group. Data were analyzed with a 1-way ANOVA with Dunnett multiple comparisons test to the control PBS group. *P ≤ .05; ****P ≤ .0001.
Passive immunization with anti-HOD mAb promotes memory lymphocyte formation. (A) General experimental design to test whether passive immunization can lead to RBC-specific lymphocyte memory formation. (B) One week posttransfusion sera from B6.IgHa recipients were collected assayed for IgG1b alloantibodies by flow crossmatch against HOD and control B6 RBCs. The percent of IgG1b+ HOD RBCs for a representative experiment is shown (C) and the number of mice with detectable memory responses across all experiments is provided (D). In parallel, splenocytes were harvested and stained with antibodies against CD19, I-Ab, GL7, and PNA to identify germinal center B cells. Representative flow plots (E) and percentage of germinal center B cells (F) are shown. Flow plots are gated on CD19+I-Ab+Thy1.1−Thy1.2− leukocytes. Each experiment was performed 3 times with 3 to 5 mice per group. Data were analyzed with a 1-way ANOVA with Dunnett multiple comparisons test to the control PBS group. *P ≤ .05; ****P ≤ .0001.
Anti-HOD mAb IgG2c enhances humoral immunity to a third-party alloantigen if it is expressed on the same RBC as HOD
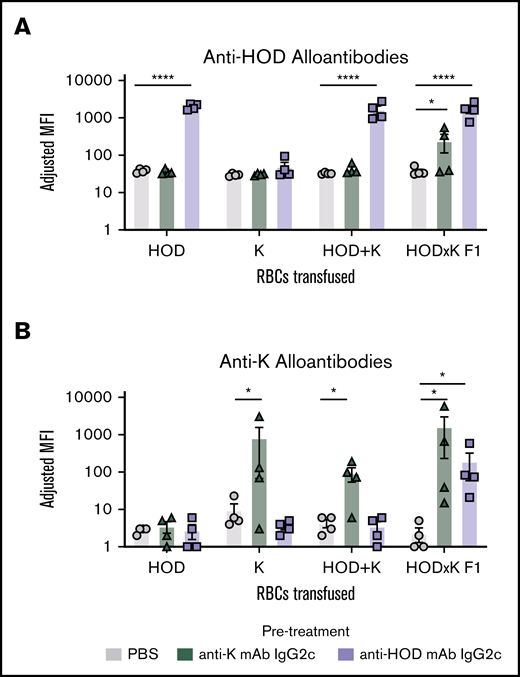
To test anti-HOD mAb enhanced immunity to a third-party alloantigen, HOD mice were crossed with K1 mice to generate F1 mice that simultaneously expressed HOD and K on RBCs (HOD × K F1 RBCs). Recipient mice were infused with either anti-HOD mAb IgG2c, anti-K mAb IgG2c, or PBS control, followed by RBCs expressing HOD, K, or HOD × K1 F1 RBCs expressing both alloantigens. An additional control group was included in which HOD and K RBCs were mixed together (HOD + K); in this case, twice the total number of RBCs were transfused to maintain the same dose of K and HOD as in the other conditions. Humoral responses were then monitored for polyclonal antibody responses to HOD or K by flow cytometry using HOD or K RBCs as targets.
Anti-HOD mAb enhanced alloantibodies to HOD in mice that were exposed to HOD on RBCs (either HOD RBCs alone, HOD + K RBCs, or F1 RBCs) (Figure 7A). Anti-K mAb had no effect upon anti-HOD alloantibodies, except in the case of F1 RBCs, in which anti-K mAb resulted in a significant enhancement of anti-HOD alloantibodies compared with PBS controls. Conversely, anti-K mAb-enhanced alloantibodies to K in mice that were exposed to K on RBCs (either K RBCs alone, HOD + K RBCs, or F1 RBCs) (Figure 7B). Anti-HOD mAb had no effect upon anti-K alloantibodies, except in the case of F1 RBCs, in which anti-HOD mAb resulted in a significant enhancement of anti-K alloantibodies compared with PBS controls. Together, these data indicate that mAbs to 1 alloantigen “cross-enhance” to a third-party antigen, but only in the case that both antigens are expressed on the same RBC. No cross enhancement was observed when the alloantigens were both present but expressed on separate RBCs.
Anti-RBC mAbs cross-enhance to third-party alloantigens only if they are expressed on the same RBCs. Recipient B6 mice were pretreated with either PBS, anti-HOD mAb, or anti-K mAb. For each pretreatment, 4 different groups were set up, each receiving a different RBC transfusion: HOD RBCs, K RBCs, HOD RBCs mixed with K RBCs (HOD+K), or F1 RBCs expressing both alloantigens. Serum was obtained 21 days after transfusion and was assayed for polyclonal anti-HOD antibodies (A) and polyclonal anti-K antibodies (B) by indirect immunofluorescence using HOD or K RBC targets, respectively. Data were analyzed with a 1-way ANOVA using a Dunnett multiple comparison test to the control PBS group. *P ≤ .05; ****P ≤ .0001.
Anti-RBC mAbs cross-enhance to third-party alloantigens only if they are expressed on the same RBCs. Recipient B6 mice were pretreated with either PBS, anti-HOD mAb, or anti-K mAb. For each pretreatment, 4 different groups were set up, each receiving a different RBC transfusion: HOD RBCs, K RBCs, HOD RBCs mixed with K RBCs (HOD+K), or F1 RBCs expressing both alloantigens. Serum was obtained 21 days after transfusion and was assayed for polyclonal anti-HOD antibodies (A) and polyclonal anti-K antibodies (B) by indirect immunofluorescence using HOD or K RBC targets, respectively. Data were analyzed with a 1-way ANOVA using a Dunnett multiple comparison test to the control PBS group. *P ≤ .05; ****P ≤ .0001.
Discussion
The data presented here reject the generalization that IgGs only suppress responses to antigens on RBCs. Moreover, the current findings also isolate IgG subtype as an independent variable that affects if an antibody enhances immunity. Although the current report is, to the best of our knowledge, the first investigation of the effect of IgG subtype on RBC alloimmunization using switch variants that maintain the same antigen-binding domain, other murine systems have used monoclonal antibodies of defined IgG subtype. Yu et al reported suppression in response to each of 6 monoclonal antibodies (4 IgG1 and 2 IgGa).19 No IgG2b, IgG2c, or IgG3 was tested in this study. Why IgG2a enhanced in the current report but suppressed in the referenced report is unclear; however, different antibodies were used with distinct epitope specificities and affinities. As such, a direct comparison cannot be made that controls for other confounders. Of course, as with all murine models, the extent to which the observed biology extends to human immunology is also not clear. However, the general phenomenon of enhancement has been reported in humans, both in the context of monoclonal and polyclonal antibodies to RBC antigens.21,25
The antibody-mediated enhancement observed in the current report requires that affinity-matured IgG be present at the initiation of an immune response. In addition to the previously discussed use of anti-RhD to suppress alloimmunization, there are at least 2 other scenarios in which passive antibody is given with the goal of facilitating immunity. First is the administration of antisera specific to a pathogen or toxin as prophylaxis to individuals at risk of exposure. Second is the administration of intravenous immunoglobulin for the treatment of humoral immunodeficiency. In both cases, the rationale behind the therapy is to transfer humoral immunity as a transient protection. However, should antigen be encountered, it may very well be that the transferred antibodies not only neutralize but also promote a more robust primary immune response and immunological memory. Thus, the current data raise the possibility that the efficacy of immunoglobulin prophylaxis may include a hitherto unappreciated mechanism of not only direct neutralization, but also promoting a primary immune response.
There are also 2 natural circumstances in which affinity-matured antibody may be present in the absence of an antecedent CD4+ T-cell response. First is when antibodies from a previous immune response cross-react to a new antigen based upon tertiary structure of proteins that are unrelated at the primary amino acid level. Second, and more likely, is transfer of maternal antibody (but not cellular immunity) to offspring. However, as neonatal immunity is an entirely different mechanistic landscape, it is not possible to extrapolate from the current data to the neonatal setting, other than speculation that may drive future experimentation.
Together, the data contained in this report demonstrate that IgG to RBCs can have an enhancing effect upon a primary immune response through an FcγR-dependent pathway that cross-presents different antigens on the same RBC. This results in increased CD4+ T-cell activation and expansion, enhanced humoral immunity, and increased memory. Although macrophages consume the majority of antibody opsonized RBCs, it is the DCs that are required for the enhancement effects. Thus, the phagocytes that are responsible for the majority of RBC clearance are separate from the APCs that drive IgG-mediated enhancement of RBC alloimmunization. These findings provide phenomenological and mechanistic insight to both natural effects of passive immunity as well as iatrogenic outcomes of antibody therapy.
Acknowledgments
The authors thank Jeanne Hendrickson, John Luckey, Steven Spitalnik, and Eldad Hod for thoughtful conversations and critical review of this manuscript.
These studies were supported in part by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL135248 [K.E.H.] and P01HL132819 [J.C.Z.]).
Authorship
Contribution: J.C.Z. and K.E.H. designed the studies and experiments; D.R.G., A.L.R., H.L.H., J.N.L., X.W., and K.E.H. carried out experiments; all authors were involved in interpretation of data; and D.R.G., K.E.H., and J.C.Z. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Krystalyn E. Hudson, Columbia University Medical Center, 630 West 168th St, Room P&S 14-511, New York, NY 10032; e-mail: keh2197@cumc.columbia.edu.
References
Author notes
J.C.Z. and K.E.H. contributed equally to this study and share senior authorship.
All electronic information will be freely provided by e-mail with the corresponding author, Krystalyn E. Hudson, at keh2197@cumc.columbia.edu. Monoclonal antibodies and mice will be provided upon request and without unreasonable restrictions.
The full-text version of this article contains a data supplement.