Key Points
Blinatumomab maintenance therapy demonstrated evidence of extended survival outcome in patients with R/R ALL.
Most responders after induction maintained their responses with additional cycles of blinatumomab; some new responses were achieved in the later cycles.
Abstract
In a phase 3 clinical study of heavily pretreated adults with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL), overall survival (OS) following blinatumomab, a BiTE (bispecific T-cell engager) immunooncology therapy, was significantly improved vs chemotherapy following induction (cycles 1 to 2). Here we report the efficacy and safety of those who received additional cycles of blinatumomab. Blinatumomab was administered as a continuous IV infusion for 4 weeks in a 6-week cycle. Patients who achieved a bone marrow response (≤5% blasts) or complete remission (full, partial, or incomplete hematological recovery) during induction could receive additional cycles of blinatumomab. OS and relapse-free survival (RFS) for consolidation (cycles 3 to 5) vs no consolidation, and maintenance (cycles ≥6) vs no maintenance were analyzed using Simon-Makuch and Mantel-Byar odds ratios. Of 267 patients who received blinatumomab induction, 86 (32%) entered consolidation and 36 (13%) entered maintenance. Evidence of longer OS was demonstrated among the maintenance group compared with no-maintenance (median OS [95% confidence interval, CI]: not reached for maintenance vs 15.5 months for no maintenance). Median RFS (months; 95% CI) was numerically longer among maintenance group (14.5; 7.1 to 21.9) compared with no-maintenance (9.8; 8.5 to 11.1). A lower incidence of adverse events was seen during maintenance (72.2%) compared with induction (97.2%) and consolidation (86.1%). Adults with R/R ALL who achieved remission following blinatumomab induction had longer survival on continuation therapy than those who discontinued blinatumomab early, supporting the use of blinatumomab as long-term therapy. No new safety signals were reported. This trial was registered at www.clinicaltrials.gov as #NCT02013167.
Introduction
Despite up to 80% of adults with acute lymphoblastic leukemia (ALL) achieving complete remission (CR) following traditional chemotherapy, 45% of adults will relapse or remain refractory. Among these patients, the prognosis has historically been poor with median overall survival (OS) of 2 to 8 months and 5-year OS rates of <10%.1,2 In recent years, significant advances in our understanding of ALL pathogenesis have led to the development of promising treatment options beyond conventional chemotherapy, such as those targeting cell surface antigens (eg, inotuzumab ozogamicin, blinatumomab, chimeric antigen receptor T-cell therapy).3
Blinatumomab is a CD19 BiTE (bispecific T-cell engager) immunooncology therapy that activates endogenous cytotoxic T cells to kill target B cells. Blinatumomab is indicated for the treatment of adults and children with relapsed/refractory (R/R) B-cell precursor (BCP) ALL and for patients with minimal residual disease (MRD) ALL, defined as at least 10−3 (0.1%) leukemic cells detected by quantitative polymerase chain reaction (PCR).4 In a randomized phase 3 study, OS with blinatumomab was superior to standard-of-care chemotherapy (SOC) in patients with R/R ALL.5 Patients who completed 2 cycles of induction therapy with blinatumomab could proceed to hematopoietic stem cell transplantation (HSCT) or opt for additional blinatumomab consolidation and maintenance cycles, provided they continued in hematological remission.5 Here we report the survival outcomes, remission rates, and safety of patients who received blinatumomab continuation therapy.5,6 OS and relapse-free survival (RFS) are compared between patients who received consolidation/maintenance cycles against those who were eligible but did not receive such cycles.
Methods
Full details regarding study (NCT02013167) methodology have been previously published.5 Here we present the analysis using final data from 3 January 2014 to 14 March 2017. The study and all amendments were reviewed by an independent ethics committee/institutional review board at each center.
Trial design and patients
In brief, this was an international, randomized, open-label, phase 3 study of blinatumomab vs SOC in adults with R/R Philadelphia chromosome–negative (Ph−) BCP-ALL. Eligible patients were refractory to primary induction or salvage therapy with intensive combination chemotherapy, first relapse within first remission <12 months, second or greater relapse, or had relapse any time after allogeneic HSCT. Additional criteria included >5% bone marrow blasts, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and adequate organ function. Key exclusion criteria have been previously reported.5
Treatments
During induction (cycles 1 to 2), blinatumomab was administered as a continuous IV infusion 4 weeks on and 2 weeks off (1 cycle) at an initial dose of 9 µg per day for days 1 to 7 of cycle 1, and then the dose was stepped up to 28 µg per day for days 8 to 28 of cycle 1 and all days of subsequent cycles. Patients who achieved a bone marrow response (≤5% bone marrow blasts) or CR, complete remission with partial hematological recovery/complete remission with incomplete hematological recovery (CRh/CRi) within the first 2 cycles could receive additional consolidation (cycles 3 to 5) and maintenance cycles (cycles 6 and above). Maintenance cycles were 4 weeks on and 8 weeks off (Figure 1). Patients discontinued blinatumomab if one of the following occurred: HSCT, investigator discretion, toxicity, relapse, or use of protocol-excluded medications.5 Dexamethasone premedication was required prior to each infusion and dose step to prevent infusion-related reactions and cytokine release syndrome (CRS). Safety follow-up visit occurred 30 days after the last dose of protocol-specified therapy, and long-term follow-up occurred every 3 months as specified in the protocol.
Induction, consolidation, and maintenance study design (blinatumomab group).aIn induction cycle 1 week 1, 9 µg per day, and 28 µg per day thereafter by continuous infusion. bObserved cycle 3 day 1 varied between day 85 and day 131 on study. cObserved maintenance day 1 varied between day 211 and day 266 on study. dMaintenance therapy was discontinued in the case of transition to HSCT, investigator discretion, toxicity, relapse, or use of protocol-excluded medications.
Induction, consolidation, and maintenance study design (blinatumomab group).aIn induction cycle 1 week 1, 9 µg per day, and 28 µg per day thereafter by continuous infusion. bObserved cycle 3 day 1 varied between day 85 and day 131 on study. cObserved maintenance day 1 varied between day 211 and day 266 on study. dMaintenance therapy was discontinued in the case of transition to HSCT, investigator discretion, toxicity, relapse, or use of protocol-excluded medications.
Response assessments
Hematological remission was defined as having ≤5% blasts in bone marrow, no evidence of disease, and peripheral blood count recovery as follows: CR was defined as full recovery with platelets >100 × 103/µL and absolute neutrophil count (ANC) >1 × 103/µL; CRh was defined as platelets >50 × 103/µL and ANC >0.5 × 103/µL; and CRi was defined as platelets >100 × 103/µL or ANC >1 × 103/µL. In addition, MRD remission was defined as MRD level <10−4 by multicolor flow cytometry or real-time quantitative PCR.
Statistical analysis
End points included OS (time from randomization until death), RFS (time from first CR/CRh/CRi to relapse or death), hematological response (CR/CRh/CRi), MRD response, and adverse events by treatment phase (ie, induction, consolidation, and maintenance). Descriptive response rates and patient incidence of treatment-emergent adverse event are provided for those who received blinatumomab consolidation and maintenance cycles.
Because patients had to survive and be in remission long enough to receive consolidation and maintenance cycles, simple comparisons of all patients would have a survivor time bias in favor of the consolidation and maintenance groups and against the no-consolidation and no-maintenance groups. Thus, only patients alive and who have the opportunity to receive these additional cycles (who may or may not have) were included in OS analyses (eg, those alive and in remission at month 3 were included in consolidation vs no-consolidation comparison; those alive and in remission at month 7 were included in the maintenance vs no-maintenance comparison). All induction responders were included in the consolidation vs no-consolidation comparison, but only patients in remission at least 5 months after first CR/CRh/CRi were included in the maintenance vs no-maintenance comparison. Because of the potential of treatment interruptions in early cycles, patients could start consolidation and maintenance cycles at different times (Figure 1). Simon-Makuch curves7 and Mantel-Byar odd ratios8 were used in the OS and RFS comparisons to correctly assign patients to comparison groups. These methods check at each time point (posteligibility cutoff date) if the patient has started consolidation or maintenance and are attributed accordingly. For example, the number of patients in the Simon-Makuch curve varies as patients who start in the no-consolidation group switch to the consolidation group when they start cycle 3. The OS and RFS analyses were not censored at HSCT as there would not have been enough observed death or relapse events for inference if the OS and RFS analyses had been censored for HSCT.
Results
Study population
In this study of 405 patients, 271 were randomized to blinatumomab and 267 received blinatumomab. Of the 119 patients who achieved CR/CRh/CRi during induction and were eligible for consolidation, 86 (32%) patients received consolidation cycles; 36 (13%) patients received maintenance cycles; and 11 (4%) patients completed maintenance (Figure 2). Baseline characteristics of patients receiving blinatumomab by randomization, consolidation, and maintenance phases are summarized in Table 1. Overall, the subset of patients who proceeded to maintenance had a higher rate of prior HSCT treatment and posttransplant relapse than those in the larger subset who entered randomization and received consolidation.
Study consort diagram (blinatumomab group).aIntention to receive HSCT (n = 18), intention to receive treatment other than allogeneic HSCT (n = 1), relapse (n = 25), failure to achieve CR/CRh/CRi in first 2 cycles (n = 1). bIntention to receive HSCT (n = 5), intention to receive treatment other than allogeneic HSCT (n = 4), relapse (n = 9).
Study consort diagram (blinatumomab group).aIntention to receive HSCT (n = 18), intention to receive treatment other than allogeneic HSCT (n = 1), relapse (n = 25), failure to achieve CR/CRh/CRi in first 2 cycles (n = 1). bIntention to receive HSCT (n = 5), intention to receive treatment other than allogeneic HSCT (n = 4), relapse (n = 9).
Demographic and baseline characteristics
| . | Blinatumomab randomization (N = 271) . | Blinatumomab consolidation (N = 86) . | Blinatumomab maintenance (N = 36) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 162 (60.0) | 43 (50.0) | 18 (50.0) |
| Female | 109 (40.0) | 43 (50.0) | 18 (50.0) |
| Race, n (%) | |||
| White | 228 (84.0) | 72 (83.7) | 33 (91.7) |
| Asian | 19 (7.0) | 7 (8.1) | 2 (5.6) |
| Black (or African American) | 5 (2.0) | 2 (2.3) | 0 (0) |
| American Indian or Alaska Native | 0 (0) | 1 (1.2) | 0 (0) |
| Other | 19 (7.0) | 4 (4.7) | 1 (2.8) |
| Median age, y (range) | 37 (18-80) | 38.5 (19-80) | 34 (19-80) |
| Age group, y, n (%) | |||
| <35 | 124 (46.0) | 38 (44.2) | 19 (52.8) |
| 35 to 54 | 80 (30.0) | 26 (30.2) | 12 (33.3) |
| 55 to 64 | 34 (13.0) | 9 (10.5) | 2 (5.6) |
| ≥65 | 33 (12.0) | 13 (15.1) | 3 (8.3) |
| ECOG performance status, n (%) | |||
| 0 | 96 (35.4) | 38 (44.2) | 13 (36.1) |
| 1 | 134 (49.4) | 37 (43) | 19 (52.8) |
| 2 | 41 (15.1) | 11 (12.8) | 4 (11.1) |
| >2 | 0 (0) | 0 (0) | 0 (0) |
| Patients with prior HSCT, n (%) | 94 (35.0) | 38 (44.2) | 24 (66.7) |
| Patients who relapsed after HSCT, n (%) | 91 (34.0) | 36 (41.9) | 23 (63.9) |
| Prior number of salvage regimens, n (%) | |||
| 0 | 114 (42.0) | 46 (53.5) | 20 (55.6) |
| 1 | 91 (34.0) | 22 (25.6) | 11 (30.6) |
| 2 | 45 (17.0) | 13 (15.1) | 3 (8.3) |
| 3 | 14 (5.0) | 3 (3.5) | 2 (5.6) |
| >3 | 7 (3.0) | 2 (2.3) | 0 (0) |
| . | Blinatumomab randomization (N = 271) . | Blinatumomab consolidation (N = 86) . | Blinatumomab maintenance (N = 36) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 162 (60.0) | 43 (50.0) | 18 (50.0) |
| Female | 109 (40.0) | 43 (50.0) | 18 (50.0) |
| Race, n (%) | |||
| White | 228 (84.0) | 72 (83.7) | 33 (91.7) |
| Asian | 19 (7.0) | 7 (8.1) | 2 (5.6) |
| Black (or African American) | 5 (2.0) | 2 (2.3) | 0 (0) |
| American Indian or Alaska Native | 0 (0) | 1 (1.2) | 0 (0) |
| Other | 19 (7.0) | 4 (4.7) | 1 (2.8) |
| Median age, y (range) | 37 (18-80) | 38.5 (19-80) | 34 (19-80) |
| Age group, y, n (%) | |||
| <35 | 124 (46.0) | 38 (44.2) | 19 (52.8) |
| 35 to 54 | 80 (30.0) | 26 (30.2) | 12 (33.3) |
| 55 to 64 | 34 (13.0) | 9 (10.5) | 2 (5.6) |
| ≥65 | 33 (12.0) | 13 (15.1) | 3 (8.3) |
| ECOG performance status, n (%) | |||
| 0 | 96 (35.4) | 38 (44.2) | 13 (36.1) |
| 1 | 134 (49.4) | 37 (43) | 19 (52.8) |
| 2 | 41 (15.1) | 11 (12.8) | 4 (11.1) |
| >2 | 0 (0) | 0 (0) | 0 (0) |
| Patients with prior HSCT, n (%) | 94 (35.0) | 38 (44.2) | 24 (66.7) |
| Patients who relapsed after HSCT, n (%) | 91 (34.0) | 36 (41.9) | 23 (63.9) |
| Prior number of salvage regimens, n (%) | |||
| 0 | 114 (42.0) | 46 (53.5) | 20 (55.6) |
| 1 | 91 (34.0) | 22 (25.6) | 11 (30.6) |
| 2 | 45 (17.0) | 13 (15.1) | 3 (8.3) |
| 3 | 14 (5.0) | 3 (3.5) | 2 (5.6) |
| >3 | 7 (3.0) | 2 (2.3) | 0 (0) |
OS
For OS consolidation vs no-consolidation, 115 patients alive and in remission at month 3 were included in the comparison (Figure 3A); 82 patients eventually received consolidation and 34 did not. For OS maintenance vs no-maintenance, 78 patients alive in remission at month 7 were included in the comparison (Figure 3B). Of these, 36 eventually received maintenance cycles. Additional details can be found in supplemental Figure 1.
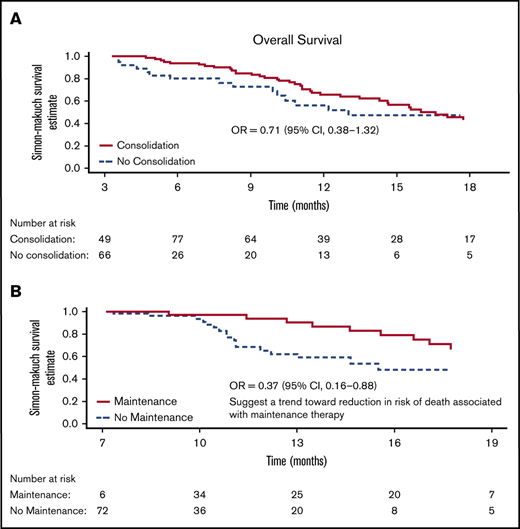
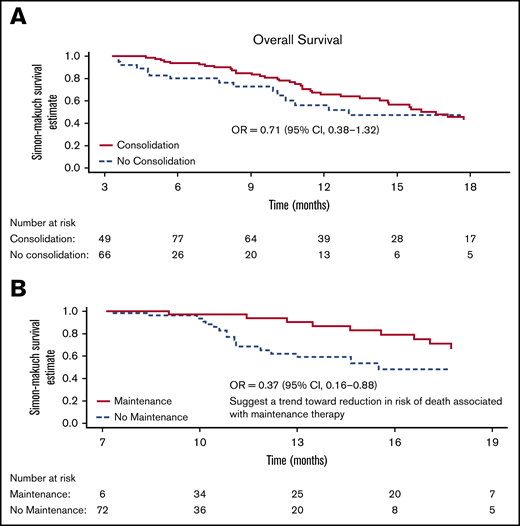
Simon-Makuch plot for OS. (A) Analysis includes 115 patients alive and in CR/CRh/CRi at month 3 (from randomization), of which, 82 patients eventually received consolidation cycles (4 other consolidations patients either die or relapse before month 3 and were not included in this analysis). Median OS is 13.0 months in the no-consolidation group and 16.6 (13.6 to 19.6) months in the consolidation group. (B) Analysis includes 78 patients alive and in CR/CRh/CRi at month 7 (from randomization), of which, 36 patients eventually received maintenance cycles. Median (95% CI) is 15.5 months in the no-maintenance group and not reached in the maintenance group. OR, odds ratio.
Simon-Makuch plot for OS. (A) Analysis includes 115 patients alive and in CR/CRh/CRi at month 3 (from randomization), of which, 82 patients eventually received consolidation cycles (4 other consolidations patients either die or relapse before month 3 and were not included in this analysis). Median OS is 13.0 months in the no-consolidation group and 16.6 (13.6 to 19.6) months in the consolidation group. (B) Analysis includes 78 patients alive and in CR/CRh/CRi at month 7 (from randomization), of which, 36 patients eventually received maintenance cycles. Median (95% CI) is 15.5 months in the no-maintenance group and not reached in the maintenance group. OR, odds ratio.
Median OS was 16.6 months (95% confidence interval [CI], 13.6-19.6) in the consolidation group and 13.0 months (95% CI, not estimable) in the no-consolidation group, with a relative odds ratio of 0.71 (95% CI, 0.38-1.32) (Figure 3A). Median OS was not reached at the end of the study in patients who received maintenance and 15.5 months (95% CI, not estimable) among those who did not receive maintenance, with a relative odds ratio of 0.37 (95% CI, 0.16-0.88) indicating a 63% reduction in the risk of death for maintenance (Figure 3B). This estimates an effect of OS benefit from maintenance cycles among patients who are alive long enough to receive such cycles.
RFS
For the RFS consolidation comparison, 119 patients who achieved CR/CRh/CRi during the first 2 cycles were included in the consolidation vs no-consolidation comparison. Of these, 82 eventually received consolidation cycles and 37 did not. For the RFS maintenance comparison, 63 remained in remission for 5 months from first CR/CRh/CRi and were included in the analysis. Of these, 34 eventually received maintenance cycles and 29 did not. Additional details can be found in supplemental Figure 1.
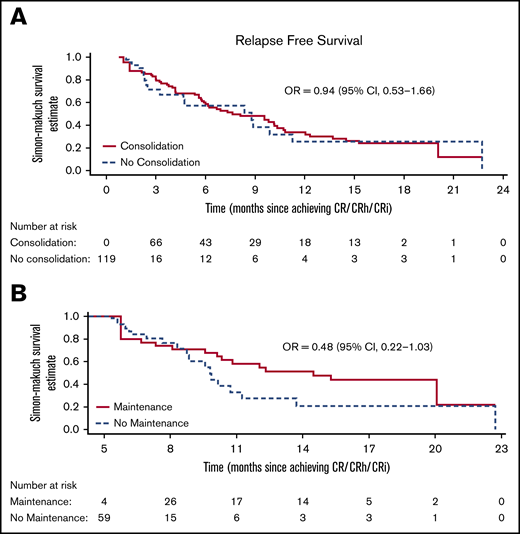
Median RFS was 7.6 months (95% CI, 3.7-11.6) in the consolidation group and 8.8 months (95% CI, 0-10.42) in the no-consolidation group, with a relative odds ratio of 0.94 (95% CI, 0.53-1.66; Figure 4A). In the maintenance comparison, the median RFS was 14.5 months (95% CI, 7.1-21.9) in the maintenance group and 9.8 months (95% CI, 8.5-11.1) in the no-maintenance group, with a relative odds ratio of 0.48 (95% CI, 0.22-1.03; Figure 4B).
Simon-Makuch plot for RFS. (A) Analysis includes 119 patients in CR/CRh/CRi from date of first response, of which, 82 eventually received consolidation cycles (4 other consolidation patients did not achieve CR/CRh/CRi in first 2 cycles and were not included in in this analysis). Median (95% CI) is 8.8 (7.6 to 9.9) months in the no-consolidation group and 7.6 (3.7-11.6) months in the consolidation group. (B) Analysis includes 63 patients alive and in CR/CRh/CRi 5 months after first hematological response, of which, 34 eventually received maintenance cycles (2 other maintenance patients relapse before 5 months and were not included in this analysis). Median (95% CI) is 9.8 (8.5-11.1) months in the no-maintenance group and 14.5 (7.1-21.9) months in the maintenance group.
Simon-Makuch plot for RFS. (A) Analysis includes 119 patients in CR/CRh/CRi from date of first response, of which, 82 eventually received consolidation cycles (4 other consolidation patients did not achieve CR/CRh/CRi in first 2 cycles and were not included in in this analysis). Median (95% CI) is 8.8 (7.6 to 9.9) months in the no-consolidation group and 7.6 (3.7-11.6) months in the consolidation group. (B) Analysis includes 63 patients alive and in CR/CRh/CRi 5 months after first hematological response, of which, 34 eventually received maintenance cycles (2 other maintenance patients relapse before 5 months and were not included in this analysis). Median (95% CI) is 9.8 (8.5-11.1) months in the no-maintenance group and 14.5 (7.1-21.9) months in the maintenance group.
Best responses for patients receiving blinatumomab
Rates of hematological response were stable between induction and consolidation phases. Among the 86 patients who started consolidation, 69 (80.2%) and 63 (73.3%) had CR during induction and consolidation, respectively; a total of 84 (97.7%) and 71 (82.6%) patients had CR/CRh/CRi (Table 2). Rates of MRD response among those who had evaluable MRD assessment were also stable between induction and consolidation phases, with 44 out of 72 (61.1%) and 29 out of 49 (59.1%) patients having MRD CR during induction and consolidation, respectively (Table 2).
Best hematological and MRD response across phases
| . | Patients who started consolidation (N = 86) . | Patients who started maintenance (N = 36) . | ||||
|---|---|---|---|---|---|---|
| . | During induction (cycles 1 to 2) . | During consolidation (cycles 3 to 5) . | During induction (cycles 1 to 2) . | During consolidation (cycles 3 to 5) . | During induction + consolidation . | During maintenance (cycles ≥6) . |
| Best response, n (%) | ||||||
| CR | 69* (80.2) | 63 (73.3) | 26 (72.2) | 34 (94.4) | 34** (94.4) | 30 (83.3) |
| CRh | 14† (16.3) | 7 (8.1) | 9 (25) | 2 (5.6) | 2 (5.6) | 1 (2.8) |
| CRi (without CRh) | 1‡ (1.2) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blast-free hypoplastic or aplastic bone marrow (without CRi) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Partial remission | 1 (1.2) | 0 (0) | 1 (2.8) | 0 (0) | 0 (0) | 0 (0) |
| Nonresponse | 1 (1.2) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hematological relapse | 0 (0) | 6 (7) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) |
| Progressive disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) |
| Unevaluable/no response data | 0 (0) | 8 (9.3) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) |
| CR/CRh/CRi | 84 (97.7) | 71 (82.6) | 35 (97.2) | 36 (100) | 36 (100) | 31 (86.1) |
| Best MRD response, n (%) | ||||||
| Evaluable MRD assessment, N1 | 72 | 49 | 30 | 23 | 30 | 14 |
| MRD CR§ | 44/72‖ (61.1) | 29/49 (59.1) | 22/30 (73.3) | 21/23 (91.3) | 24/30†† (80.0) | 8/14 (57.1) |
| MRD remission§ | 11/72¶ (15.2) | 3/49 (6.1) | 4/30 (13.3) | 1/23 (4.3) | 3/30 (10.0) | 3/14 (21.4) |
| No MRD remission§ | 14/72# (19.4) | 17/49 (34.6) | 4/30 (13.3) | 1/23 (4.3) | 3/30 (10.0) | 3/14 (21.4) |
| No MRD assessment | 17/86 (16.3) | 37/86 (43.0) | 6/36 (16.7) | 13/36 (36.1) | 6/36 (16.7) | 22/36 (61.1) |
| . | Patients who started consolidation (N = 86) . | Patients who started maintenance (N = 36) . | ||||
|---|---|---|---|---|---|---|
| . | During induction (cycles 1 to 2) . | During consolidation (cycles 3 to 5) . | During induction (cycles 1 to 2) . | During consolidation (cycles 3 to 5) . | During induction + consolidation . | During maintenance (cycles ≥6) . |
| Best response, n (%) | ||||||
| CR | 69* (80.2) | 63 (73.3) | 26 (72.2) | 34 (94.4) | 34** (94.4) | 30 (83.3) |
| CRh | 14† (16.3) | 7 (8.1) | 9 (25) | 2 (5.6) | 2 (5.6) | 1 (2.8) |
| CRi (without CRh) | 1‡ (1.2) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blast-free hypoplastic or aplastic bone marrow (without CRi) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Partial remission | 1 (1.2) | 0 (0) | 1 (2.8) | 0 (0) | 0 (0) | 0 (0) |
| Nonresponse | 1 (1.2) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hematological relapse | 0 (0) | 6 (7) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) |
| Progressive disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) |
| Unevaluable/no response data | 0 (0) | 8 (9.3) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) |
| CR/CRh/CRi | 84 (97.7) | 71 (82.6) | 35 (97.2) | 36 (100) | 36 (100) | 31 (86.1) |
| Best MRD response, n (%) | ||||||
| Evaluable MRD assessment, N1 | 72 | 49 | 30 | 23 | 30 | 14 |
| MRD CR§ | 44/72‖ (61.1) | 29/49 (59.1) | 22/30 (73.3) | 21/23 (91.3) | 24/30†† (80.0) | 8/14 (57.1) |
| MRD remission§ | 11/72¶ (15.2) | 3/49 (6.1) | 4/30 (13.3) | 1/23 (4.3) | 3/30 (10.0) | 3/14 (21.4) |
| No MRD remission§ | 14/72# (19.4) | 17/49 (34.6) | 4/30 (13.3) | 1/23 (4.3) | 3/30 (10.0) | 3/14 (21.4) |
| No MRD assessment | 17/86 (16.3) | 37/86 (43.0) | 6/36 (16.7) | 13/36 (36.1) | 6/36 (16.7) | 22/36 (61.1) |
CR was defined as 5% or less bone marrow blasts and no evidence of disease and was further characterized according to the extent of recovery of peripheral blood counts as follows: CR with full recovery (platelet count of >100 × 103/µL and ANC of >1 × 103/µL), CRh (platelet count of >50 × 103/µL and ANC of >0.5 × 103/µL), or CRi (platelet count of >100 × 103/µL or ANC of >1 × 103/µL). MRD response was defined as MRD level <10−4 measured by real-time quantitative PCR (or multicolor flow cytometry). MRD complete response was defined as no detectable leukemic cells by quantitative PCR (or flow cytometry).
Of the 69 patients with best response of CR before consolidation, 52 had best response of CR, 4 had CRh, 6 had hematological relapse, and 7 patients had unevaluable or no data during consolidation (supplemental Table 2A).
Of the 14 patients with best response of CRh before consolidation, 9 had best response of CR during consolidation (supplemental Table 2A).
This patient went on to achieve CR during blinatumomab consolidation therapy (supplemental Table 2A).
Percentage with respect to N1.
Of the 44 patients who had MRD CR before consolidation, 25 had best response of MRD CR, 1 had MRD remission, 8 lost remission, and 10 had no MRD assessment during consolidation (supplemental Table 3A).
Of the 11 patients who had best response of MRD remission before consolidation, 2 had best response of MRD CR, 1 maintained MRD remission, and 8 had no MRD assessment during consolidation (supplemental Table 3A).
All 14 patients had no MRD assessment during consolidation (supplemental Table 3A).
Of the 34 patients with best response of CR before maintenance, 30 had best response of CR, 2 relapsed, 1 had progressive disease, and 1 had unevaluable or no data during maintenance (supplemental Table 2B).
Of the 24 patients with best response of MRD complete response before maintenance, 8 had best response of MRD complete response, 1 had MRD remission, 2 had no MRD remission, and 13 had no MRD assessment during maintenance (supplemental Table 3B).
Among the 36 patients who started maintenance, best responses were assessed during induction, consolidation, and maintenance, with 26 (72.2%), 34 (94.4%), and 30 (83.3%) having CR, respectively (Table 2). Best MRD responses assessed during induction, consolidation, and maintenance identified 22 out of 30 (73.3%), 21 out of 23 (91.3%), and 8 out of 14 (57%) patients having MRD CR, respectively (Table 2). Additional details of the change in hematological and MRD responses before vs during consolidation or maintenance can be found in supplemental Tables 2A-B and 3A-B.
Transplant
Of 86 patients who entered consolidation, 23 went on to receive HSCT with median time to HSCT of 178 days (119 to 373). Among the 44 HSCT without consolidation, median time to HSCT is 94.5 days (38 to 204). Of 36 patients who entered maintenance, 4 patients went on to receive HSCT with the median time to HSCT of 340 days (281 to 373). Among the 63 HSCT without maintenance, median time to HSCT is 111 days (38 to 204).
Overall, patients receiving maintenance were less likely to have HSCT after blinatumomab than those not receiving maintenance (11.1% vs 26.8%, respectively; supplemental Table 1).
Adverse events
Treatment-emergent adverse events of interest (EOIs) in patients receiving maintenance cycles were compared across induction, consolidation, and maintenance (Table 3). Reported EOIs decreased from 97.2% to 86.1% to 72.2% of patients in induction, consolidation, and maintenance, respectively. Grade ≥3 adverse events were reported in 83.3%, 52.8%, and 38.9% of patients in induction, consolidation, and maintenance, respectively. The grade ≥3 events of most interest were neurotoxicities and CRS. Events due to neurotoxicities were reported in 11.1% of patients in induction, 0% in consolidation, and 11.1% in maintenance, whereas CRS was reported in 5.6% in induction, 0% in consolidation, and 2.8% in maintenance. Other grade ≥3 adverse events for induction, consolidation, and maintenance included neutropenia (44.4%, 33.3%, and 5.6% of patients, respectively), cytopenia (61.1%, 33.3%, 16.7%), decreased immunoglobulins (8.3%, 2.8%, 5.6%), elevated liver enzyme (13.9%, 2.8%, 2.8%), and infusion reaction considering duration (8.3%, 0%, 0%). On the other hand, infections (16.7%, 22.2%, 22.2%) and lymphopenia (0%, 2.8%, 5.6%) of grade ≥3 occurred more often during the later cycles. Serious adverse events were reported in 30.6% of patients in induction and consolidation, and in 36.1% of patients in maintenance. EOIs (any grade) are summarized in supplemental Table 4.
Patients with treatment-emergent adverse EOIs of grade ≥3 among those entering the blinatumomab maintenance phase (N = 36)
| EOI category . | Induction (cycles 1 to 2), n (%) . | Consolidation (cycles 3 to 5), n (%) . | Maintenance (cycles ≥6), n (%) . |
|---|---|---|---|
| Any adverse EOI | 35 (97.2) | 31 (86.1) | 26 (72.2) |
| Event leading to treatment discontinuation | 0 (0) | 0 (0) | 2 (5.6) |
| Serious adverse event | 11 (30.6) | 11 (30.6) | 13 (36.1) |
| Fatal adverse event | 0 (0) | 0 (0) | 1 (2.8) |
| Any adverse event of grade ≥3 | 30 (83.3) | 19 (52.8) | 14 (38.9) |
| Grade ≥3 adverse EOIs reported in at least 3% of patients | |||
| Central neuropsychiatric events due to direct neurotoxicities | 4 (11.1) | 0 (0) | 4 (11.1)* |
| CRS | 2 (5.6) | 0 (0) | 1 (2.8)† |
| Cytopenia | 22 (61.1) | 12 (33.3) | 6 (16.7) |
| Decreased immunoglobulins | 3 (8.3) | 1 (2.8) | 2 (5.6) |
| Infections | 6 (16.7) | 8 (22.2) | 8 (22.2)‡ |
| Lymphopenia | 0 (0) | 1 (2.8) | 2 (5.6) |
| Neutropenia | 16 (44.4) | 12 (33.3) | 2 (5.6) |
| Elevated liver enzyme | 5 (13.9) | 1 (2.8) | 1 (2.8) |
| Infusion reaction considering duration | 3 (8.3) | 0 (0) | 0 (0) |
| EOI category . | Induction (cycles 1 to 2), n (%) . | Consolidation (cycles 3 to 5), n (%) . | Maintenance (cycles ≥6), n (%) . |
|---|---|---|---|
| Any adverse EOI | 35 (97.2) | 31 (86.1) | 26 (72.2) |
| Event leading to treatment discontinuation | 0 (0) | 0 (0) | 2 (5.6) |
| Serious adverse event | 11 (30.6) | 11 (30.6) | 13 (36.1) |
| Fatal adverse event | 0 (0) | 0 (0) | 1 (2.8) |
| Any adverse event of grade ≥3 | 30 (83.3) | 19 (52.8) | 14 (38.9) |
| Grade ≥3 adverse EOIs reported in at least 3% of patients | |||
| Central neuropsychiatric events due to direct neurotoxicities | 4 (11.1) | 0 (0) | 4 (11.1)* |
| CRS | 2 (5.6) | 0 (0) | 1 (2.8)† |
| Cytopenia | 22 (61.1) | 12 (33.3) | 6 (16.7) |
| Decreased immunoglobulins | 3 (8.3) | 1 (2.8) | 2 (5.6) |
| Infections | 6 (16.7) | 8 (22.2) | 8 (22.2)‡ |
| Lymphopenia | 0 (0) | 1 (2.8) | 2 (5.6) |
| Neutropenia | 16 (44.4) | 12 (33.3) | 2 (5.6) |
| Elevated liver enzyme | 5 (13.9) | 1 (2.8) | 1 (2.8) |
| Infusion reaction considering duration | 3 (8.3) | 0 (0) | 0 (0) |
Ataxia (n = 1), progressive multifocal leukoencephalopathy (n = 1), sensory loss (n = 1), somnolence (n = 1).
Grade 3 macrophage activation syndrome in cycle 6, while in MRD + CR.
Device-related infection (n = 2), Clostridium difficile (n = 1), mastoiditis (n = 1), sepsis (n = 1), pneumonia (n = 1), upper respiratory tract infection (n = 1), urinary tract infection (n = 1), varicella zoster infection (n = 1).
Discussion
As previously reported, blinatumomab induction (cycles 1 to 2) resulted in significantly longer OS compared with SOC in adults with R/R BCP-ALL.5 Here we report the study outcomes for those patients entering consolidation (cycles 3 to 5) and maintenance (cycles ≥6) treated with blinatumomab. OS and RFS were numerically longer with consolidation therapy vs no-consolidation therapy (ie, stopping blinatumomab after induction), and improved survival outcomes were achieved for patients with maintenance therapy compared with no-maintenance therapy (ie, stopping blinatumomab after consolidation). A large number of patients who achieved a best hematological response of CR were able to maintain their responses with additional cycles of blinatumomab.
The safety profile of blinatumomab across induction, consolidation, and maintenance phases was generally consistent with that seen in other studies, with the incidence of most adverse events decreasing with additional exposure to blinatumomab.9,10 Given the tolerability and efficacy of blinatumomab in patients receiving consolidation and maintenance therapy, blinatumomab may be an important therapeutic option to consider for long-term treatment.
An important limitation of this ad hoc analysis is the small sample size of 86 patients from consolidation and 36 patients from maintenance. The small sample size did not provide sufficient power to detect an OS or RFS benefit from both consolidation and maintenance cycles of blinatumomab. However, the evidence of OS improvement was found in patients who received maintenance compared with those who did not, despite being eligible. As the small number of patients also made censoring at HSCT not feasible, any favorable effects of additional blinatumomab cycles might have been dampened due to the potential benefit of HSCT in those patients who received HSCT but not additional blinatumomab cycles.
In conclusion, these findings suggest that long-term blinatumomab therapy has the potential to help some patients achieve and maintain a hematological CR. However, because many patients were not assessed for MRD response in this study, it is not possible to interpret the effect of extended blinatumomab therapy on molecular remission. However, additional blinatumomab maintenance cycles were found to prolong OS and trended toward longer remission duration. Therefore, blinatumomab may be a suitable option for long-term therapy with a manageable safety profile, but larger studies are warranted to confirm these findings.
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing. E-mail the corresponding author, Alessandro Rambaldi (alessandro.rambaldi@unimi.it), or Beatrice Chiang (bchiang@amgen.com).
Acknowledgments
Beatrice Chiang and Julie Gegner, employees of Amgen Inc, provided medical writing and editing assistance. CACTUS Communications Inc (on behalf of Amgen Inc) edited the figures and performed quality check.
This study was funded by Amgen Inc.
Authorship
Contribution: A.R., F.H., P.Z., and P.C. were responsible for the patient data collection/acquisition of data and contributed to the analysis and interpretation of data; Q.T. contributed to the analysis and interpretation of data; J.F. contributed to the conception and design of study and the analysis and interpretation of data; and M.S.T. contributed to the conception and design of the study, was responsible for patient data collection/acquisition of data, and contributed to the analysis and interpretation of data.
Conflict-of-interest disclosure: A.R. received consulting/advisory fees from Amgen, Novartis, Roche/Genentech, and Italfarmaco, and travel expenses from Celgene and Sanofi. F.H. received honoraria and consulting/advisory fees from Amgen, Bristol-Myers Squibb, Novartis, Incyte, Jazz Pharma, and Pfizer. P.C. received travel support from Amgen. Q.T. is employed by and holds stock in Amgen Inc. J.F. is employed by and holds stock in Amgen Inc. M.S.T. received honoraria and speakers’ bureau fees from Amgen Inc; consulting/advisory fees from Amgen Inc, Roche, and Regeneron; and travel expenses from Amgen Inc and Roche. P.Z. declares no competing financial interests.
Correspondence: Alessandro Rambaldi, Università Statale di Milano, Milan and Azienda Socio Sanitaria Territoriale Papa Giovanni XXIII, Bergamo, Italy, Piazza OMS 1, 24127 Bergamo, Italy; e-mail: alessandro.rambaldi@unimi.it.
References
Author notes
The full-text version of this article contains a data supplement.