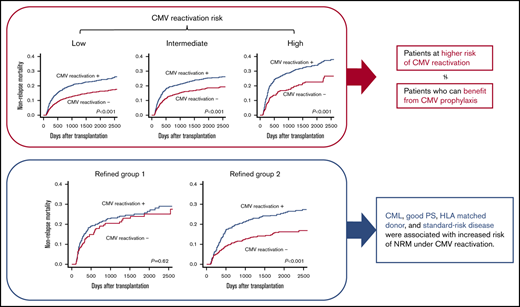
Key Points
Even among transplant recipients at low risk for CMV reactivation, reactivation was associated with higher NRM.
CML, good PS, HLA-matched donor, and standard-risk disease were associated with increased risk for NRM under CMV reactivation.
Abstract
Cytomegalovirus (CMV) infection is a major complication in allogeneic stem cell transplantation. The utility of CMV prophylaxis with letermovir has been reported; however, the specific applications remain unclear. In this study, we retrospectively analyzed large-scale registry data (N = 10 480) to clarify the risk factors for nonrelapse mortality (NRM) in connection with CMV reactivation. First, we identified risk factors for CMV reactivation using multivariate analysis and developed a scoring model. Although the model effectively stratified reactivation risk into 3 groups (43.7% vs 60.9% vs 71.5%; P < .001), the 3-year NRM was significantly higher in patients with CMV reactivation, even in the low (20.9% vs 13.0%, P < .001), intermediate (21.4% vs 15.6%; P < .001), and high (29.3% vs 18.0%; P < .001) reactivation risk groups. Next, survival analysis considering competing risks, time-dependent covariates, and interaction terms for exploring the heterogeneous impact of CMV reactivation on NRM in the training cohort revealed that chronic myeloid leukemia (CML) (hazard ratio [HR], 1.76; 95% confidence interval [CI], 1.05-2.96; P = .033), good performance status (PS) (HR, 1.42; 95% CI, 1.04-1.94; P = .028), HLA-matched donor (HR, 1.34; 95% CI, 1.06-1.70; P = .013), and standard-risk disease (HR, 1.28; 95% CI, 1.04-1.58; P = .022) were associated with increased NRM. In the test cohort, CMV reactivation was significantly associated with increased 3-year NRM among patients with 2 to 4 factors (22.1% vs 13.1%; P < .001) but was comparable among patients with 0 or 1 factor (23.2% vs 20.4%; P = .62). We propose that CMV prophylaxis should be determined based on reactivation risk, as well as these other factors.
Introduction
Cytomegalovirus (CMV) diseases are major causes of significant morbidity and mortality in allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients.1-3 Preemptive therapy with ganciclovir can effectively prevent CMV diseases,4,5 whereas prophylactic treatment with ganciclovir failed to improve survival outcomes because of drug-induced myelosuppression and increased risk for other infections.6,7 Therefore, preemptive therapy has been commonly used as a standard strategy in recent years. However, CMV infection, rather than CMV end-organ disease, remains a major complication and is known to be associated with high nonrelapse mortality (NRM) rates in allo-HSCT recipients (ie, indirect effects).8-10 Preemptive strategies could limit the incidence of many CMV diseases, but they are not sufficient to prevent CMV infection and indirect effects.11
Recent data on CMV prophylaxis with letermovir have shown that this drug effectively decreases the risk of clinically significant CMV infection and may reduce the risk of overall mortality in allo-HSCT recipients.12-15 However, there are no standard criteria for the ideal application of letermovir, and universal prophylaxis could result in overtreatment for several reasons. First, only approximately half of allo-HSCT recipients develop CMV infection without any prophylaxis, and some side effects, including gastrointestinal toxicity, may occur because of letermovir administration. Second, some reports have described breakthrough infection during letermovir prophylaxis, and excessive use can promote intrinsic resistance against letermovir.13,16,17 Third, widespread use of letermovir could lead to increased medical costs, although some studies have suggested that prophylactic letermovir is a cost-effective option.18,19 Therefore, optimization of letermovir application is necessary.
In some studies, including the phase 3 study of letermovir prophylaxis, the benefit of CMV prophylaxis was considered important in patients at higher risk for CMV reactivation.13,15,20 However, the association between CMV reactivation risk and direct/indirect mortality risk has not been clarified and whether the requirement for letermovir prophylaxis really depends on CMV reactivation risk should be elucidated.
Accordingly, in the current study, we aimed to evaluate the heterogeneous impact of CMV reactivation on NRM using large-scale registry data from the Japan Society for Hematopoietic Cell Transplantation. To avoid multiple subgroup analyses, which could lead to substantial false-positive findings, we examined whether the effects of CMV reactivation on NRM were regulated by candidate factors with significant interactions.21 We implemented this by including the interaction terms of the candidate factors and the CMV reactivation incidence in the survival analysis with competing risks and time-dependent covariates, as has been reported in the literature.9,10
Patients and methods
Data source and patient selection
Clinical data for the patients were collected through the Transplant Registry Unified Management Program, which is a nationwide data registry managed by the Japan Society for Hematopoietic Cell Transplantation and the Japanese Data Center for Hematopoietic Cell Transplantation.22 Patients ranging in age from 18 to 70 years with acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, or chronic myeloid leukemia (CML) and who underwent initial allo-HSCT from 2004 through 2016 were initially included in the study. Among these patients, those who had complete data on survival status, relapse, and preemptive therapy for CMV were used for the analysis. To focus on common conditions, patients who received double-unit cord blood (CB) or combined bone marrow and peripheral blood stem cells (PBSCs) as a graft source, those who received posttransplant cyclophosphamide for graft-versus-host disease (GVHD) prophylaxis, those who failed to achieve neutrophil engraftment or hematological complete remission after allo-HSCT, and those who received anti-CMV therapy before neutrophil engraftment were excluded. This retrospective study was performed in accordance with the Declaration of Helsinki and was approved by the data management committees of the Transplant Registry Unified Management Program and the Institutional Review Board of the Cancer and Infectious Diseases Center, Tokyo Metropolitan Komagome Hospital.
Definitions
Surveillance of pp65 antigenemia was generally started at the time of neutrophil engraftment after allo-HSCT. Few patients were evaluated by polymerase chain reaction (PCR) for CMV because PCR is not covered by the public insurance system in Japan. CMV reactivation was defined as the beginning of CMV preemptive therapy, as previously described.10 CB was considered donor negative for CMV by definition. Performance status (PS) was evaluated according to Eastern Cooperative Oncology Group criteria; PS of 0 or 1 was defined as good, whereas scores of 2 through 4 were defined as poor. Conditioning intensity was classified based on a report by the Center for International Blood and Marrow Transplant Research.23 HLA disparity was defined as HLA matched when allo-HSCT was performed from serologically HLA-A, HLA-B, HLA-C, or HLA-DR 8/8 matched related donors or allele 8/8 matched unrelated donors. Disease risk was defined as previously reported.24 T-cell depletion (TCD) in vivo was defined as the use of anti-thymocyte globulin, anti-lymphocyte globulin, or alemtuzumab before transplantation. Detailed transplant procedures are described in supplemental Methods.
Statistical analysis and establishment of a scoring model
Overall survival was defined as the time between transplantation and death owing to any cause or last follow-up. Disease-free survival was defined as the time interval from allo-HSCT to the first event (relapse or death). NRM was defined as death without relapse. The cumulative incidences of relapse and NRM were evaluated using Gray’s method by considering each risk as a competing risk.25 The cumulative incidence of CMV reactivation was also evaluated using Gray’s method by considering relapse and NRM as competing risks. Multivariate analysis for cumulative incidence of CMV reactivation, including variables associated with P ≤ .05 in univariate analysis, was performed using the method of Fine and Gray. The scoring model for CMV reactivation included variables with P ≤ .0023 in the multivariate analysis (with the exception of transplant year), based on Bonferroni corrections.
To establish and validate the heterogeneous effects of CMV reactivation on NRM, data from patients who had complete information on candidate factors were used. We divided the cohort into 2 groups: the training cohort (75%) to devise the models and the test cohort (25%) to validate the models.26 We used the survival analysis for NRM with relapse as a competing risk and CMV reactivation incidence as a time-dependent covariate among the training cohort, as previously described.27 Briefly, individuals who developed CMV reactivation after allo-HSCT were split into pseudoindividuals for pre-CMV and post-CMV reactivation periods. Next, survival analysis of pseudoindividuals was performed, and relative risks in the post-CMV to pre-CMV reactivation periods were assessed for candidate factors. This method handled left truncation, as well as right censoring for the post-CMV reactivation period appropriately. A positive (negative) coefficient for the interaction term of a candidate factor with the CMV reactivation incidence could be interpreted as evidence of the aggravating (alleviating) effects of the factor on the impact of CMV reactivation on NRM.21 Therefore, statistically significant coefficients of the interaction terms demonstrated the heterogeneous impact of CMV reactivation on NRM.
A scoring model for the heterogeneous impact of CMV reactivation on NRM was developed using variables having a coefficient of P ≤ .05 in the interaction term with CMV reactivation incidence, and data were evaluated using landmark analysis at day 100 for the test cohort, as well as CMV immunoglobulin G (IgG)–positive and -negative recipients in the whole cohort. To check the robustness, we also performed the interaction analysis excluding CB and including acute GVHD (grade II-IV) as a time-dependent covariate. All P values were 2-sided, and P ≤ .05 was considered significant. Statistical analyses were performed with R (version 3. 5. 0; The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/).
Results
Patient characteristics
From 2004 to 2016, 20 756 patients with acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, or CML who underwent initial allo-HSCT were registered in the database. Among these patients, 4356 were excluded: 1075 patients lacked data on survival status, relapse, or preemptive therapy for CMV, 1041 patients failed to achieve neutrophil engraftment or lacked data on neutrophil engraftment, 175 patients received posttransplant cyclophosphamide for GVHD prophylaxis, 66 patients received double-unit CB or combined bone marrow and PBSCs as a graft source, 1725 patients failed to show hematological remission after allo-HSCT, and 274 patients received anti-CMV therapy before neutrophil engraftment. Thus, 16 400 patients were selected (supplemental Table 1); among them, patients with complete data were included in the subsequent analyses (n = 10 480). Patient characteristics are summarized in Table 1. The median age at allo-HSCT for recipients and donors was 49 years (range, 18-70) and 34 years (range, 0-69), respectively. CMV reactivation was observed in 5743 patients (54.8%) at a median of 42 days (interquartile range, 32-52 days) after transplantation. Among these patients, CMV reactivation was found in 5629 patients (98.0%) by day 100 after transplantation. The cumulative incidence of CMV reactivation among all patients was 53.8% (95% confidence interval [CI], 52.9-54.8) at day 100 after transplantation. Overall survival, disease-free survival, cumulative incidence of relapse, and NRM were 55.5% (95% CI, 54.4-56.5), 50.0% (95% CI, 49.0-51.0), 26.9% (95% CI, 26.0-27.8), and 23.1% (95% CI, 22.3-24.0), respectively, at 3 years after transplantation.
Patient characteristics in training and test cohorts
| Variables . | All patients (N = 10 480) . | Training cohort (n = 7860) . | Test cohort (n = 2620) . | P . |
|---|---|---|---|---|
| Underlying disease | .35 | |||
| Acute myeloid leukemia | 5811 (55.4) | 4344 (55.3) | 1467 (56.0) | |
| Acute lymphoblastic leukemia | 2466 (23.5) | 1873 (23.8) | 593 (22.6) | |
| Myelodysplastic syndromes | 1801 (17.2) | 1333 (17.0) | 468 (17.9) | |
| CML | 402 (3.8) | 310 (3.9) | 92 (3.5) | |
| Patient age, median (range), y | 49 (18-70) | 49 (18-70) | 49 (18-70) | .44 |
| Patient age ≥50 y | 5154 (49.2) | 3861 (49.1) | 1293 (49.4) | .84 |
| Donor age, median (range), y | 33 (0-69) | 33 (0-69) | 33 (0-68) | .33 |
| Donor age ≥50 y | 1181 (11.3) | 889 (11.3) | 292 (11.1) | .83 |
| Patient sex, male | 6112 (58.3) | 4575 (58.2) | 1537 (58.7) | .70 |
| Donor sex, male | 6318 (60.3) | 4748 (60.4) | 1570 (59.9) | .66 |
| Female donor to male recipient | 2325 (22.2) | 1742 (22.2) | 583 (22.3) | .94 |
| Recipient/donor CMV serology | .75 | |||
| Positive/positive | 4329 (41.3) | 3236 (41.2) | 1093 (41.7) | |
| Positive/negative | 4201 (40.1) | 3143 (40.0) | 1058 (40.4) | |
| Negative/positive | 754 (7.2) | 575 (7.3) | 179 (6.8) | |
| Negative/negative | 1196 (11.4) | 906 (11.5) | 290 (11.1) | |
| Disease risk (high) | 4240 (40.5) | 3134 (39.9) | 1106 (42.2) | .036 |
| PS (poor) | 718 (6.9) | 563 (7.2) | 155 (5.9) | .029 |
| Stem cell source | .55 | |||
| Bone marrow | 5920 (56.5) | 4418 (56.2) | 1502 (57.3) | |
| PBSC | 1853 (17.7) | 1405 (17.9) | 448 (17.1) | |
| CB | 2707 (25.8) | 2037 (25.9) | 670 (25.6) | |
| Transplant from unrelated donor | 7898 (75.4) | 5928 (75.4) | 1970 (75.2) | .81 |
| HLA disparity (mismatch) | 6314 (60.2) | 4742 (60.3) | 1572 (60.0) | .77 |
| Conditioning (reduced intensity) | 2774 (26.5) | 2106 (26.8) | 668 (25.5) | .20 |
| Total body irradiation | 7521 (71.8) | 5688 (72.4) | 1833 (70.0) | .019 |
| GVHD prophylaxis | ||||
| Tacrolimus-based regimen | 7352 (70.2) | 5534 (70.4) | 1818 (69.4) | .32 |
| Mycophenolate mofetil use | 1154 (11.0) | 857 (10.9) | 297 (11.3) | .54 |
| TCD in vivo | 872 (8.3) | 676 (8.6) | 196 (7.5) | .072 |
| Transplant year | .67 | |||
| 2004-2010 | 3878 (37.0) | 2918 (37.1) | 960 (36.6) | |
| 2011-2016 | 6602 (63.0) | 4942 (62.9) | 1660 (63.4) |
| Variables . | All patients (N = 10 480) . | Training cohort (n = 7860) . | Test cohort (n = 2620) . | P . |
|---|---|---|---|---|
| Underlying disease | .35 | |||
| Acute myeloid leukemia | 5811 (55.4) | 4344 (55.3) | 1467 (56.0) | |
| Acute lymphoblastic leukemia | 2466 (23.5) | 1873 (23.8) | 593 (22.6) | |
| Myelodysplastic syndromes | 1801 (17.2) | 1333 (17.0) | 468 (17.9) | |
| CML | 402 (3.8) | 310 (3.9) | 92 (3.5) | |
| Patient age, median (range), y | 49 (18-70) | 49 (18-70) | 49 (18-70) | .44 |
| Patient age ≥50 y | 5154 (49.2) | 3861 (49.1) | 1293 (49.4) | .84 |
| Donor age, median (range), y | 33 (0-69) | 33 (0-69) | 33 (0-68) | .33 |
| Donor age ≥50 y | 1181 (11.3) | 889 (11.3) | 292 (11.1) | .83 |
| Patient sex, male | 6112 (58.3) | 4575 (58.2) | 1537 (58.7) | .70 |
| Donor sex, male | 6318 (60.3) | 4748 (60.4) | 1570 (59.9) | .66 |
| Female donor to male recipient | 2325 (22.2) | 1742 (22.2) | 583 (22.3) | .94 |
| Recipient/donor CMV serology | .75 | |||
| Positive/positive | 4329 (41.3) | 3236 (41.2) | 1093 (41.7) | |
| Positive/negative | 4201 (40.1) | 3143 (40.0) | 1058 (40.4) | |
| Negative/positive | 754 (7.2) | 575 (7.3) | 179 (6.8) | |
| Negative/negative | 1196 (11.4) | 906 (11.5) | 290 (11.1) | |
| Disease risk (high) | 4240 (40.5) | 3134 (39.9) | 1106 (42.2) | .036 |
| PS (poor) | 718 (6.9) | 563 (7.2) | 155 (5.9) | .029 |
| Stem cell source | .55 | |||
| Bone marrow | 5920 (56.5) | 4418 (56.2) | 1502 (57.3) | |
| PBSC | 1853 (17.7) | 1405 (17.9) | 448 (17.1) | |
| CB | 2707 (25.8) | 2037 (25.9) | 670 (25.6) | |
| Transplant from unrelated donor | 7898 (75.4) | 5928 (75.4) | 1970 (75.2) | .81 |
| HLA disparity (mismatch) | 6314 (60.2) | 4742 (60.3) | 1572 (60.0) | .77 |
| Conditioning (reduced intensity) | 2774 (26.5) | 2106 (26.8) | 668 (25.5) | .20 |
| Total body irradiation | 7521 (71.8) | 5688 (72.4) | 1833 (70.0) | .019 |
| GVHD prophylaxis | ||||
| Tacrolimus-based regimen | 7352 (70.2) | 5534 (70.4) | 1818 (69.4) | .32 |
| Mycophenolate mofetil use | 1154 (11.0) | 857 (10.9) | 297 (11.3) | .54 |
| TCD in vivo | 872 (8.3) | 676 (8.6) | 196 (7.5) | .072 |
| Transplant year | .67 | |||
| 2004-2010 | 3878 (37.0) | 2918 (37.1) | 960 (36.6) | |
| 2011-2016 | 6602 (63.0) | 4942 (62.9) | 1660 (63.4) |
Unless otherwise noted, all data are n (%).
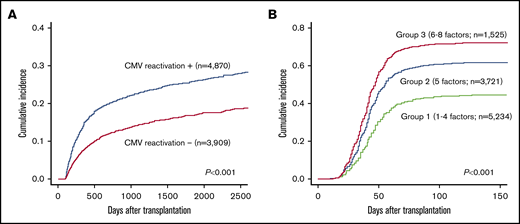
Using landmark analysis for 8779 patients who survived without relapse for 100 days after allo-HSCT, CMV reactivation by day 100 after transplantation was associated with a significant increase in NRM (incidence of NRM at 3 years after transplantation: 4870 patients with CMV reactivation vs 3909 patients without CMV reactivation, 22.7% vs 14.2%, respectively; P < .001; Figure 1A).
The impact of CMV reactivation on NRM and strarification of CMV reactivation risk after transplantation. (A) The impact of CMV reactivation on NRM after allo-HSCT by a landmark analysis at day 100. (B) Cumulative incidence of CMV reactivation stratified into 3 groups by the number of significant risk factors.
The impact of CMV reactivation on NRM and strarification of CMV reactivation risk after transplantation. (A) The impact of CMV reactivation on NRM after allo-HSCT by a landmark analysis at day 100. (B) Cumulative incidence of CMV reactivation stratified into 3 groups by the number of significant risk factors.
Scoring model for CMV reactivation
The results of univariate and multivariate analyses for CMV reactivation among the whole cohort (N = 10 480) are shown in Table 2. Upon multivariate analysis, recipient positive/donor negative CMV serology (hazard ratio [HR], 2.48; 95% CI, 2.22-2.76; P < .001), recipient positive/donor positive CMV serology (HR, 2.20; 95% CI, 1.96-2.47; P < .001), TCD in vivo (HR, 1.64; 95% CI, 1.48-1.82; P < .001), HLA disparity (HR, 1.46; 95% CI, 1.37-1.55; P < .001), age ≥50 years (HR, 1.30; 95% CI, 1.22-1.38; P < .001), transplant from an unrelated donor (HR, 1.27; 95% CI, 1.13-1.42; P < .001), total body irradiation (TBI; HR, 1.12; 95% CI, 1.06-1.19; P < .001), older transplant year (HR, 1.12; 95% CI, 1.06-1.18; P < .001), tacrolimus-based GVHD prophylaxis regimen (HR, 0.90; 95% CI, 0.84-0.96; P = .002), and CB (HR, 0.86; 95% CI, 0.79-0.93; P < .001) were significant factors. To develop a scoring model, a score of 1 was assigned to each factor (recipient CMV seropositivity, TCD in vivo, HLA disparity, older age [≥50 years], transplant from unrelated donor, and TBI). Transplant from a source other than CB (bone marrow or PBSCs) and non–tacrolimus-based GVHD prophylaxis were also assigned a score of 1 because CB and tacrolimus-based GVHD prophylaxis were inversely associated with CMV reactivation in multivariate analysis. Transplant year was excluded from the model for predicting the prognosis of future patients. The scoring model effectively stratified the patients into 3 groups: 5234 patients with scores of 1 through 4 (group 1), 3721 patients with a score of 5 (group 2), and 1525 patients with scores of 6 to 8 (group 3). The cumulative incidence of CMV reactivation at day 100 after transplantation in groups 1, 2, and 3 was 43.7% (95% CI, 42.3-45.0), 60.9% (95% CI, 59.3-62.5), and 71.5% (95% CI, 69.2-73.7), respectively (P < .001) (Figure 1B).
Univariate and multivariate analysis for cumulative incidence of CMV reactivation
| Variables . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Underlying disease* | ||||||
| Acute lymphoblastic leukemia | 0.93 | 0.87-0.99 | .035 | 1.01 | 0.94-1.09 | .79 |
| Myelodysplastic syndromes | 1.06 | 0.99-1.14 | .071 | 1.03 | 0.96-1.10 | .38 |
| CML | 0.83 | 0.72-0.95 | .009 | 0.89 | 0.78-1.02 | .10 |
| Patient age ≥50 y | 1.38 | 1.31-1.46 | <.001 | 1.30 | 1.22-1.38 | <.001 |
| Donor age ≥50 y | 0.87 | 0.80-0.94 | <.001 | 0.97 | 0.88-1.07 | .52 |
| Patient sex, male | 1.01 | 0.95-1.06 | .85 | 0.97 | 0.90-1.04 | .35 |
| Donor sex, male | 1.03 | 0.98-1.09 | .22 | 1.07 | 0.99-1.16 | .10 |
| Female donor/male recipient | 1.00 | 0.94-1.07 | .89 | 1.07 | 0.96-1.19 | .22 |
| Recipient/donor CMV serology† | ||||||
| Positive/positive | 2.17 | 1.95-2.42 | <.001 | 2.20 | 1.96-2.47 | <.001 |
| Positive/negative | 2.63 | 2.36-2.93 | <.001 | 2.48 | 2.22-2.76 | <.001 |
| Negative/positive | 1.11 | 0.95-1.30 | .20 | 1.16 | 0.98-1.36 | .080 |
| Disease risk (high) | 1.04 | 0.99-1.09 | .15 | 0.94 | 0.89-1.00 | 0.042 |
| PS (poor) | 1.03 | 0.94-1.14 | .51 | 0.95 | 0.86-1.05 | .30 |
| Stem cell source‡ | ||||||
| PBSC | 0.88 | 0.82-0.95 | <.001 | 1.02 | 0.91-1.14 | .72 |
| CB | 1.09 | 1.03-1.15 | .005 | 0.86 | 0.79-0.93 | <.001 |
| Transplant from unrelated donor | 1.29 | 1.21-1.37 | <.001 | 1.27 | 1.13-1.42 | <0.001 |
| HLA disparity (mismatch) | 1.51 | 1.43-1.59 | <.001 | 1.46 | 1.37-1.55 | <.001 |
| Conditioning (reduced intensity) | 1.25 | 1.18-1.32 | <.001 | 1.03 | 0.97-1.10 | .34 |
| TBI | 1.06 | 1.01-1.13 | .032 | 1.12 | 1.06-1.19 | <.001 |
| GVHD prophylaxis | ||||||
| Tacrolimus-based regimen§ | 1.13 | 1.06-1.19 | <.001 | 0.90 | 0.84-0.96 | .002 |
| Mycophenolate mofetil use | 0.97 | 0.89-1.05 | .41 | 0.88 | 0.80-0.96 | .006 |
| TCD in vivo | 1.73 | 1.57-1.89 | <.001 | 1.64 | 1.48-1.82 | <.001 |
| Transplant year (2004-2010)¶ | 1.10 | 1.04-1.16 | <.001 | 1.12 | 1.06-1.18 | <.001 |
| Variables . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Underlying disease* | ||||||
| Acute lymphoblastic leukemia | 0.93 | 0.87-0.99 | .035 | 1.01 | 0.94-1.09 | .79 |
| Myelodysplastic syndromes | 1.06 | 0.99-1.14 | .071 | 1.03 | 0.96-1.10 | .38 |
| CML | 0.83 | 0.72-0.95 | .009 | 0.89 | 0.78-1.02 | .10 |
| Patient age ≥50 y | 1.38 | 1.31-1.46 | <.001 | 1.30 | 1.22-1.38 | <.001 |
| Donor age ≥50 y | 0.87 | 0.80-0.94 | <.001 | 0.97 | 0.88-1.07 | .52 |
| Patient sex, male | 1.01 | 0.95-1.06 | .85 | 0.97 | 0.90-1.04 | .35 |
| Donor sex, male | 1.03 | 0.98-1.09 | .22 | 1.07 | 0.99-1.16 | .10 |
| Female donor/male recipient | 1.00 | 0.94-1.07 | .89 | 1.07 | 0.96-1.19 | .22 |
| Recipient/donor CMV serology† | ||||||
| Positive/positive | 2.17 | 1.95-2.42 | <.001 | 2.20 | 1.96-2.47 | <.001 |
| Positive/negative | 2.63 | 2.36-2.93 | <.001 | 2.48 | 2.22-2.76 | <.001 |
| Negative/positive | 1.11 | 0.95-1.30 | .20 | 1.16 | 0.98-1.36 | .080 |
| Disease risk (high) | 1.04 | 0.99-1.09 | .15 | 0.94 | 0.89-1.00 | 0.042 |
| PS (poor) | 1.03 | 0.94-1.14 | .51 | 0.95 | 0.86-1.05 | .30 |
| Stem cell source‡ | ||||||
| PBSC | 0.88 | 0.82-0.95 | <.001 | 1.02 | 0.91-1.14 | .72 |
| CB | 1.09 | 1.03-1.15 | .005 | 0.86 | 0.79-0.93 | <.001 |
| Transplant from unrelated donor | 1.29 | 1.21-1.37 | <.001 | 1.27 | 1.13-1.42 | <0.001 |
| HLA disparity (mismatch) | 1.51 | 1.43-1.59 | <.001 | 1.46 | 1.37-1.55 | <.001 |
| Conditioning (reduced intensity) | 1.25 | 1.18-1.32 | <.001 | 1.03 | 0.97-1.10 | .34 |
| TBI | 1.06 | 1.01-1.13 | .032 | 1.12 | 1.06-1.19 | <.001 |
| GVHD prophylaxis | ||||||
| Tacrolimus-based regimen§ | 1.13 | 1.06-1.19 | <.001 | 0.90 | 0.84-0.96 | .002 |
| Mycophenolate mofetil use | 0.97 | 0.89-1.05 | .41 | 0.88 | 0.80-0.96 | .006 |
| TCD in vivo | 1.73 | 1.57-1.89 | <.001 | 1.64 | 1.48-1.82 | <.001 |
| Transplant year (2004-2010)¶ | 1.10 | 1.04-1.16 | <.001 | 1.12 | 1.06-1.18 | <.001 |
Acute myeloid leukemia was treated as reference.
Negative/negative CMV serology was treated as reference.
Bone marrow transplantation was treated as reference.
Cyclosporine-based regimen was treated as reference.
Transplant year (2011-2016) was treated as reference.
Association between CMV reactivation and its impact on NRM
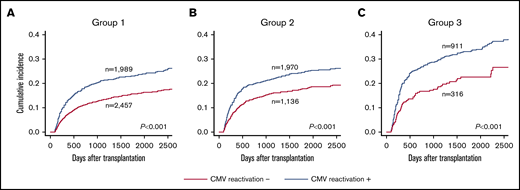
Next, we examined the impact of CMV reactivation on NRM in 3 groups, which were divided based on the scoring model described above, using landmark analysis on day 100. CMV reactivation was associated with increased NRM among patients in group 1 (3-year NRM with CMV reactivation vs without CMV reactivation, 20.9% vs 13.0%; P < .001), group 2 (21.4% vs 15.6%; P < .001), and group 3 (29.3% vs 18.0%; P < .001) (Figure 2). Even among transplant recipients at low risk for CMV reactivation, the adverse impact observed on NRM was significant. Therefore, NRM risk associated with CMV reactivation may have to be evaluated separately from reactivation risk itself.
The impact of CMV reactivation on NRM among the patients in each group by a landmark analysis at day 100.(A) Group1 (low reactivation risk group). (B) Group 2 (intermediate risk group). (C) Group 3 (high risk group).
The impact of CMV reactivation on NRM among the patients in each group by a landmark analysis at day 100.(A) Group1 (low reactivation risk group). (B) Group 2 (intermediate risk group). (C) Group 3 (high risk group).
Heterogeneous impact of CMV reactivation on NRM
To identify the risk factors associated with NRM in connection with CMV reactivation, the impact of CMV reactivation on NRM was evaluated considering the interaction terms of the candidate factors and CMV reactivation incidence in the survival analysis.20 Among the whole cohort (N = 10 480), three fourths of the patients (n = 7860) were randomly allocated into the training cohort to devise the models, and the remaining one fourth (n = 2620) was allocated to the test cohort to validate the models. Patient characteristics for each cohort are summarized in Table 1. Interaction analysis of training cohort data revealed that CML (HR, 1.76; 95% CI, 1.05-2.96; P = .033) was significantly associated with an increase in NRM under CMV reactivation. Moreover, poor PS (HR, 0.70; 95% CI, 0.52-0.96; P = .028), transplantation from HLA-mismatched donors (HR, 0.74; 95% CI, 0.59-0.94; P = .013), and high disease risk (HR, 0.78; 95% CI, 0.63-0.97; P = .022) were inversely associated with increased NRM under CMV reactivation (Table 3).
Multivariate analysis on NRM considering the interaction between CMV reactivation and the other factors
| Variables . | Baseline . | Interaction term . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| CMV reactivation | 1.92 | 1.05-3.50 | .035 | |||
| Underlying disease* | ||||||
| Acute lymphoblastic leukemia | 1.09 | 0.89-1.32 | .41 | 1.15 | 0.89-1.47 | .29 |
| Myelodysplastic syndromes | 1.05 | 0.86-1.29 | .64 | 1.13 | 0.87-1.46 | .36 |
| CML | 0.74 | 0.48-1.14 | .17 | 1.76 | 1.05-2.96 | .033 |
| Patient age ≥ 50 y | 1.51 | 1.26-1.80 | <.001 | 1.11 | 0.89-1.40 | .35 |
| Donor age ≥ 50 y | 1.35 | 1.05-1.74 | .020 | 0.90 | 0.65-1.24 | .51 |
| Patient sex, male | 1.23 | 1.00-1.51 | .050 | 0.96 | 0.75-1.24 | .78 |
| Donor sex, male | 0.99 | 0.77-1.27 | .92 | 1.29 | 0.89-1.69 | .21 |
| Female donor/male recipient | 1.01 | 0.74-1.38 | .96 | 1.30 | 0.87-1.95 | .20 |
| Recipient/donor CMV serology† | ||||||
| Positive/positive | 0.93 | 0.73-1.19 | .56 | 0.93 | 0.66-1.31 | .67 |
| Positive/negative | 0.97 | 0.78-1.22 | .83 | 0.79 | 0.57-1.08 | .14 |
| Negative/positive | 1.15 | 0.84-1.56 | .39 | 0.68 | 0.43-1.08 | .10 |
| Disease risk (high) | 1.53 | 1.30-1.81 | <.001 | 0.78 | 0.63-0.97 | .022 |
| PS (poor) | 1.78 | 1.41-2.25 | <.001 | 0.70 | 0.52-0.96 | .028 |
| Stem cell source‡ | ||||||
| PBSC | 1.26 | 0.92-1.72 | .15 | 1.18 | 0.78-1.78 | .43 |
| CB | 0.76 | 0.60-0.98 | .035 | 0.85 | 0.62-1.16 | .30 |
| Transplant from unrelated donor | 1.49 | 1.08-2.06 | .016 | 0.98 | 0.64-1.48 | .91 |
| HLA disparity (mismatch) | 1.87 | 1.56-2.25 | <.001 | 0.74 | 0.59-0.94 | .013 |
| Conditioning (reduced intensity) | 1.04 | 0.86-1.24 | .70 | 0.98 | 0.78-1.22 | .83 |
| TBI | 0.93 | 0.78-1.10 | .40 | 1.17 | 0.94-1.46 | .15 |
| GVHD prophylaxis | ||||||
| Tacrolimus-based regimen§ | 1.07 | 0.87-1.30 | .52 | 0.83 | 0.65-1.07 | .15 |
| Mycophenolate mofetil use | 1.21 | 0.95-1.54 | .13 | 1.15 | 0.84-1.56 | .38 |
| TCD in vivo | 0.69 | 0.50-0.97 | .032 | 1.36 | 0.92-2.01 | .13 |
| Transplant year (2004-2010) | 1.05 | 0.89-1.23 | .55 | 1.04 | 0.85-1.27 | .69 |
| Variables . | Baseline . | Interaction term . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| CMV reactivation | 1.92 | 1.05-3.50 | .035 | |||
| Underlying disease* | ||||||
| Acute lymphoblastic leukemia | 1.09 | 0.89-1.32 | .41 | 1.15 | 0.89-1.47 | .29 |
| Myelodysplastic syndromes | 1.05 | 0.86-1.29 | .64 | 1.13 | 0.87-1.46 | .36 |
| CML | 0.74 | 0.48-1.14 | .17 | 1.76 | 1.05-2.96 | .033 |
| Patient age ≥ 50 y | 1.51 | 1.26-1.80 | <.001 | 1.11 | 0.89-1.40 | .35 |
| Donor age ≥ 50 y | 1.35 | 1.05-1.74 | .020 | 0.90 | 0.65-1.24 | .51 |
| Patient sex, male | 1.23 | 1.00-1.51 | .050 | 0.96 | 0.75-1.24 | .78 |
| Donor sex, male | 0.99 | 0.77-1.27 | .92 | 1.29 | 0.89-1.69 | .21 |
| Female donor/male recipient | 1.01 | 0.74-1.38 | .96 | 1.30 | 0.87-1.95 | .20 |
| Recipient/donor CMV serology† | ||||||
| Positive/positive | 0.93 | 0.73-1.19 | .56 | 0.93 | 0.66-1.31 | .67 |
| Positive/negative | 0.97 | 0.78-1.22 | .83 | 0.79 | 0.57-1.08 | .14 |
| Negative/positive | 1.15 | 0.84-1.56 | .39 | 0.68 | 0.43-1.08 | .10 |
| Disease risk (high) | 1.53 | 1.30-1.81 | <.001 | 0.78 | 0.63-0.97 | .022 |
| PS (poor) | 1.78 | 1.41-2.25 | <.001 | 0.70 | 0.52-0.96 | .028 |
| Stem cell source‡ | ||||||
| PBSC | 1.26 | 0.92-1.72 | .15 | 1.18 | 0.78-1.78 | .43 |
| CB | 0.76 | 0.60-0.98 | .035 | 0.85 | 0.62-1.16 | .30 |
| Transplant from unrelated donor | 1.49 | 1.08-2.06 | .016 | 0.98 | 0.64-1.48 | .91 |
| HLA disparity (mismatch) | 1.87 | 1.56-2.25 | <.001 | 0.74 | 0.59-0.94 | .013 |
| Conditioning (reduced intensity) | 1.04 | 0.86-1.24 | .70 | 0.98 | 0.78-1.22 | .83 |
| TBI | 0.93 | 0.78-1.10 | .40 | 1.17 | 0.94-1.46 | .15 |
| GVHD prophylaxis | ||||||
| Tacrolimus-based regimen§ | 1.07 | 0.87-1.30 | .52 | 0.83 | 0.65-1.07 | .15 |
| Mycophenolate mofetil use | 1.21 | 0.95-1.54 | .13 | 1.15 | 0.84-1.56 | .38 |
| TCD in vivo | 0.69 | 0.50-0.97 | .032 | 1.36 | 0.92-2.01 | .13 |
| Transplant year (2004-2010) | 1.05 | 0.89-1.23 | .55 | 1.04 | 0.85-1.27 | .69 |
Acute myeloid leukemia was treated as reference.
Negative/negative CMV serology was treated as reference.
Bone marrow transplantation was treated as reference.
Cyclosporine-based regimen was treated as reference.
Validation of the heterogeneous impact in the test cohort
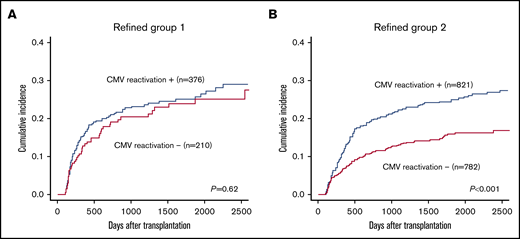
Finally, we validated the results of the interaction analysis by landmark analysis on day 100 using the test cohort dataset. Patients were divided into 3 refined groups based on the results for 4 factors (ie, CML, good PS, transplantation from an HLA-matched donor, and standard-risk disease), as identified in the interaction analysis. CMV reactivation was not significantly associated with increased NRM among the 586 patients with 0 or 1 factor (refined group 1: 3-year NRM with CMV reactivation vs without CMV reactivation, 23.2% vs 20.4%; P = .62). On the contrary, CMV reactivation was significantly associated with increased NRM among the 1603 patients with 2 to 4 factors (refined group 2: 22.1% vs 13.1%; P < .001; Figure 3). There were no obvious differences in the specific causes of death according to CMV reactivation or refined risk (Table 4).
The impact of CMV reactivation on NRM among the patients in each refined group by a landmark analysis at day 100. (A) Group 1. (B) Group 2.
The impact of CMV reactivation on NRM among the patients in each refined group by a landmark analysis at day 100. (A) Group 1. (B) Group 2.
Broad categories of causes of death among patients who survived without disease relapse at day 100
| Cause of death . | Whole cohort (N = 8779)* . | Refined group 1 (n = 2253)† . | Refined group 2 (n = 6526)‡ . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | CMV− (n = 188) . | % . | CMV+ (n = 369) . | % . | CMV− (n = 408) . | % . | CMV+ (n = 786) . | % . | |
| IPS/ARDS | 237 | 13.5 | 21 | 11.2 | 46 | 12.5 | 49 | 12.0 | 121 | 26.8 |
| Chronic GVHD | 156 | 8.9 | 10 | 5.3 | 25 | 6.8 | 50 | 12.3 | 71 | 15.4 |
| Acute GVHD | 103 | 5.9 | 11 | 5.9 | 20 | 5.4 | 16 | 3.9 | 56 | 9.0 |
| Renal failure/TMA | 82 | 4.7 | 6 | 3.2 | 20 | 5.4 | 19 | 4.7 | 37 | 7.1 |
| Liver failure/VOD/SOS | 63 | 3.6 | 7 | 3.7 | 14 | 3.8 | 20 | 4.9 | 22 | 4.7 |
| Bleeding | 59 | 3.4 | 5 | 2.7 | 12 | 3.3 | 12 | 2.9 | 30 | 2.8 |
| Secondary malignancy | 51 | 2.9 | 2 | 1.1 | 12 | 3.3 | 14 | 3.4 | 23 | 3.8 |
| Other organ failure | 43 | 2.5 | 7 | 3.7 | 12 | 3.3 | 8 | 2.0 | 16 | 2.9 |
| Heart failure | 25 | 1.4 | 5 | 2.7 | 5 | 1.4 | 7 | 1.7 | 8 | 2.0 |
| Consciousness disturbance | 25 | 1.4 | 2 | 1.1 | 8 | 2.2 | 5 | 1.2 | 10 | 1.0 |
| Secondary graft failure | 13 | 0.7 | 0 | 0 | 2 | 0.5 | 5 | 1.2 | 6 | 1.3 |
| Not available | 418 | 23.9 | 60 | 31.9 | 74 | 20.1 | 109 | 26.7 | 175 | 0.8 |
| Cause of death . | Whole cohort (N = 8779)* . | Refined group 1 (n = 2253)† . | Refined group 2 (n = 6526)‡ . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | CMV− (n = 188) . | % . | CMV+ (n = 369) . | % . | CMV− (n = 408) . | % . | CMV+ (n = 786) . | % . | |
| IPS/ARDS | 237 | 13.5 | 21 | 11.2 | 46 | 12.5 | 49 | 12.0 | 121 | 26.8 |
| Chronic GVHD | 156 | 8.9 | 10 | 5.3 | 25 | 6.8 | 50 | 12.3 | 71 | 15.4 |
| Acute GVHD | 103 | 5.9 | 11 | 5.9 | 20 | 5.4 | 16 | 3.9 | 56 | 9.0 |
| Renal failure/TMA | 82 | 4.7 | 6 | 3.2 | 20 | 5.4 | 19 | 4.7 | 37 | 7.1 |
| Liver failure/VOD/SOS | 63 | 3.6 | 7 | 3.7 | 14 | 3.8 | 20 | 4.9 | 22 | 4.7 |
| Bleeding | 59 | 3.4 | 5 | 2.7 | 12 | 3.3 | 12 | 2.9 | 30 | 2.8 |
| Secondary malignancy | 51 | 2.9 | 2 | 1.1 | 12 | 3.3 | 14 | 3.4 | 23 | 3.8 |
| Other organ failure | 43 | 2.5 | 7 | 3.7 | 12 | 3.3 | 8 | 2.0 | 16 | 2.9 |
| Heart failure | 25 | 1.4 | 5 | 2.7 | 5 | 1.4 | 7 | 1.7 | 8 | 2.0 |
| Consciousness disturbance | 25 | 1.4 | 2 | 1.1 | 8 | 2.2 | 5 | 1.2 | 10 | 1.0 |
| Secondary graft failure | 13 | 0.7 | 0 | 0 | 2 | 0.5 | 5 | 1.2 | 6 | 1.3 |
| Not available | 418 | 23.9 | 60 | 31.9 | 74 | 20.1 | 109 | 26.7 | 175 | 0.8 |
ARDS, acute respiratory distress syndrome; IPS, idiopathic pneumonia syndrome; SOS, sinusoidal obstruction syndrome; TMA, thrombotic microangiopathy; VOD, veno-occlusive disease.
A total of 1751 (19.9%) patients experienced NRM.
A total of 557 (24.7%) patients experienced NRM.
A total of 1194 (18.3%) patients experienced NRM.
Sensitivity analysis for the interaction model
We also performed interaction analysis excluding CB. In this analysis, poor PS (HR, 0.65; 95% CI, 0.44-0.98; P = .039) and transplantation from HLA-mismatched donors (HR, 0.68; 95% CI, 0.54-0.86; P = .002) were inversely associated with increased NRM under CMV reactivation (supplemental Table 2).
Next, we performed the interaction analysis including acute GVHD (grade II to IV) as a time-dependent covariate, because acute GVHD is an important post-transplant risk factor for CMV reactivation and NRM. In this analysis, transplantation from HLA-mismatched donors (HR, 0.60; 95% CI, 0.47-0.75; P < .001) was significant, as well as TBI (HR, 1.26; 95% CI, 1.01-1.55; P = .039) (supplemental Table 3).
Validation of our scoring model in an alternative cohort
Our scoring model was also effective in the whole cohort (N = 10 840), because CMV reactivation was not significantly associated with increased NRM in refined group 1, whereas it was significant in refined group 2 (supplemental Figure 1).
When we restricted the analysis to CMV IgG+ recipients in the test cohort (n = 1798), our stratification was also effective (supplemental Figure 2). We also evaluated whether the impact of CMV reactivation on NRM in CMV-seropositive recipients could be changed depending on donor CMV serology. We found that the 3-year NRM was significantly higher in patients with CMV reactivation when transplanted from a CMV-seropositive donor (23.4% vs 11.4%; P < .001), whereas it was not significantly higher when transplanted from a CMV-seronegative donor (21.7% vs 17.1%; P = .43) (supplemental Figure 3).
When we analyzed only CMV-seronegative transplant recipients in the test cohort (n = 391), our stratification appeared to be effective, because CMV infection was not significantly associated with increased NRM in refined group 1 (n = 94; 15.3% vs 17.9%; P = .58), whereas it tended to be associated with higher NRM in refined group 2 (n = 297; 24.0% vs 16.3%; P = .050) (supplemental Figure 4).
With respect to stem cell source, CMV reactivation was associated with increased NRM among recipients of bone marrow (23.0% vs 13.2%; P < .001) or PBSCs (26.2% vs 15.2%; P = .019), whereas there was no significant difference among CB recipients (19.4% vs 18.1%; P = .98) in the test cohort (supplemental Figure 5). Cox regression analysis for CB recipients in the test cohort also revealed that CMV reactivation was not significantly associated with NRM (HR, 1.08; 95% CI, 0.76-1.52; P = .67). The score for CMV impact on NRM was significantly lower in CB recipients, and most CB recipients were assigned to refined group 1 (P < .001) (supplemental Table 4).
Discussion
CMV diseases had been major causes of death among allo-HSCT recipients. Thereafter, a preemptive strategy was developed that could effectively decrease the risk of death directly associated with CMV diseases, but it could not prevent CMV infection and the indirect effects associated with CMV reactivation. In 2017, Marty et al clearly showed that letermovir safely and effectively prevented clinically significant CMV infection in CMV-seropositive transplant recipients.13 However, it is unclear whether all patients should be administered letermovir after allo-HSCT. Universal prophylaxis could cause some problems, including letermovir side effects, resistant infections, and, possibly, increased medical costs. In the current study, we retrospectively analyzed large-scale registry data and identified the risk factors for CMV reactivation, consistent with several previous studies.28-31 Nevertheless, our data clearly indicated that the adverse effects of CMV reactivation on NRM were not consistent with the risk stratification of CMV reactivation. Even in patients with low reactivation risk, CMV reactivation was relatively common (∼40%) and was significantly associated with an increased risk for NRM. Therefore, application of CMV prophylaxis probably should not be determined based simply on the risk of CMV reactivation.
To clarify the characteristics of patients who could show survival benefits with CMV prophylaxis, we performed an additional analysis considering the interactive effects of candidate factors with CMV reactivation. Although evaluation of the effects of interaction terms is not a new method in clinical research,21 this approach has rarely been used in combination with survival analysis incorporating competing risks and time-dependent covariates, a common statistical method in the field of clinical hematology.9,10 Thus, we believe that the interaction analysis used in this study may be useful for elucidating the heterogeneous effects of treatment in this field and can be used in future research.
Interaction analysis revealed that CML, good PS, transplantation from an HLA-matched donor, and standard-risk disease were significant risk factors for higher NRM under CMV reactivation. HLA-mismatched donors and high-risk disease are well-known risk factors for CMV reactivation,10,32 and these factors are also known to be associated with GVHD and higher NRM.33,34 Poor PS is also a well-known risk factor for NRM.35 Therefore, in patients with poor PS, HLA-mismatched donors, and/or high-risk disease, the impact of CMV infection on NRM may be relatively minor compared with allo-HSCT recipients with good PS, HLA-matched donors, or standard-risk disease. Meanwhile, CML was also associated with increased NRM under CMV reactivation. Tyrosine kinase inhibitors were administered to most patients with CML in our cohort, which could be a risk factor for CMV reactivation and diseases,36-38 but the exact mechanism is unclear.
There were no obvious differences in the specific causes of death according to CMV reactivation, as previously reported.9 CMV reactivation could be associated with various nonrelapse causes of death, including GVHD and infection, which were presumably increased by the indirect effects of CMV reactivation and the side effects of anti-CMV drugs.39-41
When we evaluated the impact of CMV reactivation on NRM in patients with CMV IgG+, CMV reactivation was associated with higher NRM when transplanted from a CMV-seropositive donor but not from a CMV-seronegative donor. This result was apparently surprising, because transplantation from a seronegative donor to a seropositive recipient is reported to be associated with delayed CMV-specific immune reconstitution.42 However, large-scale database studies also showed comparable NRM between transplantation from CMV-seropositive donors and that from CMV-seronegative donors for CMV-seropositive recipients.9,43 The landmark analysis method might also affect the results, because donor CMV-seropositivity/recipient CMV-seropositivity patients who suffered from CMV reactivation before day 100 might have unfavorable characteristics.
Marty et al restricted the participants of a phase 3 clinical trial to CMV IgG+ allo-HSCT recipients.13 Therefore, the usefulness of letermovir prophylaxis in CMV-seronegative patients is unclear; however, they are at lower risk for CMV antigenemia.9,44 In this study, 19% of the patients were CMV seronegative, and ∼30% of them suffered from clinically significant CMV infection; however, our scoring model could not stratify the impact of CMV infection. It may be necessary to interpret the data with caution, because the cumulative incidence of CMV antigenemia found in this study was somewhat higher in patients with donor CMV-seronegativity/recipient CMV-seronegativity compared with previous reports in western countries.9 However, Takenaka et al10 also reported a comparable frequency of clinically significant CMV infection in donor CMV-seronegativity/recipient CMV-seronegative patients, and, at least in Japanese transplant recipients, CMV infection is often observed in CMV-seronegative recipients. It seems unlikely that CMV was transmitted to CMV-seronegative recipients via transfusion, because transfusion filters were routinely used in Japan during the study period, but the underlying mechanism was unclear. Further investigations, including prospective trials, are warranted.
Our data indicated that NRM did not differ according to CMV reactivation in CB recipients, but it was significantly different in bone marrow or PBSC recipients. The specific mechanism is still unclear; however, the study by Marty et al included only a small number of patients who received CB transplant (12 in the letermovir group and 11 in the placebo group).13 Accordingly, the benefits of letermovir should be evaluated in future clinical trials. The analytical method may have also affected the results, because CB transplant is associated with a high risk for early death compared with other transplants, and landmark analysis intrinsically excluded death before grouping. However, Cox regression analysis for CB recipients, which included patients with early death or relapse after transplantation, supported the results.
In our cohort, in vivo TCD was rare (7%). The association among TCD, CMV antigenemia, and NRM should be interpreted with caution because TCD is rarely used in Japan, primarily as a result of the low incidence of GVHD in Japanese patients.45-47
Some previous studies have reported that CMV reactivation was associated with a lower risk for relapse of primary hematological malignancy after transplantation.8,10,48 However, no obvious difference in relapse was observed in the phase 3 trial,13 and we do not believe that the CMV prophylactic strategy should be changed based on relapse risk.
Our study had several limitations. First, we excluded patients with engraftment failure, because patients in Japan generally undergo surveillance for pp65 antigenemia, which can only be performed after neutrophil engraftment, rather than by PCR testing. This exclusion might affect the results, because a recent report has shown that CMV viremia can be detected before neutrophil engraftment.49 Second, this was a retrospective analysis, and it is unclear whether we can extrapolate these data to clinical practice, because prophylaxis for CMV cannot completely prevent CMV reactivation. Additionally, it is unclear whether CMV prophylaxis completely negates the indirect effects. Moreover, in this study we focused on NRM. However, CMV infection is also associated with side effects of antiviral drugs, increased medical costs, and prolonged hospitalization.50,51 Therefore, we cannot deny the usefulness of CMV prophylaxis for patients at high risk for CMV reactivation. In addition, in this study, well-known posttransplant risk factors for CMV reactivation, such as GVHD or administration of additional immunosuppressants, including systemic corticosteroids, were not included as potential covariates,31,52,53 because such factors are not useful for determining the application of CMV prophylaxis before or at the time of transplantation. This is a major limitation of our study. Therefore, we performed an additional analysis that included acute GVHD as a time-dependent covariate and confirmed the robustness of our analysis.
In conclusion, our current findings revealed that the risk of CMV reactivation was not consistent with the risk of increased NRM under CMV reactivation. The use of CMV prophylaxis probably should not be determined solely by CMV reactivation risk. Patients who received transplantation from an HLA-matched donor, as well as patients in good PS, with CML and/or standard-risk disease may be suitable candidates for CMV prophylaxis. We believe that our current study could be a milestone in managing CMV infection among allo-HSCT recipients.
Data sharing requests should be sent to Takashi Toya (tooya-tky@umin.ac.jp).
Acknowledgments
The authors thank all physicians and staff at the transplant centers who provided clinical data to the Transplant Registry Unified Management Program of the Japan Society of Hematopoietic Cell Transplantation. They also express gratitude to the staff at the Japan Society of Hematopoietic Cell Transplantation and the Japanese Data Center for Hematopoietic Cell Transplantation for their dedication to the organization and management of the data.
This study was supported by the Japanese Initiative for Progress of Research on Infectious Diseases for Global Epidemic from the Agency for Medical Research and Development (19fm0208013h0003). This study was also facilitated by collaborating with the Transplant Complications Working Group of the Japanese Society for Hematopoietic Cell Transplantation.
Authorship
Contribution: S.K., Y.N., K.H., T.T., T.N., M.O., and A.T. designed the study; N.U., J.M., T.F., Y.O., M.T., K.I., Y.K., T.K., J.K., and Y.A. contributed to data collection; S.K. and Y.N. analyzed the data; K.H. supervised data management; T.T., A.T. and K.O. supervised the research; S.K., K.H., and T.T. wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.K. has received lecture fees from MSD. The remaining authors declare no competing financial interests.
Correspondence: Takashi Toya, Hematology Division, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113-8677, Japan; e-mail: tooya-tky@umin.ac.jp; and Ayumi Taguchi, Gynecology Division, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113-8677, Japan; e-mail: aytaguchi-tky@umin.ac.jp.
References
Author notes
S.K. and Y.N. contributed equally to this work.
The full-text version of this article contains a data supplement.