Key Points
Targeted RNA sequencing detected a cryptic G3BP1-PDGFRB rearrangement in a myeloid neoplasm with eosinophilia and normal FISH studies.
Consistent with the patient’s response to imatinib, we demonstrate this rearrangement is oncogenic and sensitive to TKI in cell culture.
Introduction
Cytogenetic or molecular detection of an oncogenic driver rearrangement is required for the World Health Organization diagnosis of “myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB, FGFR1, or with PCM1-JAK2.” High rates of durable responses to imatinib therapy have changed the natural history of PDGFRA- and PDGFRB-rearranged myeloid neoplasms1,2 ; however, the molecular heterogeneity of the rearrangements can be a diagnostic challenge. ETV6 was the first PDGFRB fusion partner to be discovered and remains the most common recurrent translocation t(5;12)(q32;p13.2).3,4 Subsequently, 5′ partner genes with oligomerization domains have been shown to rearrange with the 3′ region of PDGFRB encoding the kinase domain to form constitutively active tyrosine kinase fusion oncoproteins. Given that >30 diverse genes have been identified in rearrangements with PDGFRB,4-7 standard cytogenetic and molecular methods may not identify all actionable PDGFRB fusions. Here, we present a case of a patient with a myeloid neoplasm with eosinophilia (MLN-EO) and a novel cryptic rearrangement involving exons 12 to 23 of PDGFRB at 5q32 and the 5′ (UTR) of G3BP1 at 5q33.1, which is also undetectable by PDGFRB break-apart fluorescence in situ hybridization (FISH). In a cell culture model, we showed that the fusion is oncogenic and sensitive to tyrosine kinase inhibition. The patient had a dramatic response to imatinib that was persistent at 16 months but has not achieved molecular remission.
Case description
A 22-year-old man with a history of childhood giant cell hepatitis, on therapy for the past 5 years with azathioprine, and a family history only notable for celiac disease in the mother, was referred for evaluation of hypereosinophilia in the setting of anemia and thrombocytopenia (white blood cell count, 9.83 × 109/L; 27.9% eosinophils; absolute eosinophil count, 2.74 × 109/L; hemoglobin, 11.9 g/dL; platelet count, 69 × 109/L). Three months prior to referral, his complete blood cell was normal. His azathioprine was discontinued.
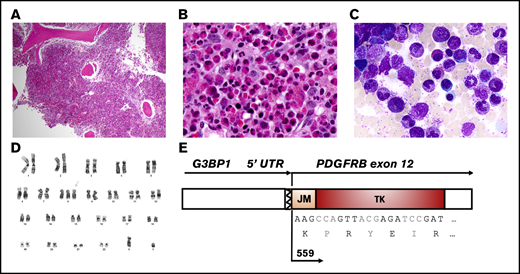
Although initially asymptomatic, the patient rapidly developed night sweats, abdominal pain, weight loss, malaise, and fatigue, and examination revealed tender splenomegaly that was palpable 10 cm below the left costal margin. A bone marrow biopsy revealed a markedly hypercellular marrow (∼95% cellularity), with myeloid-predominant trilineage hematopoiesis, markedly increased eosinophilic forms (41% of cellularity), and no increase in blasts (Figure 1A-C). Many eosinophils appeared immature and showed uneven granulation. The findings were suggestive of an MLN-EO.
Histologic, cytogenetic, and molecular characteristics of the MLN-EO and a G3BP1-PDGFRB fusion. Hematoxylin and eosin–stained bone marrow core biopsy at low magnification (×4) (A) and high magnification (×40) (B), showing a hypercellular myeloid-predominant marrow with markedly increased eosinophilic forms. (C) May-Grünwald-Giemsa–stained bone marrow aspirate showing eosinophils in various stages of differentiation (magnification, ×100). (D) G-banding karyotype showing trisomy 8. (E) Schematic representation of the fusion transcript between the 5′UTR of G3BP1 and exons 12 of PDGFRB, detected by targeted AMP sequencing off RNA.
Histologic, cytogenetic, and molecular characteristics of the MLN-EO and a G3BP1-PDGFRB fusion. Hematoxylin and eosin–stained bone marrow core biopsy at low magnification (×4) (A) and high magnification (×40) (B), showing a hypercellular myeloid-predominant marrow with markedly increased eosinophilic forms. (C) May-Grünwald-Giemsa–stained bone marrow aspirate showing eosinophils in various stages of differentiation (magnification, ×100). (D) G-banding karyotype showing trisomy 8. (E) Schematic representation of the fusion transcript between the 5′UTR of G3BP1 and exons 12 of PDGFRB, detected by targeted AMP sequencing off RNA.
Conventional bone marrow cytogenetic analysis was notable for trisomy 8: 47,XY,+8[11]/46,XY[9] (Figure 1D). Interphase PDGFRB break-apart FISH did not identify a PDGFRB rearrangement. A targeted DNA-sequencing panel for genes recurrently mutated in hematologic malignancies did not reveal any putative driver mutations. However, a targeted RNA-sequencing panel for hematologic malignancies identified chimeric fusion transcripts involving the 5′UTR of G3BP1 (5′) and exon 12 of PDGFRB (3′) (Figure 1E).
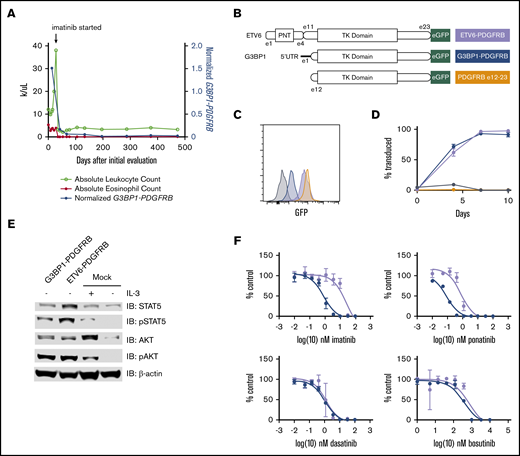
The patient had a dramatic response to treatment with imatinib (400 mg/d), with rapid resolution of leukocytosis, eosinophilia, and splenomegaly; a 6-pound weight gain; and significant improvement in fatigue, loss of appetite, and night sweats. Although our heme fusion targeted RNA-sequencing assay is, in clinical practice, a qualitative assay for the presence or absence of fusion transcripts, G3BP1-PDGFRB fusion reads were markedly reduced, but still detected, upon repeat molecular testing after initiation of imatinib (Figure 2A). At the time of writing, the patient is in hematologic remission after 16 months of therapy.
The novel oncogenic G3BP1-PDGFRB fusion protein responds to targeted inhibition. (A) Treatment course of the index patient. The G3BP1-PDGFRB fusion transcript was detectable in all heme fusion targeted RNA-sequencing assays, albeit at lower levels, after initiation of imatinib. To normalize for sequencing depth across different assays, the G3BP1-PDGFRB transcript count was normalized to a highly expressed transcript, PICALM. (B) Schematic representation of the constructs used to overexpress fusion transcripts ETV6-PDGFRB, G3BP1-PDGFRB, and truncated PDGFRB (exons 12-23) in Ba/F3 cell lines. (C) GFP expression measured by flow cytometric analysis confirms overexpression of transduced PDGFRB constructs. Dark gray = untransduced. (D) Outgrowth of transduced cells over the indicated time frame, starting from 1% to 5% transduced cell populations. Black = empty vector. (E) Lysate of Ba/F3 cells with G3BP1-PDGFRB, ETV6-PDGFRB, or empty vector (mock) with or without IL-3. (F) Viability of Ba/F3 cells exposed to ranges of tyrosine kinase inhibitors assessed by CellTiter-Glo. Experiments were performed in triplicate. Error bars indicate standard deviation.
The novel oncogenic G3BP1-PDGFRB fusion protein responds to targeted inhibition. (A) Treatment course of the index patient. The G3BP1-PDGFRB fusion transcript was detectable in all heme fusion targeted RNA-sequencing assays, albeit at lower levels, after initiation of imatinib. To normalize for sequencing depth across different assays, the G3BP1-PDGFRB transcript count was normalized to a highly expressed transcript, PICALM. (B) Schematic representation of the constructs used to overexpress fusion transcripts ETV6-PDGFRB, G3BP1-PDGFRB, and truncated PDGFRB (exons 12-23) in Ba/F3 cell lines. (C) GFP expression measured by flow cytometric analysis confirms overexpression of transduced PDGFRB constructs. Dark gray = untransduced. (D) Outgrowth of transduced cells over the indicated time frame, starting from 1% to 5% transduced cell populations. Black = empty vector. (E) Lysate of Ba/F3 cells with G3BP1-PDGFRB, ETV6-PDGFRB, or empty vector (mock) with or without IL-3. (F) Viability of Ba/F3 cells exposed to ranges of tyrosine kinase inhibitors assessed by CellTiter-Glo. Experiments were performed in triplicate. Error bars indicate standard deviation.
Methods
Patient
The subject was treated per standard of care with imatinib.
Karyotyping and FISH evaluation
G-banded karyotype was performed per standard procedures. FISH testing for BCR-ABL1, PDGFRA, and PDFGRB rearrangements (Abbott Vysis probes) was performed using a dual color–dual fusion probe, a 4q12 Tri-Color Rearrangement, and a break-apart probe, respectively.
Molecular testing
Total nucleic acid isolated from bone marrow aspirate and peripheral blood specimens was subjected to targeted Illumina NextSeq next-generation sequencing using 2 clinically validated anchored multiplex polymerase chain reaction (AMP) assays:8 1 (heme SNaPshot) DNA based, to detect single nucleotide variants and insertions/deletions in 103 genes and the other one (heme fusion) RNA based, to detect fusion transcripts involving 82 genes.
Cell culture and immunoblotting assays
Ba/F3 cells were maintained in media containing 10 ng/mL murine interleukin-3 (IL-3; PeproTech; catalog number 213-13). Transgenes were synthesized and cloned into a pSFFV–entry site–GFP-IRES-mCherry-pEF1-Puro lentivector. A CellTiter-Glo assay (Promega) was used, according to the manufacturer’s instructions, to measure viability of Ba/F3 cells carrying the ETV6-PDGFRB or the G3BP1-PDGFRB fusion gene after 48 hours of exposure to tyrosine kinase inhibitor or vehicle control. The following antibodies were used for immunoblotting: phosphorylated STAT5 (4322S), STAT5 (94205S), phosphorylated AKT (4060S), AKT (4685S), GFP (2555S), and β-actin (3700S) (all from Cell Signaling Technologies).
Results and discussion
The IL-3–dependent murine cell line Ba/F3 was transduced with lentivirus to express the G3BP1-PDGFRB fusion transcript, a truncated PDGFRB exon 12_23 transcript, or the canonical oncogenic ETV6 exon 1_4-PDGFRB exon 11_23 transcript (Figure 2B-C). When deprived of IL-3, only cells carrying ETV6-PDGFRB or G3BP1-PDGFRB constructs could outcompete nontransduced cells (Figure 2D) and maintain constitutive JAK/STAT pathway activation (Figure 2E). In this model, the 5′UTR of G3BP1 was necessary to confer oncogenic properties to truncated PDGFRB. Compared with cells expressing the ETV6-PDGFRB fusion, cells expressing G3BP1-PDGFRB were more sensitive to treatment with imatinib and ponatinib and equally sensitive to treatment with dasatinib and bosutinib (Figure 2F).
Here, we describe a patient with clinicopathologic features consistent with an MLN-EO and a novel and cryptic G3BP1-PDGFRB rearrangement. The patient’s disease rapidly improved after tyrosine kinase inhibition with imatinib. Karyotype and interphase FISH analysis failed to identify rearrangement of the PDGFRB gene (the 2 genes are <2 Mb apart and are joined through a paracentric inversion). However, Massachusetts General Hospital's clinically validated targeted RNA-sequencing assay identified a fusion transcript linking the G3BP1 5′UTR and PDGFRB exon 12. Cell culture studies confirmed that this fusion was oncogenic and sensitive to ABL1/PDGFRB kinase inhibition.
G3BP stress granule assembly factor 1 (G3BP1; also known as Ras-GTPase activating protein SH3 domain-binding protein 1) is an RNA-binding protein involved in the regulation of cellular functions, including stress granule formation, in response to extracellular stimuli, promotion of S-phase entry, regulation of cell apoptosis, and modulation of RAS signaling.9,10 To our knowledge, this is the first report of an MLN-EO linked to a genetic alteration involving G3BP1. In addition, the fusion does not involve the coding sequence of G3BP1 but its 5′UTR. Fusions with UTRs, particularly 5′UTRs, are not commonly reported or characterized, although they have been described in ∼5% of gene fusions based on a large study that included 33 cancer types.11
Multiple reports have detailed the successful and rapid clinical response to different doses of imatinib (100-400 mg/d) in patients with PDGFRB-rearranged MPN1,2,6,12 leading to durable long-term remissions, a 6-year progression-free survival rate of 88% (95% confidence interval, 65-97), and a 10-year overall survival rate of 90% (95% confidence interval, 64-97). Although reports are limited, all patients achieving hematologic response did so within 2 months of therapy initiation. In addition, all patients who achieved a molecular remission (36%) sustained this remission without the development of resistance and without the need to treat with second-generation tyrosine kinase inhibitors.6 Insufficient data are available to evaluate the potential for imatinib withdrawal in this patient population. Indeed, imatinib discontinuation in patients with PDGFRA-rearranged MPN has been linked to rapid disease relapse.13,14 Therefore, the patient, particularly in light of persistent molecular evidence of disease (Figure 2A), remains on treatment with imatinib.
In summary, these findings illuminate an unusual mode of proto-oncogene activation and highlight the importance of comprehensive morphologic, cytogenetic, and molecular diagnostic evaluation for patients with hypereosinophilia who may benefit from targeted therapy.
Acknowledgments
The authors thank the patient and his family for their cooperation with this study.
This work was supported by National Institutes of Health, National Cancer Institute Project K-12-CA087723 (G.S.H.).
Authorship
Contribution: M.J., D.E.G., J.A.V., G.S.H., V.N., and D.B.S. wrote the manuscript; V.N., M.J., D.E.G., B.L.E., A.J.I., and P.D.C. designed the study, performed experiments, and interpreted data; G.S.H. and D.B.S. were responsible for patient care and provided clinical data; and all authors reviewed, edited, and approved the final manuscript.
Conflict-of-interest disclosure: A.J.I. has partial ownership in, and has acted as an advisor for, Archer Diagnostics. D.B.S. is a cofounder and holds equity in Clear Creek Bio. B.L.E. has received research funding from Celgene and Deerfield, has received consulting fees from GRAIL, and serves on the scientific advisory boards for Skyhawk Therapeutics and Exo Therapeutics. G.S.H. has received research funding through Bayer, Merck, and Incyte and has acted as a consultant for Incyte, Celgene, Agios, and Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Valentina Nardi, Massachusetts General Hospital, MGH Pathology Service, Warren 219, 55 Fruit St, Boston, MA 02114; e-mail: vnardi@partners.org.
References
Author notes
Cell culture sequences and reagents are available from Valentina Nardi (vnardi@partners.org). The targeted RNA sequencing reported here is clinical testing not for public deposition.