Key Points
The ACPA risk score model was established to help recognize ITP patients with a high risk of hospitalization for CAP.
The ACPA model was demonstrated to have good discrimination and calibration power by internal and external validation.
Abstract
Infection is one of the primary causes of death from immune thrombocytopenia (ITP), and the lungs are the most common site of infection. We identified the factors associated with hospitalization for community-acquired pneumonia (CAP) in nonsplenectomized adults with ITP and established the ACPA prediction model to predict the incidence of hospitalization for CAP. This was a retrospective study of nonsplenectomized adult patients with ITP from 10 large medical centers in China. The derivation cohort included 145 ITP inpatients with CAP and 1360 inpatients without CAP from 5 medical centers, and the validation cohort included the remaining 63 ITP inpatients with CAP and 526 inpatients without CAP from the other 5 centers. The 4-item ACPA model, which included age, Charlson Comorbidity Index score, initial platelet count, and initial absolute lymphocyte count, was established by multivariable analysis of the derivation cohort. Internal and external validation were conducted to assess the performance of the model. The ACPA model had an area under the curve of 0.853 (95% confidence interval [CI], 0.818-0.889) in the derivation cohort and 0.862 (95% CI, 0.807-0.916) in the validation cohort, which indicated the good discrimination power of the model. Calibration plots showed high agreement between the estimated and observed probabilities. Decision curve analysis indicated that ITP patients could benefit from the clinical application of the ACPA model. To summarize, the ACPA model was developed and validated to predict the occurrence of hospitalization for CAP, which might help identify ITP patients with a high risk of hospitalization for CAP.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune bleeding disorder characterized by antibody-induced destruction of platelets and decreased production of platelets because of impaired thrombopoiesis.1-3 Corticosteroids are the first-line treatment for ITP. In emergency situations or when patients are intolerant to corticosteroids, intravenous immunoglobulins are considered.4,5 Rituximab, thrombopoietin receptor agonists, immunosuppressive agents, and splenectomy are second-line treatments for ITP.4,6-8 It has been reported that up to 90% of patients have an initial response to treatment, but many patients relapse upon cessation of corticosteroids or intravenous immunoglobulins, which indicates that chronic or continued treatment is required.4,9 Several population-based studies have revealed an increased incidence of infections in ITP patients resulting from immune dysfunction from the disease itself and immunosuppression caused by long-term treatment.6,10-15 Several studies have reported other risk factors for infection in adult ITP patients, but the risks factors have not yet been clearly defined.10,16,17
Infection is one of the primary causes of death in ITP, and the mortality associated with infection in ITP patients has increased over time.16,18,19 The lungs were the most common site of infection in ITP patients in previous studies (40.0%-54.0%).6,10,14 Although there are no data on mortality caused by pneumonia in ITP patients, the data on the general population are discouraging: community-acquired pneumonia (CAP) is a disease with a high mortality rate and a short-term mortality rate of 14% to 32%.20-22 Therefore, the early identification and management of CAP in ITP patients is essential. However, there is no information on the risk factors for CAP in nonsplenectomized ITP patients. Therefore, we conducted a multicenter, retrospective cohort study to develop and validate a risk score model to predict the probability of hospitalization for CAP in nonsplenectomized ITP patients with the goals of early identification of disease and timely treatment of patients.
Methods
Patients
A multicenter, retrospective cohort study was conducted to evaluate ITP patients at 10 large Chinese medical centers from December 2002 to September 2019: the 5 centers for the derivation cohort were Peking University People’s Hospital, Shandong University Qilu Hospital, Second Affiliated Hospital of Shanxi Medical University, The Second Affiliated Hospital of Kunming Medical University, and Heping Hospital Affiliated to Changzhi Medical College; the 5 centers for the validation cohort were Affiliated Shanxi Big Hospital of Shanxi Medical University, Beijing Hospital, Chinese PLA General Hospital, Peking University Shenzhen Hospital, and Peking University First Hospital. The study population included nonsplenectomized primary ITP inpatients 18 years of age or older. Patients who had a diagnosis of connective tissue disease, cancer (solid tumor or leukemia), or primary immune deficiency were excluded,2,23 along with those who had a diagnosis of infection before confirmation of ITP.
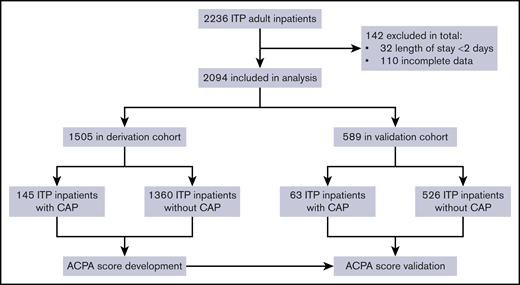
In all, 208 ITP inpatients diagnosed with CAP were included in the study (Figure 1). The derivation cohort included 145 inpatients from 5 centers, and the validation cohort included 63 inpatients from the other 5 centers, which were located in the northern, southwestern, and southeastern geographical regions of China. Medical records were collected from the inpatient clinic databases at each hospital. When a patient had more than 1 admission or infection episode, only data from the first admission or infection were analyzed. The study was conducted in accordance with the standards of the ethics committees at the 10 hospitals.
Demographic data, including age, sex, underlying comorbidities, previous bleeding events, history of smoking, infection records during hospitalization, laboratory results at the time of ITP diagnosis, length of hospital stay, and treatment plans before hospitalization were collected. Considering the time-dependence of the drugs’ effects in patients, collected information about exposures to drugs was time-varying. As assessed in the French Adult Immune Thrombocytopenia: a French Pharmacoepidemiological Study (FAITH) cohort and in a rheumatoid arthritis cohort, exposure to corticosteroids was defined as the 1-month period after treatment with corticosteroids.10,11,24,25 Because B cells were typically depleted for a 6-month period during which patients had a high risk of developing infection, duration of exposure to rituximab was considered as 6 months.10,26 For all other drugs, a 1-month exposure period was considered, in accordance the exposure period reported in a previous study by Moulis et al.10 Clinical outcomes for patient survival or mortality at 6 months after hospitalization were obtained by reviewing the clinical records or through telephone interviews. The study protocol was approved by the Central Institutional Review Board of the Peking University People’s Hospital (Beijing, China) and the ethic committees or institutional review boards of all the other participating hospitals.
Definitions and diagnosis
All patients included in our analysis received an initial primary clinical diagnosis of CAP within 48 hours after admission. CAP was confirmed by radiographic evidence of a pulmonary infiltrate with no other known cause and (1) the presence of at least 1 of the following newly acquired respiratory symptoms or signs: cough, sputum, dyspnea, and/or auscultatory findings of abnormal breath sounds and crackles,27-29 or 1 of the following nonrespiratory symptoms or signs such as confusion, worsening of underlying chronic illnesses, or tachypnea, or (2) evidence of infection, which included fever ≥38.0°C, leukocytosis, or a decline in oxygenation.28-31 Each patient in the study was evaluated by respiratory specialists and hematologists on the basis of the patient’s clinical features, laboratory examinations, chest radiography, etiologic data, and results of laboratory cultures. The final diagnosis of CAP was made according to the opinions of all involved experts.
Coinfection was defined as infection with copathogens identified within 2 days of hospital admission.32 The Charlson Comorbidity Index (CCI) score was used to assess baseline comorbidities at the time of ITP diagnosis.33 Clinical complications referred to a subset of unintended and undesirable events that harmed the patients’ health or required additional monitoring or intervention.34,35 Our analysis focused mainly on bleeding complications in ITP patients. Severe bleeding was defined as mucosal bleeding leading to a decrease in hemoglobin of >2 g/dL or as suspected internal hemorrhage.4
Statistical analysis
Patients with or without CAP in the derivation cohort were compared by univariable analysis using a logistic regression model. Variables with a value of P < .10 in the univariable analysis were included in the multivariable analysis. Variables with a value of P > .10 in the multivariable analysis were removed from the model during the backward stepwise selection based on the estimation of the Akaike information criterion. According to the outcomes of the multivariable analysis, we developed a scoring system to predict the probability of CAP, and we assigned point values to the risk factors on the basis of regression coefficients.36 We performed a bootstrap internal validation procedure with 1000 bootstrap resamples. In addition, a geographically independent cohort was used for external validation. We evaluated the discrimination and calibration power of the prediction model using the area under the receiver operating characteristic curve (AUC) and calibration plot, respectively. We also determined the net benefit by using decision curve analysis. We validated our model in patients with chronic ITP in the derivation and validation cohorts, and we compared the absolute lymphocyte count (ALC) and the platelet count between ITP and CAP diagnosis according to the Wilcoxon signed-rank test. To investigate the associations between clinical features, including bleeding complications, smoking, treatment exposures, and the risk for CAP, we conducted a subgroup analysis using a multivariable logistic regression model adjusted for the identified baseline risk factors in the univariable logistic regression model.
Data were computed with IBM SPSS 24.0 and R version 4.0.0 software. Clinical features were summarized by using descriptive statistics. Continuous variables were summarized as median values, and categorical variables were reported as counts (%). Categorical variables were compared using the χ2 test or Fisher’s exact test. The Kaplan-Meier method was used to estimate the 6-month survival of ITP inpatients. The log-rank test was used to compare survival among groups. All P values were 2-sided, and a value of P < .05 was considered to indicate statistical significance.
Results
Patient characteristics
From December 2002 to September 2019, 2094 eligible nonsplenectomized ITP adult inpatients from 10 medical centers in China were included. The mean age of the population was 52.4 years (range, 18-96 years), with a female:male ratio of 1.7:1. Of the total patient population, 767 patients (36.6%) were newly diagnosed with ITP, and 1327 patients (63.4%) had persistent and chronic ITP.
There were 208 ITP inpatients with CAP. The derivation cohort included 145 ITP inpatients with CAP and 1360 ITP patients without CAP from 5 medical centers. The validation cohort consisted of the remaining 63 ITP inpatients with CAP and 526 ITP inpatients without CAP from the other 5 centers. Table 1 summarizes patients’ characteristics. Age at ITP diagnosis, comorbidities, and initial ALC at ITP diagnosis were similar in the both cohorts (P > .05). There was no significant difference between ALC at ITP diagnosis and ALC at CAP diagnosis among 208 ITP patients with CAP according to the Wilcoxon signed-rank test (P = .132). The derivation cohort included patients with significantly more persistent and chronic ITP (P < .001) and had a higher male:female ratio (P = .014). In addition, the initial platelet count at ITP diagnosis in the derivation cohort was significantly higher than that in the validation cohort (P < .001). The initial platelet count was significantly different from the platelet count at the time of CAP diagnosis (P < .001).
Clinical characteristics of nonsplenectomized adult ITP inpatients with and without CAP
| Characteristic . | Derivation cohort (n = 1505) . | Validation cohort (n = 589) . | Difference between derivation and validation cohorts, P . | ||||
|---|---|---|---|---|---|---|---|
| With CAP (n = 145) . | Without CAP (n = 1360) . | P . | With CAP (n = 63) . | Without CAP (n = 526) . | P . | ||
| Age at diagnosis of ITP, y | <.001 | <.001 | .571 | ||||
| ≥60, n (%) | 72 (49.7) | 402 (29.6) | 28 (60.3) | 140 (26.6) | |||
| Median | 56.3 | 46.5 | 60.0 | 45.1 | |||
| Type of ITP, n (%) | .718 | .148 | <.001 | ||||
| Newly diagnosed | 43 (29.7) | 384 (28.2) | 31 (49.2) | 309 (58.7) | |||
| Persistent and chronic | 102 (70.3) | 976 (71.8) | 32 (50.8) | 217 (41.3) | |||
| Male sex, n (%) | 74 (51.0) | 465 (34.2) | <.001 | 32 (50.8) | 213 (40.5) | .117 | .014 |
| CCI score | <.001 | <.001 | .991 | ||||
| ≥3, n (%) | 39 (26.9) | 63 (4.6) | 25 (39.7) | 15 (2.9) | |||
| Median (range) | 1.3 (0-6) | 0.6 (0-5) | 1.5 (0-4) | 0.3 (0-4) | |||
| Initialplatelet count* | <.001 | .070 | <.001 | ||||
| <20 × 109/L, n (%) | 82 (56.9) | 446 (32.8) | 49 (79.0) | 77 (67.8) | |||
| Median | 24.8 | 38.0 | 14.4 | 15.7 | |||
| Initial ALC† | <.001 | <.001 | .063 | ||||
| <1 × 109/L, n (%) | 89 (66.4) | 199 (16.3) | 46 (74.2) | 83 (18.5) | |||
| Median | 1.1 | 2.7 | 1.1 | 1.8 | |||
| Characteristic . | Derivation cohort (n = 1505) . | Validation cohort (n = 589) . | Difference between derivation and validation cohorts, P . | ||||
|---|---|---|---|---|---|---|---|
| With CAP (n = 145) . | Without CAP (n = 1360) . | P . | With CAP (n = 63) . | Without CAP (n = 526) . | P . | ||
| Age at diagnosis of ITP, y | <.001 | <.001 | .571 | ||||
| ≥60, n (%) | 72 (49.7) | 402 (29.6) | 28 (60.3) | 140 (26.6) | |||
| Median | 56.3 | 46.5 | 60.0 | 45.1 | |||
| Type of ITP, n (%) | .718 | .148 | <.001 | ||||
| Newly diagnosed | 43 (29.7) | 384 (28.2) | 31 (49.2) | 309 (58.7) | |||
| Persistent and chronic | 102 (70.3) | 976 (71.8) | 32 (50.8) | 217 (41.3) | |||
| Male sex, n (%) | 74 (51.0) | 465 (34.2) | <.001 | 32 (50.8) | 213 (40.5) | .117 | .014 |
| CCI score | <.001 | <.001 | .991 | ||||
| ≥3, n (%) | 39 (26.9) | 63 (4.6) | 25 (39.7) | 15 (2.9) | |||
| Median (range) | 1.3 (0-6) | 0.6 (0-5) | 1.5 (0-4) | 0.3 (0-4) | |||
| Initialplatelet count* | <.001 | .070 | <.001 | ||||
| <20 × 109/L, n (%) | 82 (56.9) | 446 (32.8) | 49 (79.0) | 77 (67.8) | |||
| Median | 24.8 | 38.0 | 14.4 | 15.7 | |||
| Initial ALC† | <.001 | <.001 | .063 | ||||
| <1 × 109/L, n (%) | 89 (66.4) | 199 (16.3) | 46 (74.2) | 83 (18.5) | |||
| Median | 1.1 | 2.7 | 1.1 | 1.8 | |||
Fifteen patients (derivation cohort, 3; validation cohort, 12) had no records of initial platelet count.
A total of 226 patients (derivation cohort, 148; validation cohort, 78) had no records of initial ALC.
Infection profiles
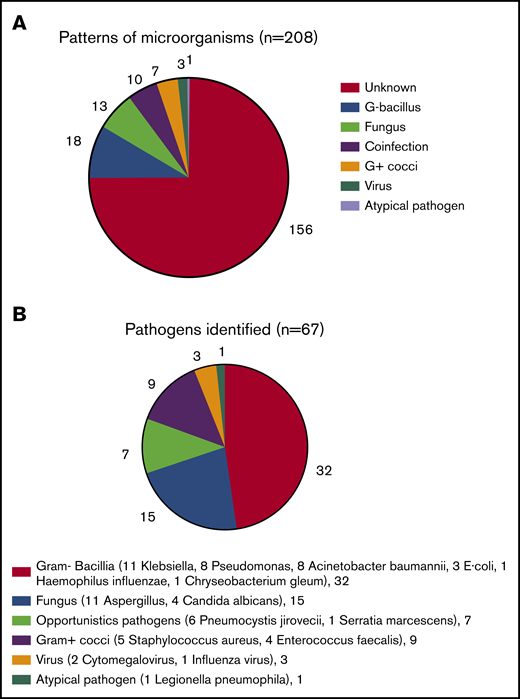
In all, 208 ITP inpatients had CAP. Pathogens were identified in 52 patients (25.0%) (Figure 2A), of whom 42 patients had a single infection, and 10 patients had coinfections. Six patients had 2 infection events, 3 patients had 3 infection events, and 1 patient had 4 infection events. Gram-negative bacilli were the main pathogens identified; there were 11 patients with Klebsiella pneumoniae, 8 with Pseudomonas aeruginosa, 8 with Acinetobacter baumannii, 3 with Escherichia coli, 1 with Haemophilus influenzae, and 1 with Chryseobacterium gleum infections. Fifteen patients had fungal infections: 11 with Aspergillus and 4 Candida albicans infections. Opportunistic infections were identified according to the definition for opportunistic nonviral microorganisms37 : 6 patients had Pneumocystis jirovecii and 1 had Serratia marcescens infections. Nine patients had gram-positive cocci infections: 5 with Staphylococcus aureus and 4 with Enterococcus faecalis. Three patients had viral infections: 2 with cytomegalovirus and 1 with influenza virus. One patient was infected by Legionella pneumophila, an atypical pathogen (Figure 2B).
Profiles of CAP in nonsplenectomized adult ITP. (A) Patterns of microorganisms. The infective pathogens in 52 of 208 ITP inpatients with CAP were identified. (B) Pathogens identified. The majority of bacilli were Gram-negative (G–). E coli, Escherichia coli; G+, Gram-positive.
Profiles of CAP in nonsplenectomized adult ITP. (A) Patterns of microorganisms. The infective pathogens in 52 of 208 ITP inpatients with CAP were identified. (B) Pathogens identified. The majority of bacilli were Gram-negative (G–). E coli, Escherichia coli; G+, Gram-positive.
Risk factors for being hospitalized for CAP
We conducted univariable analysis of baseline characteristics of CAP in the derivation cohort (Table 1). Five variables with a value of P < .10 were included in the multivariable analysis: male sex (P < .001), age 60 years or older (P < .001), CCI score of 3 or more (P < .001), initial platelet count of <20 × 109/L (P < .001), and initial ALC of <1 × 109/L, all measured at the time of ITP diagnosis (P < .001).
Multivariable analysis identified the following 4 independent risk factors (Table 2): age 60 years or older (odds ratio [OR], 1.901; 95% confidence interval [CI], 1.242-2.910; P = .003), CCI score of 3 or more (OR, 9.328; 95% CI, 5.240-16.604; P < .001), initial platelet count of <20 × 109/L (OR, 3.776; 95% CI, 2.425-5.879; P < .001), and initial ALC of <1 × 109/L (OR, 8.932; 95% CI, 5.893-13.539; P < .001), all measured at the time of ITP diagnosis.
Coefficients and points for the ACPA risk score model for hospitalization for CAP using independent variables at the time of ITP diagnosis in the derivation cohort
| ACPA variables . | Multivariable analysis . | . | . | |
|---|---|---|---|---|
| OR (95% CI) . | P . | Coefficients . | Points . | |
| Age ≥60 vs <60 y | 1.901 (1.242-2.910 | .003 | 0.642 | 1 |
| CCI score ≥3 vs <3 | 9.328 (5.240-16.604) | <.001 | 2.233 | 3.5 |
| Platelet count <20 vs ≥20 × 109/L | 3.776 (2.425-5.879) | <.001 | 1.239 | 2 |
| ALC <1 vs ≥1 × 109/L | 8.932 (5.893-13.539) | <.001 | 2.190 | 3.5 |
| ACPA variables . | Multivariable analysis . | . | . | |
|---|---|---|---|---|
| OR (95% CI) . | P . | Coefficients . | Points . | |
| Age ≥60 vs <60 y | 1.901 (1.242-2.910 | .003 | 0.642 | 1 |
| CCI score ≥3 vs <3 | 9.328 (5.240-16.604) | <.001 | 2.233 | 3.5 |
| Platelet count <20 vs ≥20 × 109/L | 3.776 (2.425-5.879) | <.001 | 1.239 | 2 |
| ALC <1 vs ≥1 × 109/L | 8.932 (5.893-13.539) | <.001 | 2.190 | 3.5 |
In addition, we completed the statistical analysis of the association between the main baseline comorbidities (with a proportion greater than 3%) and the risk of hospitalization for CAP by using a multivariable logistic regression model adjusted for the identified baseline risk factors in the univariable logistic regression model (supplemental Table II) to find the weights of the comorbidities included in the CCI score in relation to hospitalization for CAP, and we found that chronic pulmonary disease was associated with an increased risk of hospitalization for CAP (OR, 3.249; 95% CI, 1.157-9.126; P = .025).
Establishing the scoring system
The value assignment was completed according to the regression coefficient of each identified independent risk factor for CAP in the derivation cohort to establish the ACPA risk score model: Age + Charlson Comorbidity Index score + Platelet count + Absolute lymphocyte count (Table 2). The variables in the ACPA risk score model (all measured at the time of ITP diagnosis) were given points as follows: age 60 years or older, 1 point; CCI score of 3 or more, 3.5 points; platelet count <20 × 109/L, 2 points; and ALC <1 × 109/L, 3.5 points. With the establishment of the ACPA model, an increasing risk of CAP was observed in ITP patients. Forty-six (4.1%) of 1118 patients with a score of 0 to 3.5, 77 (33.9%) of 227 patients with a score of 4.5 to 8, and 11 (91.7%) of 12 patients with a score of 9 to 10 developed CAP (Table 3). The estimated rates of CAP in ITP patients are shown in supplemental Table I. Patients who received a score of 0 had an estimated 1.4% probability of developing CAP, and those who received a score of 10 had an estimated 99.9% probability of developing CAP.
Observed rates of hospitalization for CAP for ITP inpatients in the derivation and validation cohorts
| . | Derivation cohort risk group (n = 1357)* . | Validation cohort risk group (n = 511)* . | ||||
|---|---|---|---|---|---|---|
| Low . | Medium . | High . | Low . | Medium . | High . | |
| Risk score | 0-3.5 | 4.5-8 | 9-10 | 0-3.5 | 4.5-8 | 9-10 |
| Patients without CAP | 1072 (79.0) | 150 (11.1) | 1 (1.9) | 373 (73.0) | 74 (14.5) | 2 (0.4) |
| Patients with CAP | 46 (3.4) | 77 (5.7) | 11 (5.9) | 14 (2.7) | 32 (6.3) | 16 (3.1) |
| Frequency of CAP | 46/1118 (4.1) | 77/227 (33.9) | 11/12 (91.7) | 14/387 (3.6) | 32/106 (30.2) | 16/18 (88.9) |
| . | Derivation cohort risk group (n = 1357)* . | Validation cohort risk group (n = 511)* . | ||||
|---|---|---|---|---|---|---|
| Low . | Medium . | High . | Low . | Medium . | High . | |
| Risk score | 0-3.5 | 4.5-8 | 9-10 | 0-3.5 | 4.5-8 | 9-10 |
| Patients without CAP | 1072 (79.0) | 150 (11.1) | 1 (1.9) | 373 (73.0) | 74 (14.5) | 2 (0.4) |
| Patients with CAP | 46 (3.4) | 77 (5.7) | 11 (5.9) | 14 (2.7) | 32 (6.3) | 16 (3.1) |
| Frequency of CAP | 46/1118 (4.1) | 77/227 (33.9) | 11/12 (91.7) | 14/387 (3.6) | 32/106 (30.2) | 16/18 (88.9) |
All data are no. (%) unless otherwise specified.
A total of 148 patients in the derivation cohort and 78 patients in the validation cohort had no score because of lack of data for initial ALC.
Internal and external validation of the scoring system
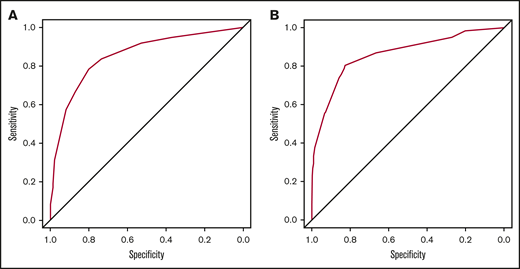
We performed a bootstrap internal validation procedure with 1000 bootstrap repetitions. The AUC was 0.853 (95% CI, 0.818-0.889) in the derivation cohort, indicating that the ACPA model had good discrimination power for predicting the probability of CAP in ITP patients (Figure 3A). In addition, good agreement between the observed and estimated risks of CAP in ITP patients was demonstrated by the calibration plot (Figure 4A).
Receiver operating characteristic (ROC) curve of the ACPA model in the derivation and validation cohorts. (A) In the derivation cohort, the area under the ROC curve (AUC) of the ACPA model was 0.853 (95% CI, 0.818-0.889). (B) In the validation cohort, the AUC of the ACPA model was 0.862 (95% CI, 0.807-0.916).
Receiver operating characteristic (ROC) curve of the ACPA model in the derivation and validation cohorts. (A) In the derivation cohort, the area under the ROC curve (AUC) of the ACPA model was 0.853 (95% CI, 0.818-0.889). (B) In the validation cohort, the AUC of the ACPA model was 0.862 (95% CI, 0.807-0.916).
Calibration plot of the ACPA scoring system for predicting hospitalization for CAP. The ACPA score in the derivation cohort (A) and the validation cohort (B). The model-predicted probability of CAP was plotted on the x-axis; actual CAP was plotted on the y-axis. An ideal calibration plot is indicated by a 45° diagonal line.
Calibration plot of the ACPA scoring system for predicting hospitalization for CAP. The ACPA score in the derivation cohort (A) and the validation cohort (B). The model-predicted probability of CAP was plotted on the x-axis; actual CAP was plotted on the y-axis. An ideal calibration plot is indicated by a 45° diagonal line.
In the validation cohort, there was also an increasing risk of CAP in ITP patients. Fourteen (3.6%) of 387 patients with a score of 0 to 3.5, 32 (30.2%) of 106 patients with a score of 4.5 to 8, and 16 (88.9%) of 18 patients with a score of 9 to 10 had CAP. For the validation cohort, the AUC was 0.862 (95% CI, 0.807-0.916), demonstrating very good discrimination capability (Figure 3B). In addition, the calibration plot in the validation cohort displayed good agreement between the observed and actual probabilities (Figure 4B). Moreover, we evaluated the net benefit for patients in clinical practice by applying the 4-item ACPA scoring system to predict the probability of CAP in ITP patients by performing decision curve analysis. It was found that patients could benefit from the application of the ACPA model (Figure 5). We also internally and externally validated the ACPA model in patients with chronic ITP and showed its generalizability and robust predictive performance (supplemental Figures 2-4).
Decision curve analysis of the ACPA score for predicting hospitalization for patients with CAP in the derivation cohort. Black line: assumes that no patient has CAP; gray line: assumes all patients have CAP. These 2 lines serve as references.
Decision curve analysis of the ACPA score for predicting hospitalization for patients with CAP in the derivation cohort. Black line: assumes that no patient has CAP; gray line: assumes all patients have CAP. These 2 lines serve as references.
Clinical features and treatments associated with the risk for hospitalization for CAP
The subgroup analysis used a multivariable logistic regression model adjusted for the identified baseline risk factors in the univariable logistic regression model (male sex, age 60 years or older, CCI score of 3 or more, initial platelet count <20 × 109/L, and initial ALC of <1 × 109/L, all at the time of ITP diagnosis). Patients with a history of severe bleeding (OR, 2.495; 95% CI, 1.726-3.608; P < .001), history of smoking (OR, 2.182; 95% CI, 1.411-3.374; P < .001), exposure to corticosteroids (OR, 3.080; 95% CI, 2.058-4.608; P < .001), and exposure to immunosuppressive agents (OR, 1.910; 95% CI, 1.043-3.498; P = .036) in the 1 month before diagnosis of pneumonia had a significantly increased risk of CAP compared with patients who did not have a history of bleeding or an exposure to treatment (Table 4).
Multivariable logistic model assessment of clinical features and exposure to treatment as risk factors for hospitalization for CAP in patients with ITP
| Variable . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Severe bleeding | 3.683 (2.749-4.935) | <.001 | 2.495 (1.726-3.608) | <.001 |
| Ever smoking | 2.211 (1.589-3.077) | <.001 | 2.182 (1.411-3.374) | <.001 |
| Exposure to corticosteroids | 2.723 (2.037-3.639) | <.001 | 3.080 (2.058-4.608) | <.001 |
| Exposure to danazol | 2.986 (1.724-5.173) | <.001 | 1.603 (0.732-3.508) | .238 |
| Exposure to intravenous immunoglobulin | 2.167 (1.449-3.240) | <.001 | 1.193 (0.659-2.158) | .560 |
| Exposure to immunosuppressive agents | 2.993 (1.959-4.573) | <.001 | 1.910 (1.043-3.498) | .036 |
| Exposure to rituximab | 3.367 (1.550-7.313) | .002 | 2.421 (0.705-8.308) | .160 |
| Variable . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Severe bleeding | 3.683 (2.749-4.935) | <.001 | 2.495 (1.726-3.608) | <.001 |
| Ever smoking | 2.211 (1.589-3.077) | <.001 | 2.182 (1.411-3.374) | <.001 |
| Exposure to corticosteroids | 2.723 (2.037-3.639) | <.001 | 3.080 (2.058-4.608) | <.001 |
| Exposure to danazol | 2.986 (1.724-5.173) | <.001 | 1.603 (0.732-3.508) | .238 |
| Exposure to intravenous immunoglobulin | 2.167 (1.449-3.240) | <.001 | 1.193 (0.659-2.158) | .560 |
| Exposure to immunosuppressive agents | 2.993 (1.959-4.573) | <.001 | 1.910 (1.043-3.498) | .036 |
| Exposure to rituximab | 3.367 (1.550-7.313) | .002 | 2.421 (0.705-8.308) | .160 |
The model was adjusted by the identified risk factors in the univariable logistic regression model (male sex, age 60 years or older, CCI score of 3 or more, initial platelet count of <20 × 109/L, and initial ALC of <1 × 109/L, all at ITP diagnosis).
Clinical outcomes of CAP
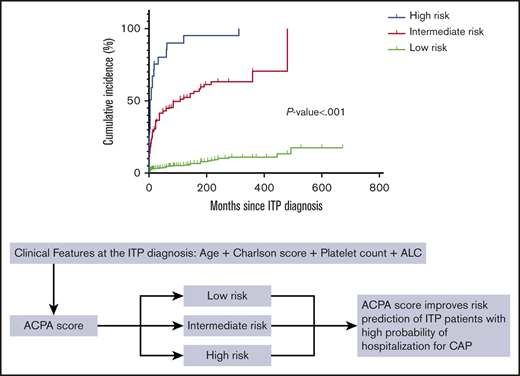
The average 6-month all-cause mortality rate for CAP in nonsplenectomized adult ITP inpatients in all 10 centers was 25.0%. The association between CAP and overall survival is illustrated in Figure 6A. CAP was significantly associated with higher 6-month all-cause mortality and poor prognosis (P < .001). We also compared the 6-month overall survival of ITP inpatients among different ACPA groups and found that patients in the high-risk group had higher 6-month all-cause mortality than patients in the intermediate-risk group (P < .001), and patients in the intermediate-risk group had higher 6-month all-cause mortality than patients in the low-risk group (P < .001) (Figure 6B).
Comparison of 6-month overall survival of ITP inpatients with and without CAP. CAP was significantly associated with higher mortality (P < .001).
Comparison of 6-month overall survival of ITP inpatients with and without CAP. CAP was significantly associated with higher mortality (P < .001).
Discussion
This study was the first series of nonsplenectomized adult ITP inpatients with CAP, and the findings demonstrated their clinical features, risk factors, and outcomes. We established and validated the ACPA risk score model to predict the occurrence of hospitalization for CAP, and we reported extremely high short-term mortality, which highlighted that ITP inpatients with CAP should be closely monitored. The clinical features of the sex ratio and age of the patients were in accordance with the epidemiology of ITP in France.38 The incidence of CAP in our study was 9.9%, which was comparable with the incidence reported in previous studies (3.9% to 11.4%).10,14,17,39
We identified 4 baseline factors associated with a higher risk of CAP in adult inpatients with ITP. Age 60 years or older at the time of ITP diagnosis was identified as a risk factor in our analysis, and older age was also recognized as a risk factor for serious infection in adult ITP patients in a previous study by Moulis et al.10 Aging was associated with a decline in the immune response,40,41 which might explain why age was found to be a risk factor. The elderly were found to be at high risk for influenza infection and pneumococcal pneumonia, and the efficacy of vaccines in that group was only 30% to 40% compared with an efficacy of 70% to 80% in younger populations.42,43 Therefore, we recommend more comprehensive clinical care for elderly ITP patients who should be monitored for infection.
A CCI score of 3 or more at the time of ITP diagnosis was an independent risk factor for hospitalization for CAP in ITP patients, indicating that more severe comorbidities would increase the risk of CAP. To find the weights of the comorbidities included in the CCI score in relation to hospitalization for CAP, we performed a statistical analysis of the association between the main baseline comorbidities (with a proportion greater than 3%) and the risk of hospitalization for CAP by using a multivariable logistic regression model adjusted for the identified baseline risk factors in the univariable logistic regression model (supplemental Table II). Our analysis demonstrated a significantly increased risk of hospitalization for CAP in adult ITP inpatients with chronic pulmonary disease (OR, 3.249; 95% CI, 1.157-9.126; P = .025), as indicated in the Moulis et al tudy.10 Some possible underlying mechanisms include decreased phagocytic responses,44 increased virus binding and entry owing to upregulation of adhesion molecules,45 outgrowth of bacteria after virus infections,46 the influx of inflammatory cells into the lung causing alteration of airway structures, or reduced ciliary function.47 Although we did not discover that other comorbidities, including diabetes, chronic kidney disease, cerebrovascular disease, and chronic heart disease, were independent baseline risk factors for hospitalization for CAP in ITP patients, there have been many studies demonstrating that these comorbidities were associated with an increase in the risk of the incidence of CAP in patients with distinct underlying medical conditions.10,48-51 There have been few studies that have explored the specific comorbidities that could lead to an increased incidence of CAP in ITP patients, and thus no consensus has been reached. We considered using CCI score to evaluate the overall status of baseline comorbidities in ITP patients and to investigate the association of the overall status of comorbidities with the risk of hospitalization for CAP.
Our analysis found that an initial platelet count <20 × 109/L at the time of ITP diagnosis was a risk factor for CAP in ITP inpatients. There is increasing evidence that platelet counts play a key role in inflammation and infection by promoting neutrophil recruitment and the formation of neutrophil extracellular traps52 and by interacting with monocytes or lymphocytes to kill pathogens.53 A previous experiment in mice demonstrated that a decrease in the platelet count impaired the host’s defense against pulmonary infection,54 but few studies have focused on the association between platelet count and the risk of infection. Qu et al55 reported that the platelet count at 1 month after ITP diagnosis but not that at the time of ITP diagnosis was significantly correlated with infection. Nevertheless, it was difficult to explain the pathophysiological causal link between the initial platelet count and the long-term risk of pneumonia. A possible explanation is that an initial low platelet count might have an impact on the immunosuppressive treatment strategies recommended by guidelines or by international consensus reports,4,9,56 and immunosuppressive treatment might in turn significantly affect the frequency of infection. Further studies shouldt focus on the links among the initial platelet count, immunosuppressive treatment, and the risk of CAP.
A lower ALC (<1 × 109/L) at the time of ITP diagnosis was associated with hospitalization for CAP, and we found that there was no significant difference between ALC at the time of ITP diagnosis and ALC at the time of CAP diagnosis among 208 ITP patients with CAP, which suggested that a low initial ALC at the time of ITP diagnosis might be a reliable factor for predicting the risk of developing CAP, which was similar to the results of a previous study by Hu et al.17 Moreover, several studies elucidated the association of baseline lymphopenia with the risk of infection in the general population57 or in transplant patients.58 In studies involving HIV-positive patients, it was demonstrated that the ALC was a good predictor of the CD4 count, and the CD4 count was a strong predictor of opportunistic infection.59 In the study by Hu et al,17 it was proposed that the ALC might be a surrogate marker that reflects a state of immune deficiency or immune dysfunction. In other studies, treatment with corticosteroids was reported to induce a decrease in the ALC,60,61 which might complicate preexisting lymphopenia and increase susceptibility to infection. However, since few studies have focused on the risk of CAP or infections in ITP patients and the reason for the association between the initial ALC and the risk of developing CAP in ITP patients remains unknown, further studies are required to confirm this and to investigate the underlying mechanisms.
Several models to predict CAP, including Yende’s risk score model,62 Socan’s prediction model,63 and Miyashita’s diagnostic score model,64 have been proposed, but because these models all had limitations, we established a scoring system to better predict CAP in ITP patients. Socan’s prediction model and Miyashita’s diagnostic score model focused on specific pathogens (Chlamydia pneumoniae and Legionella pneumophila), which limited their predictive power when other pathogens were present. Yende’s risk score model included an assessment of lung function, which was not easy or convenient to obtain. In addition, none of these models were designed specifically for ITP patients. Our ACPA score combined the identified independent risk factors based on the results of multivariable analysis to identify patients with a high risk of CAP. It is the first available risk score model in nonsplenectomized ITP patients with CAP, and it integrates several clinical parameters, including age, comorbidities, and laboratory parameters. The ACPA scoring system was demonstrated to have good discrimination and calibration power and to be clinically generalizable and credible by internal and external validation. In addition, all of the items included in the scoring system could be conveniently obtained through detailed interrogation and routine blood tests so that patients with a high risk of CAP could be easily identified. We recommend that patients with a high risk of CAP according to the ACPA risk score model receive more attention so early identification and treatment can be implemented to avoid the occurrence of life-threatening events. Further large prospective studies are needed to verify the applicability of the ACPA model to ITP patients and to address the question of whether this score model would help improve prognosis.
We performed a subgroup analysis to determine the association of the risk of hospitalization for CAP with clinical features and treatment exposure by using a multivariable logistic regression model adjusted for the identified baseline risk factors in the univariable logistic regression model. Exposure to corticosteroid and immunosuppressive agents in the previous 1-month period were both associated with CAP, similar to the results of the study by Moulis et al.10 That study also revealed that rituximab treatment was a patient-related risk factor for serious infection in adult ITP patients. Our multivariable analysis indicated that there was no association between rituximab treatment and hospitalization for CAP, but it had limited power to elucidate this component because rituximab was used in only a few patients (34 [1.6%] of 2094). Further studies are required to investigate the association between rituximab treatment and risk of infection. A history of severe bleeding was also found to be a risk factor for CAP. The Moulis et al10 study suggested that mucosal bleeding was associated with serious infection. Moreover, previously published animal data suggested that massive hemorrhage was limited to the area of inflammation. Internal hemorrhage suggested a higher inflammatory response and immune dysfunction in the area of hemorrhage, which might partly explain these findings.65 However, the association between a history of bleeding and CAP remains a mystery because of the lack of supporting data. Further studies have been proposed to elucidate this association.
Ever smoking emerged as one of the independent risk factors in our analysis, which was reasonable because exposure to cigarette smoke could markedly impact not only the innate but also the adaptive immune system, especially in the lung, which would compromise an individual’s ability to mount an appropriate immune response.66 The negative effects of smoking on the immune system were demonstrated by observing the impaired function of leukocytes,67 decreased levels of immunoglobulins,68 increased levels of inflammatory mediators,69 and increased oxidative stress.70 But to date, there have been no data regarding the association of ever smoking and the risk of CAP in ITP patients. Therefore, more studies are needed to demonstrate the association between smoking and CAP in ITP patients.
We could not adjust the analysis for some factors, including exposure to pneumococcus and influenza vaccines that might affect susceptibility to infections, because no patients were reported to have exposure to them. However, in the Moulis et al10 study, the pneumococcus and influenza vaccines showed protective effects against both serious and nonserious infections in nonsplenectomized patients with persistent or chronic ITP, which suggested that it might be important to promote the use of those vaccines in ITP patients to help reduce the risk of infections. Further studies should focus on the effects of vaccines.
Several limitations of our study need to be considered. First, the retrospective nature of the study limited the study power. Second, we could not analyze outpatients in each center in our analysis because they had incomplete medical records, which might have caused bias because our model might select more serious cases of CAP. Third, all of the patients in our analysis came from China, which might limit the applicability of our results to broader centers.
To our knowledge, this is the first study to include such a large sample size to investigate a risk model to predict the incidence of hospitalization for CAP in nonsplenectomized ITP patients. Our data indicate that the ACPA score can accurately assess the probability of hospitalization for CAP in nonsplenectomized ITP patients, as determined by internal and external validation. The identification of ITP patients who have a high risk for hospitalization for CAP might allow timely treatment and improve patient survival and outcomes.
For original data, please contact Ye-Jun Wu at wyejun1999@pku.edu.cn.
Acknowledgments
The authors thank the patients and medical staff who participated in this study, all of the investigators of the Cooperative ITP Working Group who provided their patient data, and the Department of Medical Records Library for providing medical records.
This work was supported by grants from the National Natural Science Foundation of China (81730004, 81670116, and 81970113), Beijing Natural Science Foundation (H2018206423 and 7171013), Beijing Municipal Science and Technology Commission (Z171100001017084), the National Key Research and Development Program of China (2017YFA0105500 and 2017YFA0105503), and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81621001).
The funders of the study had no role in designing the study, collecting, analyzing, or interpreting the data, or writing the report.
Authorship
Contribution: Y.-J.W. and X.-H.Z. designed the research, collected, analyzed, and interpreted the data, and wrote the manuscript; M.H. and H.-X.L. collected, analyzed, and interpreted the data, and contributed to writing the manuscript; and J.P., L.-M.M., L.-H.Y., R.F., H.L., Y.L., J.F., H.-Y.Z., Z.-P.Z., W.-S.W., X.-L.S., P.Z., H.-X.F., Q.-Z.Z., X.-L.W., Q.-S.H., Y.H., Q.J., H.J., J.L., X.-Y.Z., X.-S.Z., Y.-J.C., L.-P.X., Y.-Y.L., and Q.-F.W. contributed to data collection and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Hui Zhang, Peking University People’s Hospital, No.11 Xizhimen South St, Xicheng District, Beijing 100044, People’s Republic of China; e-mail: zhangxh@bjmu.edu.cn.
Appendix
Members of the Cooperative ITP Working Group include Zhilin Gao (Affiliated Shanxi Big Hospital of Shanxi Medical University, Taiyuan, China), Linlin Shao (Qilu Hospital, Shandong University, Jinan, China), Ruijuan Zhang (Second Affiliated Hospital of Shanxi Medical University, Taiyuan, China), Yunfeng Hao (The Second Affiliated Hospital of Kunming Medical University, Kunming, China), Chan Li (Heping Hospital Affiliated to Changzhi Medical College, Changzhi, China), and Fengqi Liu, Gao-Chao Zhang, and Xueyan Sun (Peking University People’s Hospital, Peking University Institute of Hematology, Beijing, China).
References
Author notes
Y.-J.W., M.H., and H.-X.L. contributed equally to this study.
The full-text version of this article contains a data supplement.