Key Points
DM, an autoimmune inflammatory myopathy, can be associated with a number of malignancies, including, rarely, myelodysplastic syndromes.
Allo-HCT presents a novel approach to treat refractory DM in patients with a coexisting malignancy through the GvA effect.
Introduction
Dermatomyositis (DM) is an autoimmune disease (AD) that is characterized by cutaneous symptoms and proximal muscle weakness.1 Up to 30% of cases arise secondary to malignancy, most commonly ovarian, breast, lung, and gastrointestinal cancers and non-Hodgkin lymphoma.2
Fewer than 20 cases of DM have been reported with myelodysplastic syndromes (MDSs).3,4 MDS occurs with ADs in 10% to 20% of cases.5 This may be due to defects in immune cells or cytokine production that result in inadequate clearance of apoptotic bodies, stimulating autoimmune responses. We present a follow-up report regarding a patient with anti–transcription intermediary factor-1γ (anti-TIF1γ) DM and MDS.6 Although his DM improved with chemotherapy, it did not clear until a recent allogeneic hematopoietic stem cell transplant (allo-HCT). Because only 4 reports of anti-TIF1γ DM and MDS have been published, this case offers new insights on managing these rare, but possibly life-threatening, diseases.
Case description
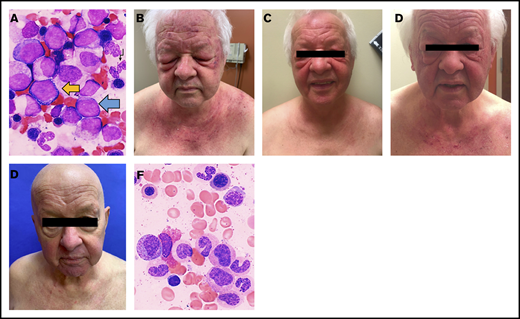
A 70-year-old male with hypertension, type 2 diabetes mellitus, and osteoarthritis with recent hip arthroplasty presented to his primary care physician with a subacute pruritic flaking rash on the scalp. He was prescribed 2% ketoconazole cream and tar gel shampoo for suspected seborrheic dermatitis. His symptoms persisted, and he developed poikiloderma and erythematous plaques on the neck and chest and violaceous discoloration of the upper eyelids resembling the shawl sign and heliotrope rash of DM, respectively. Skin biopsy of the chest exhibited focal epidermal atrophy with interface dermatitis, confirming DM. He denied muscle weakness at the time and had negative antinuclear antibodies with normal creatine kinase, aldolase, C-reactive protein, and erythrocyte sedimentation rate. Oral prednisone was initiated for presumed amyopathic DM but was quickly switched to topical steroids because of anxiety and mania. His DM was severe on presentation to our Dermatology Clinic 2 weeks later, with upper extremity and facial involvement (Figure 1B).
Significant improvements in physical examination findings and bone marrow aspirates of DM with allo-HCT. (A) Bone marrow aspirate prior to chemotherapy (1000× magnification, Wright-Giemsa stain): MDS with excess blasts-2 (MDS:EB2). Blue arrow, myeloblast; black arrow, hypogranular neutrophil; yellow arrow, proerythroblast. (B) Dermatologic findings prior to beginning chemotherapy (C), following 3 cycles of chemotherapy (D), following 6 cycles of chemotherapy and prior to allo-HCT (E), and 8 weeks following allo-HCT. (F) Bone marrow aspirate post–allo-HCT (1000× magnification, Wright-Giemsa stain): normal morphology for granulocytes and erythroid precursors.
Significant improvements in physical examination findings and bone marrow aspirates of DM with allo-HCT. (A) Bone marrow aspirate prior to chemotherapy (1000× magnification, Wright-Giemsa stain): MDS with excess blasts-2 (MDS:EB2). Blue arrow, myeloblast; black arrow, hypogranular neutrophil; yellow arrow, proerythroblast. (B) Dermatologic findings prior to beginning chemotherapy (C), following 3 cycles of chemotherapy (D), following 6 cycles of chemotherapy and prior to allo-HCT (E), and 8 weeks following allo-HCT. (F) Bone marrow aspirate post–allo-HCT (1000× magnification, Wright-Giemsa stain): normal morphology for granulocytes and erythroid precursors.
Laboratory workup demonstrated pancytopenia, concerning for malignancy. Peripheral blood smear was notable for neutropenia, hypogranular neutrophils with atypical chromatin segmentation, and normochromic normocytic anemia. Bone marrow aspiration and biopsy confirmed MDS:EB-2 (13% blasts) (Figure 1A). Cytogenetics were normal. Multiple high-frequency pathogenic mutations (ASXL1, IDH2, and STAG2 with a variant allele frequency of 38%, 48%, and 94%, respectively) were identified in the Myeloid Mutation Panel. His Revised International Prognostic Scoring System score was 4 (intermediate risk). There were no premorbid records available to ascertain how long his MDS had been present. Further workup identified anti-TIF1γ antibodies.
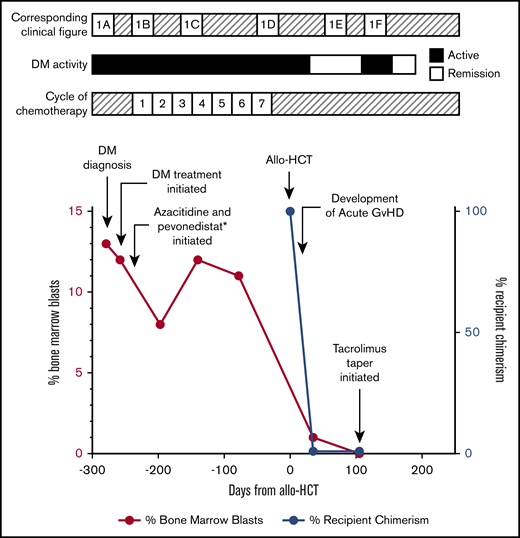
The patient was enrolled in a randomized controlled open-label trial (NCT03268954) of 75 mg/m2 per day of azacitidine and 20 mg/m2 per day of pevonedistat (TAK-924/MLN4924), a novel inhibitor of NEDD8-activating enzyme under investigation for myeloid malignancies, including MDS. Systemic DM treatments were deferred per trial protocol. His cutaneous symptoms improved rapidly, and his blast count fell to 8% (Figure 1B). However, his erythema and scalp pruritus persisted, requiring intermittent topical steroids. He also developed weakness and myalgias in the proximal right lower extremity, with elevated aldolase (10.7 U/L). Magnetic resonance imaging demonstrated inflammatory changes in the bilateral iliopsoas and sartorius and right tensor fascia latae muscles. He was given mycophenolate mofetil, 500 mg twice daily, but self-discontinued because of severe pharyngitis. After cycle 6 of azacitidine/pevonedistat, his skin worsened significantly (Figure 1C), with 11% blasts. Further systemic DM medications were deferred because of plans for allo-HCT, given its curative potential in MDS.
After cycle 7 of chemotherapy, he demonstrated hematologic improvement and stable bone marrow response. He underwent allo-HCT from a matched unrelated donor after reduced intensity conditioning with fludarabine and melphalan and without anti-thymocyte globulin. The melphalan dose was reduced to 100 mg/m2 because of concerns for toxicity, given his age and comorbidities.7-9 Tacrolimus and methotrexate were given for graft-versus-host disease (GVHD) prophylaxis. His posttransplant course was complicated by myocardial infarction, febrile neutropenia, and sepsis of unknown origin. On day +15, he developed large-volume diarrhea, presumably secondary to clinical grade 2 GVHD that improved with oral budesonide and prednisone, and cytomegalovirus reactivation managed with valganciclovir. Despite these complications, the transplant was successful, with trilineage hematopoiesis, no evidence of MDS, and bone marrow aspirate showing normal morphology (Figure 1F). His chimerism by molecular tests showed <1% recipient DNA (day +30 and day +100). Remarkably, 2 months post–allo-HCT, his DM had improved dramatically. Only a faint violaceous hue on the fingers, elbows, and knees and mild scalp pruritus remained, despite not using topical steroids (Figure 1D). On day +100, a mild rash appeared on the chest and upper back, with epidermal atrophy and vacuolar changes at the dermoepidermal junction on biopsy, consistent with DM rather than GVHD. This resolved by day +180 with 0.1% triamcinolone ointment (Figure 2). Tacrolimus taper was held initially, given concern for GVHD-related rash; however, it was resumed on day +110 and completed on day +180. Currently, 8 months post–allo-HCT, his DM remains in remission without specific intervention.
Timeline of events. MDS and DM activity surrounding allo-HCT (day 0). *Pevonedistat is a trial drug (novel inhibitor of NEDD8-activating enzyme).
Timeline of events. MDS and DM activity surrounding allo-HCT (day 0). *Pevonedistat is a trial drug (novel inhibitor of NEDD8-activating enzyme).
Methods
A retrospective review of medical records from the University of Rochester was performed for 1 patient with anti-TIF1 gamma DM and MDS:EB2. The patient provided written consent for study procedures and publication per the Declaration of Helsinki.
Results and discussion
Despite improvement with chemotherapy and topical steroids, our patient’s DM did not clear until after allo-HCT. This is the first report of allo-HCT providing a dramatically favorable outcome in DM.
Understanding the association between DM and malignancy can elucidate how treatment of malignancy can improve DM. First, antineoplastic immune responses may inadvertently target muscle tissue, leading to inflammation and necrosis.10 This is supported by the relatively short time interval between diagnosis of DM and that of the malignancy, and treatment of malignancy and improvement in DM. Second, malignancies may express mutated forms of antigens found in the body, resulting in autoantibody production.11 Specifically, >80% of cancer-associated DM is characterized by 2 autoantibodies: anti-TIF1γ and anti-nuclear matrix protein.12 Notably, anti-TIF1γ antibody titers have not been shown to definitively correlate with disease severity or activity, decreasing with treatment in some patients but not others.13,14 This may explain why our patient remained anti-TIF1γ+ post–allo-HCT. Third, chronic inflammation and tissue damage in DM may predispose to malignancy.11 This is supported by the correlation between AD activity and malignancy risk.
Hematopoietic stem cell transplantation has proven favorable for severe ADs resistant to conventional therapies. The majority of patients received high-dose chemotherapy, followed by autologous HCT (auto-HCT) because of its feasibility.15 In contrast, experience with allo-HCT in ADs is limited given its inherent risks of transplant-related mortality and GVHD.16 However, auto-HCT does not offer the same potential for healthy immune reconstitution as allo-HCT. Allo-HCT induces remission through immunosuppressive conditioning, as well as by replacing the host’s immune system through the graft-versus-autoimmunity (GvA) effect. GvA involves engrafting alloreactive donor T cells, eradicating autoreactive host T cells responsible for ADs.17 This results in a less toxic nonmyeloablative (as opposed to ablative) conditioning regimen while retaining the therapeutic potential of transplantation.16
In 1 multicenter retrospective study, allo-HCT yielded long-term control of ADs.18 However, there is no literature describing allo-HCT for coexisting malignancy and DM.19 Our case provides unique insights on this topic. Although improvement of our patient’s DM may be due in part to reduced-intensity conditioning and immunosuppressants, allo-HCT allowed for immunomodulation through GvA. Accordingly, his DM flare on day +100 improved, despite taper of immunosuppressive tacrolimus (Figure 2), suggesting that immune restoration helped to clear his DM. The use of allo-HCT as a primary treatment strategy for severe refractory ADs, including DM, needs further exploration through randomized prospective clinical trials. Trials should aim to provide direction regarding selection of candidates and conditioning regimens, GVHD prophylaxis, long-term outcomes, and toxicity.
Authorship
Contribution: All authors contributed to the conception and design, drafting and revision, and final approval of the manuscript and agree to be accountable for all aspects of this report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bushra Tbakhi, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642; e-mail: bushra_tbakhi@urmc.rochester.edu.