Key Points
We report the first identified case of chronic neutrophilic leukemia with transformation to B-lymphoblastic leukemia.
Genetic alterations involving CSF3R, ASXL1, SRSF2, and RUNX1 contributed to the unusual progression and may drive B-cell leukemogenesis.
Introduction
Chronic neutrophilic leukemia (CNL) is a rare myeloid neoplasm, which is strongly associated with mutations in colony-stimulating factor 3 receptor (CSF3R). The activated receptor leads to dysregulated JAK/STAT signaling, sustained granulopoiesis, and characteristic findings: prominent neutrophilia, bone marrow hypercellularity, and hepatosplenomegaly.1,2 Although hydroxyurea is frequently the primary treatment option, numerous reports have demonstrated clinical responses with JAK/STAT inhibitors such as ruxolitinib.3-7
Although CNL is often considered a slowly progressive disorder, recent studies have demonstrated that additional genetic lesions involving ASXL1, SRSF2, and SETBP1 may portend a worse prognosis.8-10 Indeed, the largest clinical series have demonstrated a highly variable, but generally aggressive course, with an overall survival ranging from 6 months to >20 years. In these series, blastic transformation occurred in ∼15% of patients and took the form exclusively of acute myeloid leukemia (AML).11-13
Case description
An 80-year-old man with polymyalgia rheumatica and giant cell arteritis receiving prednisone was referred for outpatient consultation by the Hematology service for persistent leukocytosis. The leukocytosis started 1 year prior and coincided with initiation of corticosteroid therapy. Physical examination was unremarkable with no evidence of bruising, hepatosplenomegaly, or lymphadenopathy. A complete blood count (CBC) at the time of initial evaluation revealed the following: white blood cell count (WBC) 27.3 × 109/L (normal range [NR], 3.8 to 11.2 × 109/L); hemoglobin (Hb) 12.6 g/dL (NR,13.4 to 17.2 g/dL); platelet count (plt) 247 × 109/L (NR, 150 to 450 × 109/L). The WBC differential was predominantly neutrophilic (neutrophils 91%). Peripheral blood polymerase chain reaction analysis was negative for BCR-ABL. Overall, the leukocytosis with neutrophilia was initially attributed to chronic prednisone and underlying inflammatory disorders (polymyalgia rheumatica and giant cell arteritis).
Three months later, the patient was hospitalized for shortness of breath, diagnosed with community-acquired pneumonia, and treated with broad-spectrum antibiotics. Imaging detected mild bilateral pleural effusions and hepatosplenomegaly. Blood cultures were negative for growth. Compared with the prior CBC, laboratory evaluation demonstrated prominent leukocytosis with a left-shift in the setting of acute infection: WBC 54.4 × 109/L, Hb 11.0 g/dL; plt 211 × 109/L. The peripheral smear was congruent and showed marked neutrophilia (Figure 1A). The Hematology-Oncology service was consulted for persistent, worsened leukocytosis.
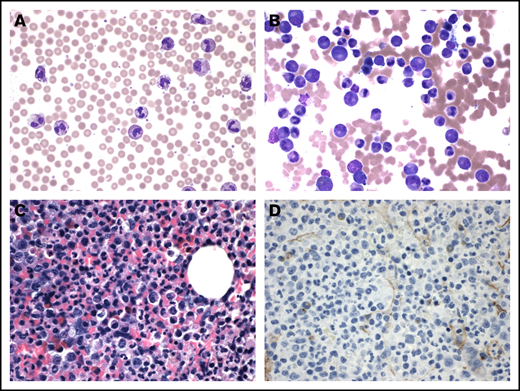
CNL characterized by increased neutrophils and myeloid hyperplasia. The peripheral smear shows prominent neutrophilia (A; original magnification ×600; Wright's stain). The accompanying bone marrow aspirate illustrates myeloid hyperplasia with complete maturation (B; original magnification ×600; Wright's stain). The core biopsy is hypercellular and dominated by maturing myeloid elements (C; original magnification ×600) with no increase in CD34-positive blasts (D; original magnification ×600; hematoxylin and eosin stain).
CNL characterized by increased neutrophils and myeloid hyperplasia. The peripheral smear shows prominent neutrophilia (A; original magnification ×600; Wright's stain). The accompanying bone marrow aspirate illustrates myeloid hyperplasia with complete maturation (B; original magnification ×600; Wright's stain). The core biopsy is hypercellular and dominated by maturing myeloid elements (C; original magnification ×600) with no increase in CD34-positive blasts (D; original magnification ×600; hematoxylin and eosin stain).
A bone marrow biopsy was performed and demonstrated a markedly hypercellular marrow (>80%), composed almost entirely of myeloid elements. There was no significant dysplasia (Figure 1B-C) and no reticulin fibrosis. CD34+ blasts were not increased (Figure 1D). Fluorescence in situ hybridization analysis was negative for BCR-ABL. Chromosome analysis showed a normal karyotype. Targeted evaluation for alterations involving a panel of up to 237 genes identified 2 mutations involving CSF3R (c.2427dupC;p.S810Qfs*6, 37% allele frequency; c.1853C>T;p.T618I, 42%). Additional mutations were detected in ASXL1 and SRSF2 exons. Genetic alterations were not detected in the remainder of assay, including CALR, EZH2, JAK2, MPL, RUNX1, and SETBP1. The overall clinical and pathologic findings were consistent with CNL.
Ruxolitinib was recommended as first-line therapy; however, due to financial toxicity of this agent, the patient opted for and was treated with hydroxyurea. Hematology analysis performed at 7-month follow-up showed normocytic anemia and improved leukocytosis: WBC 20.4 × 109/L with 88% neutrophils, Hb 10.8 g/dL; plt 348 × 109/L.
Nine months following initiation of hydroxyurea therapy, the patient presented with a nonhealing lower-extremity ulcer and progressive fatigue. The CBC demonstrated peripheral cytopenias and circulating blasts (Figure 2A): WBC 18.6 × 109/L; Hb 6.9 g/dL; plt 80 × 109/L; WBC differential with 39% blasts, 37% neutrophils.
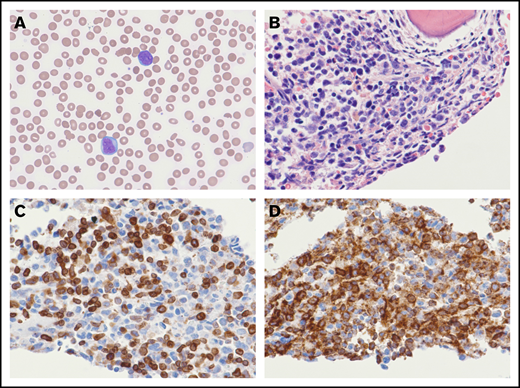
B-LBL arising out of CNL. The peripheral smear demonstrates circulating blasts with single to multiple prominent nucleoli (A; original magnification ×600; Wright's stain). The bone marrow biopsy is markedly hypercellular with an increase in blasts (B; original magnification ×600; hematoxylin and eosin stain). By immunohistochemistry, the blasts coexpress CD79a (C; original magnification ×600) and CD34 (D; original magnification ×600).
B-LBL arising out of CNL. The peripheral smear demonstrates circulating blasts with single to multiple prominent nucleoli (A; original magnification ×600; Wright's stain). The bone marrow biopsy is markedly hypercellular with an increase in blasts (B; original magnification ×600; hematoxylin and eosin stain). By immunohistochemistry, the blasts coexpress CD79a (C; original magnification ×600) and CD34 (D; original magnification ×600).
The bone marrow biopsy demonstrated a hypercellular marrow (>99%) and a marked increase in blasts, which comprised 70% of the cellularity (Figure 2B). The blasts were immunoreactive for CD79a ([Figure 2C), PAX5, CD34 (Figure 2D), CD15, and TdT, but they were negative for CD117, CD3, MPO, CD61, CD14, and CD163. By flow cytometric analysis, the blasts coexpressed CD33, HLA-DR, CD13 (partial), CD19 (dim), and TdT (dim, partial), without expression of CD20, CD22, CD10, MPO, CD64, CD5, CD2, cytoplasmic CD3, and κ and λ light chains. T- and B-cell clonality assays were performed and demonstrated a clonal T-cell receptor β-gene rearrangement. This finding supports lymphoid differentiation and has been reported in up to 70% of B-lymphoblastic leukemias (B-LBLs). There was no evidence of a B-cell gene rearrangement. Fluorescence in situ hybridization analysis was negative for BCR-ABL. Whole-genome single nucleotide polymorphism microarray identified: loss of chromosomes 3q, 7q, and 17p; gain of chromosome 11q; and copy-neutral loss of heterozygosity of chromosome 21q.
Compared with the prior CNL, comprehensive next-generation sequencing of the transformed leukemia detected a new missense mutation involving RUNX1 (D198G, c593A>G, 47% allele frequency). Persistent genetic alterations involving CSF3R (SQ810Qfs*6, 32%; T6181, 47%), SRSF2 (48%), and ASXL1 (48%) were also identified. Mutations were not detected in other genes interrogated by the assay, including CEBPA, FLT3, IDH1, IDH2, NPM1, or TP53. Overall, the clinical and pathologic findings were consistent with B-LBL arising in a patient with a history of CNL. Sadly, the patient rapidly deteriorated and succumbed to myocardial infarction before treatment was administered.
Methods
Patient data were collected via retrospective chart review, which included clinical characteristics; comprehensive hematopathologic examination, including flow cytometric, immunohistochemical, molecular, and cytogenetic analysis; and clinical outcome. The case report was approved by the institutional review board of Hawaii Pacific Health Research Institute.
Results and discussion
The CNL with transformation to B-LBL described here demonstrated 2 CSF3R mutations with comparable allele frequency. The T618I point mutation targets the extracellular domain of the receptor and represents the most common CSF3R mutation in CNL.14 The additional frameshift mutation is not well characterized but is expected to be pathogenic. Additional mutations involving ASXL1 and SRSF2 likely disrupted the epigenetic and/or splicing processes.8 Finally, the RUNX1 missense mutation highlights a unique, clonal evolution event, which was detected only in the subsequent blast transformation (B-LBL). RUNX1 is an important transcription factor, critical for hematopoietic stem cell development. Notably, RUNX1-ETO and ETV6-RUNX1 are among the most common translocations seen in AML and B-LBL with recurrent genetic abnormalities, respectively.2 Although the precise mechanism or mechanisms are uncertain, the uniquely combined and/or elevated mutational burden contributed to the highly unusual progression to B-LBL.
In summary, we report a case of CNL with numerous genetic alterations, which rapidly progressed to acute leukemia. These observations highlight the often aggressive clinical course of this rare myeloid neoplasm and further support the growing body of evidence that additional mutations involving ASXL1 and SRSF2 may blunt response to treatment with hydroxyurea and/or portend a worse prognosis. The prognostic and therapeutic implications of CNL harboring multiple CSF3R mutations are uncertain and require further investigation. Finally, transformation to AML is well described, but, to our knowledge, this is the first reported case of B-LBL arising in a patient with CNL. Together with the recent observation describing CSF3R truncation mutations in a patient with B-LBL,15 these longitudinal findings illustrate a unique nexus between CNL and B-LBL and suggest the combination of CSF3R, ASXL1, SRSF2, and RUNX1 mutations may drive B-cell leukemogenesis.
Data from this paper may be acquired by contacting the corresponding author, Joseph Aoki, at joseph.aoki@hawaiilabs.com.
Acknowledgments
The authors thank their colleagues at Hawaii Pacific Health, Hawaii Pacific Health Research Institute, Stanford Medical Center, and Sonic Healthcare USA for collaboration on the care of the patient.
Authorship
Contribution: C.S.B., B.T.T., and J.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph Aoki, Sonic Healthcare USA, 99-193 Aiea Heights Dr, Aiea, HI 96701; e-mail: joseph.aoki@hawaiilabs.com.