Key Points
BRAFV600E or mutant MAP2K1 expression in human CB CD34+ HSPCs lead to Langerhans cell–like histiocytosis in immune-deficient mice.
BRAFV600E-expressing human CB CD34+ HSPCs did not generate hairy cell leukemia in xenograft mouse models.
Introduction
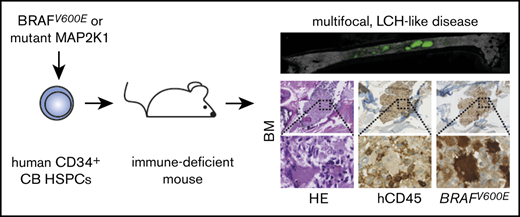
Langerhans cell histiocytosis (LCH) is the most common histiocytic disorder in humans, manifesting with a broad clinical spectrum.1,2 Although initially regarded as a reactive disorder, Langerhans cell histiocytes in disease lesions were proven to be clonal,3,4 carrying in most cases mutually exclusive BRAFV600E5 (>60%) or MAP2K1 (>10%) mutations.6-8 Detection of the BRAFV600E mutation in CD34+ hematopoietic stem and progenitor cells (HSPCs) correlates with high-risk disease and poor clinical outcome.9-11 In mice, BRAFV600E expression in dendritic cell progenitors leads to aggressive, multifocal LCH, whereas expression in more mature Langerhans cells (LCs) causes less aggressive disease.9 To prospectively test the effects of constitutive MAPK pathway activation in human early hematopoiesis, we here expressed BRAFV600E or mutated MAP2K1 in primary human CD34+ HSPCs and evaluated their differentiation patterns in vitro and in vivo.
Methods
Cord blood (CB) samples were collected after obtaining informed consent. CD34+ HSPCs were immuno-magnetically selected from cryopreserved CB and lentivirally transduced using a bidirectional promoter vector modified from,12 harboring BRAFV600E and GFP (supplemental Figure 1). As control, the BRAFV600E construct was replaced by a luciferase gene. Transduced cells were either kept in vitro and subjected to immunostaining or RNA sequencing (GSE153831) or transplanted into adult MISTRG13 or NSG mice, which were then analyzed 4 to 15 weeks after transplantation. The study was approved by the ethics commission of the canton of Zurich and the cantonal veterinary office. For further details, see supplemental Methods.
Results and discussion
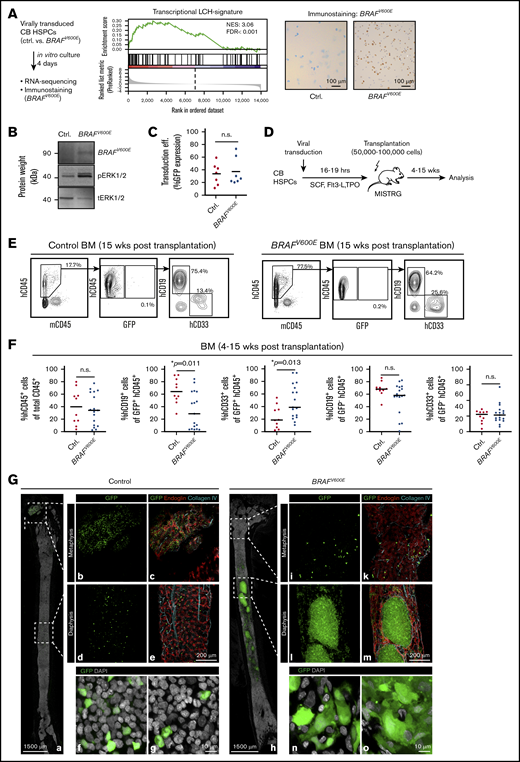
On transduction of HSPCs with a BRAFV600E-coding virus and in vitro culture for 4 days, cells showed a transcriptional profile with high analogy to previously described LCH signatures (Figure 1A; supplemental Figure 2A). The amount of BRAF transcripts (combined mutated and wild type) was increased about eightfold (supplemental Figure 2D), indicating that the described data represent a combined overexpression and constitutive BRAF activation model. Accordingly, the mutated protein was detectable in green fluorescent protein (GFP+)-sorted cells by antibody staining (Figure 1A). Furthermore, enhanced phosphorylation of the BRAF downstream target ERK was detectable on transduction of HeLa cells, demonstrating functional activity of the gene product (Figure 1B). The transduction efficiency in HSPCs was determined by GFP expression levels and was similar between control and BRAFV600E constructs (Figure 1C). Because MAPK pathway activation was demonstrated to enhance myelo-monopoiesis in murine HSPCs,14,15 we evaluated the transcriptional pattern and the lineage differentiation potential of BRAFV600E-expressing HSPCs in vitro. BRAFV600E-transduced CB HSPCs showed an enrichment in genes associated with myeloid development (supplemental Figure 2B-C). We then evaluated myeloid colony-forming unit activity. Although control virus-transduced GFP+ cells generated all types of colonies (with some alteration of distribution compared with nontransduced cells), BRAFV600E transduced GFP+ cells did not generate any burst forming units-erythroid, whereas colony-forming unit–granulocyte, erythrocyte, monocyte, megakaryocyte were increased (supplemental Figure 3A-B). When plated in myeloid differentiation-permissive liquid cell culture, BRAFV600E GFP+ cells differentiated more into spindle-shaped, plastic-adherent cells, whereas no substantial differences in CD14, BDCA-1, or BDCA-3 expression could be detected (supplemental Figure 3C-E). From these in vitro results, we conclude that BRAFV600E expression in HSPCs might influence lineage differentiation, paralleling observations in LCH patients.9
CB HSPCs expressing BRAFV600Eengraft MISTRG mice and form clusters with an inflammatory signature in the BM. (A) CB HSPCs were transduced with control or BRAFV600E virus, sorted according to GFP expression and to RNA sequencing and immunostaining with a specific anti-BRAFV600E antibody. (B) Western blot analysis showing expression of BRAFV600E in HeLa cells 5 days after transduction. Expression of BRAFV600E, phosphorylated Erk (pErk), and total expression of Erk (tErk) were evaluated using specific antibodies. (C) Transduction efficiency of CB HSPCs with control or BRAFV600E constructs determined by GFP expression level using flow cytometry at day 4 after transduction. (D) Schematic presentation of in vivo experimental procedure. (E) Representative BM FACS analysis of mice 5 weeks after transplantation with control-GFP or BRAFV600E-GFP transduced human CB HSPCs. FACS plots show frequency of hCD45+ (human) cells vs mCD45+ (mouse) cells (left panels) and percentage of GFP+ cells within hCD45+ cells (middle panels). Right panels represent expression of hCD19+ and hCD33+ cells among GFP+ cells. (F) Frequency of engrafted human hCD45+ cells, percentage of GFP+ cells among hCD45+ cells and percentage of hCD19+, hCD33+ among GFP+ cells in BM of adult MISTRG mice. Bars indicate mean values; n = 10 Ctrl, n = 17 BRAFV600E. (G) Representative BM images of MISTRG mice transplanted with control or BRAFV600E-transduced human CB HSPCs and analyzed 5 weeks later. Three-dimensional images of entire longitudinal femoral BM slices of mice transplanted with control (a-g) and BRAFV600E (h-o) CB HSPCs. Low-magnification panoramic views of marrow cavities (a,h) and enlarged, high-resolution images of metaphysis and diaphysis (b-g,i-o). Femoral slices were immuno-stained for collagen IV (turquoise) and endoglin (red). Green color represents GFP, indicating transduced human CB HSPC-derived cells. The distinct morphology of engrafted GFP+ cells in mice transplanted with control (f-g) or BRAFV600E (n-o) is shown (GFP = green, DAPI = white). Imaging depth for Figure 1G: a-e and h-m, 36 µm; f-g and n-o, 4 µm.
CB HSPCs expressing BRAFV600Eengraft MISTRG mice and form clusters with an inflammatory signature in the BM. (A) CB HSPCs were transduced with control or BRAFV600E virus, sorted according to GFP expression and to RNA sequencing and immunostaining with a specific anti-BRAFV600E antibody. (B) Western blot analysis showing expression of BRAFV600E in HeLa cells 5 days after transduction. Expression of BRAFV600E, phosphorylated Erk (pErk), and total expression of Erk (tErk) were evaluated using specific antibodies. (C) Transduction efficiency of CB HSPCs with control or BRAFV600E constructs determined by GFP expression level using flow cytometry at day 4 after transduction. (D) Schematic presentation of in vivo experimental procedure. (E) Representative BM FACS analysis of mice 5 weeks after transplantation with control-GFP or BRAFV600E-GFP transduced human CB HSPCs. FACS plots show frequency of hCD45+ (human) cells vs mCD45+ (mouse) cells (left panels) and percentage of GFP+ cells within hCD45+ cells (middle panels). Right panels represent expression of hCD19+ and hCD33+ cells among GFP+ cells. (F) Frequency of engrafted human hCD45+ cells, percentage of GFP+ cells among hCD45+ cells and percentage of hCD19+, hCD33+ among GFP+ cells in BM of adult MISTRG mice. Bars indicate mean values; n = 10 Ctrl, n = 17 BRAFV600E. (G) Representative BM images of MISTRG mice transplanted with control or BRAFV600E-transduced human CB HSPCs and analyzed 5 weeks later. Three-dimensional images of entire longitudinal femoral BM slices of mice transplanted with control (a-g) and BRAFV600E (h-o) CB HSPCs. Low-magnification panoramic views of marrow cavities (a,h) and enlarged, high-resolution images of metaphysis and diaphysis (b-g,i-o). Femoral slices were immuno-stained for collagen IV (turquoise) and endoglin (red). Green color represents GFP, indicating transduced human CB HSPC-derived cells. The distinct morphology of engrafted GFP+ cells in mice transplanted with control (f-g) or BRAFV600E (n-o) is shown (GFP = green, DAPI = white). Imaging depth for Figure 1G: a-e and h-m, 36 µm; f-g and n-o, 4 µm.
To test whether BRAFV600E HSPCs can initiate LCH-like disease in vivo, we next transplanted them into immune-deficient mice (Figure 1D). MISTRG mice carry human homologs of cytokines (macrophage colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interleukin-3, thrombopoietin) and a transgene for human SIRPα on a Rag2−/−Il2rg−/− background. They support enhanced in vivo myeloid and lymphoid development from human HSPCs and engraftment of favorable-risk acute myeloid leukemia.13,16,17 Control and BRAFV600E-transplanted mice showed overall similar engraftment levels of human CD45+ cells (Figure 1E-F). Although only a small fraction of single cell–suspended GFP+ bone marrow (BM) cells were detectable by flow cytometry, there was a significant bias toward generation of myeloid lineages in BRAFV600E HSPCs. In strong contrast to flow cytometry, ex vivo three-dimensional imaging of BM from control and BRAFV600E-transplanted mice revealed numerous clusters of engrafted GFP+ cells, which displayed typical dendritic- or macrophage-like morphology (Figure 1G). These were not detectable in mice transplanted with control-transduced HSPCs. To test whether these cellular accumulations would lead to bone erosions recurrently observed in LCH, we analyzed 6 bones by microcomputed tomography. However, we thus far did not detect differences in bone structure between BRAFV600E- or control HSPC-transplanted mice (supplemental Figure 4). Interestingly, however, in the BM of BRAFV600E HSPC-transplanted mice, regions infiltrated by GFP+ cells were found completely devoid of microvessels. Strong signs of microvascular damage, similar to those observed in inflammatory settings, could be observed in noninfiltrated surrounding tissue areas18 (Figure 1G).
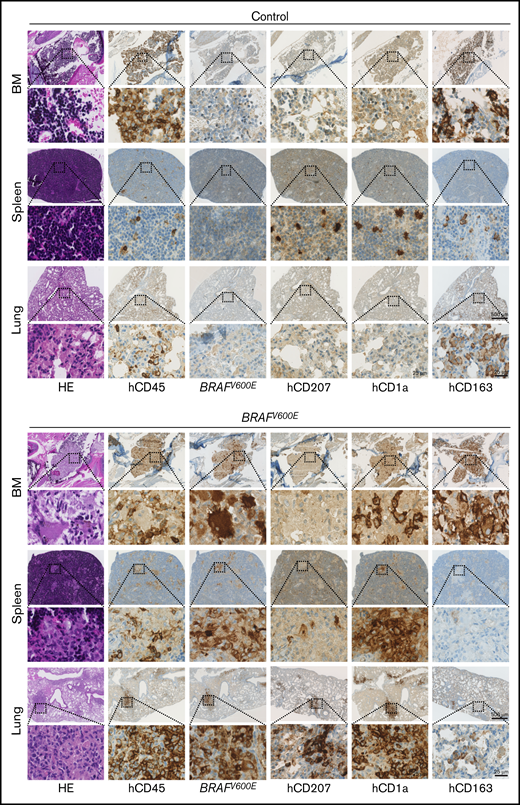
Immunohistochemical analysis of BM, spleen, lungs, and liver revealed multiorgan infiltration with clusters of atypical, mutated BRAF-expressing cells with LC-like morphology in BRAFV600E but not in control mice (Figure 2; supplemental Figure 5A-C). Some contained multiple nuclei, reminiscent of multinucleated giant cells, which are observed in some LCH lesions.19 Both organ distribution and staining of BM, spleen, and lung lesions with CD207 (langerin), CD1a, and S100 were most consistent with human aggressive, multifocal LCH (Figure 2; supplemental Figure 5D).
Expression of BRAFV600Ein human HSPCs induces Langerhans cell histiocytosis-like disease in MISTRG mice. Immunohistologic staining of BM, spleen, and lung of representative MISTRG mice, transplanted with Ctrl.-GFP or BRAFV600E-GFP transduced human CB HSPCs and analyzed 5 weeks later. Areas of magnification are indicated by respective squares. Scale bars represent 2 different magnifications. H&E, hematoxylin and eosin; hCD45, anti-human lymphocyte common antigen; BRAFV600E, monoclonal antibody against BRAFV600E.
Expression of BRAFV600Ein human HSPCs induces Langerhans cell histiocytosis-like disease in MISTRG mice. Immunohistologic staining of BM, spleen, and lung of representative MISTRG mice, transplanted with Ctrl.-GFP or BRAFV600E-GFP transduced human CB HSPCs and analyzed 5 weeks later. Areas of magnification are indicated by respective squares. Scale bars represent 2 different magnifications. H&E, hematoxylin and eosin; hCD45, anti-human lymphocyte common antigen; BRAFV600E, monoclonal antibody against BRAFV600E.
Because BRAFWT LCH lesions frequently carry mutations in MAP2K1,6,7,9 we also transduced HSPCs with an activating MAP2K1 deletion mutant (c.172_186del) (MAP2K1del), previously identified in LCH patients.20 MISTRG mice transplanted with MAP2K1del-transduced HSPCs developed LCH-like lesions in BM and lungs (supplemental Figure 6A-B). Interestingly, we observed more prominent expression of pErk in pathologic cells compared with control and BRAFV600E-transplanted mice, which possibly indicates stronger downstream signaling by this mutation (supplemental Figure 6B).
Besides in LCH, the BRAFV600E mutation is detectable in almost all classical hairy cell leukemia (HCL) cases (ie, in a hematologic malignancy of mature B cells).21 We detected GFP+CD19+ cells in both control and BRAFV600E HSPC-transplanted MISTRG mice (Figure 1E-F). However, although it was demonstrated that transgenic expression of BRAFV600E in mouse HSPCs and the BRAFV600E mutation in human, non-B-cell–committed patient-derived HSPCs leads to HCL,22 we were not able to detect typical HCL features by histology analysis in transplanted MISTRG mice, making a development of HCL in our model over time unlikely (supplemental Figure 5E). To test the possible relevance of human myeloid differentiation supporting cytokine expression in MISTRG mice on LCH vs HCL development, we also transplanted BRAFV600E and control HSPCs into nonobese diabetic/severe combined immune-deficient Il2rg−/− (NSG) mice. Similar to MISTRG mice, NSG mice developed LCH lesions, but HCL was not detectable (supplemental Figure 7A-B). These findings indicate that enhanced cell-intrinsic BRAFV600E signaling in human HSPCs is sufficient to generate LCH-like disease, and human extrinsic myeloid cytokines are not essential in this experimental in vivo setting. Absence of detectable HCL development in NSG mice also indicates that extrinsic human myeloid cytokines present in MISTRG mice likely do not prevent HCL development, although we cannot exclude that HCL might still develop with longer latency, exceeding the current observation period of maximally 15 weeks after transplantation.
Taken together, we established a xenotransplantation mouse model for LCH-like disease, originating from human BRAFV600E and MAP2K1 deletion mutant-transduced human HSPCs. The model displays classical features of human LCH, including disseminated infiltrates in BM, lung, and liver. However, it is important to note that, because of the absence of a full human immune system and the absence of a mouse adaptive immune system in the humanized mouse model, the model does not faithfully reproduce the interplay between LCH-like cells and other immune cells in patients with LCH.
Importantly, the data sustain the observation from mouse models and indirect evidence from primary human disease that aggressive LCH can arise from constitutive activating MAPK pathway mutations in human HSPCs. It further suggests that MAPK pathway activation likely promotes myeloid skewing and differentiation of human HSPCs. The disseminated, aggressive LCH-like disease in the described setting of HSPC transduction further support a model where LCH severity is driven by the cellular context in which the mutation occurs. In this, HSPC transformation leads to aggressive disease, and mature cell transformation leads to less aggressive, local disease.9 Our model might serve to further analyze LCH pathobiology and to test LCH-directed novel therapeutic approaches.
For data, please contact the corresponding author at markus.manz@usz.ch.
Acknowledgments
The authors thank Peter van Galen and J. E. Dick for providing the pSMAL vector.
This study was supported by the Swiss National Science Foundation (grants 310030B_166673/1 and 310030_184747/1), the University of Zurich Clinical Research Priority Program “Human Hemato-Lymphatic Diseases,” and the National Institutes of Health, National Cancer Institute (grant CA156689) (M.G.M.).
Authorship
Contribution: A.R. and C.M.W. designed research, performed experiments, analyzed data, and wrote the manuscript; P.M.H., R.M., Y.S., E.H., D.S., and R.C. performed experiments and analyzed data; M.M., C.E.A., and C.N.-A. designed research and wrote the manuscript; and M.G.M. directed the study and wrote the manuscript.
Conflict-of-interest disclosure: M.M. serves on the scientific advisory board of Compugen Inc, Innate Pharma Inc, Morphic Therapeutic, Myeloid Therapeutics, and Celsius Therapeutics and receives funding from Genentech Inc, Regeneron Inc, Boerhringer Ingelheim Inc, and Takeda Inc. The remaining authors declare no competing financial interests.
Correspondence: Markus G. Manz, Department of Medical Oncology and Hematology, University Hospital and University of Zurich, Raemistr 100, CH-8091 Zurich, Switzerland; e-mail: markus.manz@usz.ch.
References
Author notes
A.R. and C.M.W. contributed equally to this work.
The full-text version of this article contains a data supplement.