Key Points
Due to increased relapse risk, survival after HCT in MPN-BP is inferior to de novo AML in remission and post-MDS AML.
Survival after HCT in MPN-BP with active leukemia is similar to de novo AML with active leukemia.
Abstract
Comparative outcomes of allogeneic hematopoietic cell transplantation (HCT) for BCR-ABL1− myeloproliferative neoplasms (MPNs) in blast phase (MPN-BP) vs de novo acute myeloid leukemia (AML), and AML with prior myelodysplastic syndromes (MDSs; post-MDS AML), are unknown. Using the Center for International Blood and Marrow Transplant Research (CIBMTR) database, we compared HCT outcomes in 177 MPN-BP patients with 4749 patients with de novo AML, and 1104 patients with post-MDS AML, using multivariate regression analysis in 2 separate comparisons. In a multivariate Cox model, no difference in overall survival (OS) or relapse was observed in patients with MPN-BP vs de novo AML with active leukemia at HCT. Patients with MPN-BP in remission had inferior OS in comparison with de novo AML in remission (hazard ratio [HR], 1.40 [95% confidence interval [CI], 1.12-1.76]) due to higher relapse rate (HR, 2.18 [95% CI, 1.69-2.80]). MPN-BP patients had inferior OS (HR, 1.19 [95% CI, 1.00-1.43]) and increased relapse (HR, 1.60 [95% CI, 1.31-1.96]) compared with post-MDS AML. Poor-risk cytogenetics were associated with increased relapse in both comparisons. Peripheral blood grafts were associated with decreased relapse in MPN-BP and post-MDS AML (HR, 0.70 [95% CI, 0.57-0.86]). Nonrelapse mortality (NRM) was similar between MPN-BP vs de novo AML, and MPN-BP vs post-MDS AML. Total-body irradiation–based myeloablative conditioning was associated with higher NRM in both comparisons. Survival of MPN-BP after HCT is inferior to de novo AML in remission and post-MDS AML due to increased relapse. Relapse-prevention strategies are required to optimize HCT outcomes in MPN-BP.
Introduction
Essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF) are the 3 classical BCR-ABL1− myeloproliferative neoplasms (MPNs) characterized by recurrent mutations in JAK2, CALR, and MPL genes. Blastic transformation is 1 of the dreaded complications of MPNs, and is defined as ≥20% blasts in peripheral blood or bone marrow. This transformation is mostly myeloblastic transformation, although lymphoblastic transformation has been described. This phase of the disease is also referred to as the blast phase (BP) of MPN (MPN-BP).1 MPN-BP has an extremely poor prognosis, with median survival <6 months2-4 ; allogeneic hematopoietic cell transplantation (HCT) is the only known modality associated with long-term remission in selected patients.
There is no prospective study evaluating the outcomes of HCT in MPN-BP, and data are limited to small retrospective studies. Few retrospective studies have compared the outcomes of secondary acute myeloid leukemia (AML) with de novo AML. Due to the small number of patients, these studies have reported the results of HCT in MPN-BP combined with other forms of secondary AML, such as AML after prior history of myelodysplastic syndrome (MDS; post-MDS AML) or AML after bone marrow failure.5-7 The results from these studies are contradictory as a single-center study described similar outcomes of secondary AML in first remission (CR1) compared with de novo AML.5 However, a large study from the European Society for Blood and Marrow Transplantation (EBMT) group described inferior results of secondary AML in CR1 in comparison with de novo AML.7 Both of these studies had a small proportion of MPN-BP patients, and categorization of MDS/MPN and bone marrow failure disorders as 1 disease entity may have been oversimplification and not representative of the true biology of underlying antecedent hematological disorders. Although there are no direct comparisons, patients with MPN-BP appear to have a distinct biological profile compared with de novo AML. The spectrum of genetic abnormalities in MPN-BP is different from de novo AML, as a significant proportion of MPN-BP patients have poor-risk cytogenetics and mutations such as TP53 are enriched in this cohort (McNamara et al8 and V.G., J. A. Kennedy, J. Capo-Chichi, manuscript submitted, July 2020). Moreover, MPN patients also have a higher burden of inflammation- and thrombosis-related comorbid conditions.9
Whether the modest outcomes of HCT in MPN-BP are related to high-risk biological features of underlying disease, a higher burden of associated comorbid conditions, or both is unclear. Therefore, understanding the outcomes of HCT in MPN-BP in comparison with de novo AML and post-MDS AML after adjusting for patient-, disease-, and transplant-related factors is of clinical interest. Using the Center for International Blood and Marrow Transplant Research (CIBMTR) database, we therefore evaluated the HCT outcomes in MPN-BP with de novo AML and with post-MDS AML in 2 separate comparisons using multivariate regression analysis.
Patients and methods
Data source
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program, forming a voluntary network of >500 transplantation centers worldwide that contributes detailed data on allogeneic and autologous HCTs to a centralized statistical center. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in performance of such studies is collected and maintained in CIBMTR’s capacity as a public health authority under the US Health Insurance Portability and Accountability Act regulations.
Study cohorts
The study cohort included patients ≥40 years undergoing first allogeneic HCT between 2001 and 2015 for MPN-BP. MPN diagnoses were ET, PV, PMF, post-ET myelofibrosis, post-PV myelofibrosis, or unclassifiable MPN (MPN-U). BP was defined as ≥20% myeloblasts in peripheral blood and/or bone marrow.1 Patients with Philadelphia-positive or BCR-ABL+ MPNs, autologous transplants, transplants from syngeneic donors, cord blood transplants, or ex vivo T-cell depletion were excluded. The same inclusion and exclusion criteria were applied for selection of de novo AML and post-MDS AML cohorts. Unrelated donor (URD) transplant recipients were classified based on available HLA typing as previously described; patients with no known disparity at HLA-A, -B, -C, or -DRB1 were defined as well matched, and those with 1 locus known or probable disparate with the donors were defined as mismatched.10 The intensity of conditioning regimens was defined according to CIBMTR consensus criteria.11 Results of cytogenetic testing provided by the transplantation center were reviewed and classified as favorable, intermediate, poor risk, and missing.12 Patients who had peripheral blood and bone marrow blasts <5% prior to HCT were considered as being “in remission,” whereas those with peripheral blood and/or bone marrow blasts ≥5% were considered as having “active leukemia.”
End points
The primary end point of the study was overall survival (OS), defined as time from HCT to death from any cause. Patients were censored at the last follow-up. Other end points of interest were acute graft-versus-host disease (aGVHD), chronic GVHD (cGVHD), nonrelapse mortality (NRM), relapse, and progression-free survival (PFS). aGVHD and cGVHD were diagnosed and graded according to consensus criteria.13,14 Relapse was reported by the transplantation centers, with NRM considered a competing event. NRM was defined as death from any cause other than relapse without evidence of disease relapse, and relapse was considered a competing event. PFS was defined as time from HCT to progression, relapse, or death.
Statistical analysis
Descriptive statistical tables of patients including demographics, disease-related factors, and transplant-related factors of the study cohorts were prepared. The univariate probability of OS and PFS was calculated using the Kaplan-Meier estimator, with the variance estimated by the Greenwood formula and P value from the log-rank test. Univariate probabilities of aGVHD, cGVHD, NRM, and relapse were calculated based on cumulative incidence rates to accommodate competing risks. The Gray test P values were calculated to evaluate the differences across cumulative incidence functions. Multivariable regression analysis was performed using the Cox proportional hazards model. The comparisons of MPN-BP vs de novo AML, and MPN-BP vs post-MDS AML, were investigated separately for the following outcomes: OS, aGVHD, cGVHD, NRM, relapse, and PFS with adjustments for patient-related, disease-related, and transplant-related variables. The proportionality assumption of the Cox regression model was tested using time-dependent covariate of the main effect (MPN-BP vs de novo AML, and MPN-BP vs post-MDS AML). A forward stepwise selection procedure was used to identify significant covariates. Potential interactions between the main effect and other significant covariates were tested.
Variables considered in the multivariate analysis
The following patient-, disease-, and transplant-related variables were included in the multivariate analysis: age at HCT, sex, performance scores (<90 vs ≥90), cytogenetic abnormalities (favorable/intermediate vs poor vs missing), disease status prior to HCT (in remission vs active leukemia), donor type (matched sibling donor vs well-matched URD vs partially or mismatched URD vs other related donor), conditioning intensity (total-body irradiation [TBI]-based myeloablative vs chemotherapy-based myeloablative vs reduced intensity), graft type (peripheral blood vs bone marrow), GVHD prophylaxis (calcineurin inhibitor [CNI] plus methotrexate plus or minus others vs CNI plus mycophenolate mofetil [MMF] plus or minus others vs CNI alone vs others), antithymocyte globulin or alemtuzumab (Campath) (yes vs no), donor-recipient sex match (male-male vs male-female vs female-male vs female-female), donor-recipient cytomegalovirus (CMV) status (+/+ vs +/− vs −/+ vs −/−), and year of HCT (2001-2005 vs 2006-2010 vs 2011-2015).
Results
Patient, disease, and transplantation characteristics
A total of 6030 patients met study eligibility criteria as follows: 177 post-MPN BP, 4749 de novo AML, and 1104 post-MDS AML (Tables 1 and 2). Median follow-up of survivors was 71 months, 78 months, and 94 months in the MPN-BP, de novo AML, and post-MDS AML cohorts, respectively. Tables 1 and 2 shows detailed patient-, disease-, and transplantation-related characteristics of the study cohorts. A higher proportion of patients with MPN-BP had poor-risk karyotype (38%) in comparison with de novo AML (23%) and post-MDS AML (34%). Active leukemia at HCT was seen in 43%, 26%, and 38% of patients with MPN-BP, de novo AML, and post-MDS AML, respectively (P < .001) (Tables 1 and 2). There was significant reduction in patients undergoing HCT with active leukemia (MPN-BP, 61%, 40%, and 35% [P = .02]; de novo AML, 51%, 37%, and 23% [P < .01]; post-MDS AML, 34%, 25%, and 17% [P < .01]) in the 3 time periods between 2001 and 2005, 2006 and 2010, and 2011 and 2015, respectively. Comorbidity data as assessed by the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) were available after 2007 and HCT-CI scores ≥3 were seen in 28%, 18%, and 22% in patients with MPN-BP, de novo AML, and post-MDS AML, respectively (Tables 1 and 2). The median duration of underlying hematologic disorder was significantly longer in MPN-BP patients compared with post-MDS AML (3 years vs 0.29 years; P < .001) (Tables 1 and 2).
Baseline characteristics of study cohorts with MPN-BP and de novo AML
| Characteristic . | MPN-BP . | De novo AML . | P . |
|---|---|---|---|
| No. of patients | 177 | 4749 | |
| No. of centers | 73 | 221 | |
| Patient-related | |||
| Age at HCT, median (range), y | <.01* | ||
| Median (min-max) | 59 (40-82) | 54 (40-78) | |
| Age at HCT, n (%), y | <.01† | ||
| 40-49 | 30 (17) | 1658 (35) | |
| 50-59 | 73 (41) | 1894 (40) | |
| 60-69 | 59 (33) | 1077 (23) | |
| ≥70 | 15 (8) | 120 (3) | |
| Sex, n (%) | <.01† | ||
| Male | 116 (66) | 2552 (54) | |
| Female | 61 (34) | 2197 (46) | |
| Karnofsky score, n (%) | <.01† | ||
| 90-100 | 88 (50) | 2888 (61) | |
| <90 | 84 (47) | 1664 (35) | |
| Missing | 5 (3) | 197 (4) | |
| HCT-CI, n (%) | <.01† | ||
| 0 | 33 (19) | 705 (15) | |
| 1-2 | 31 (18) | 736 (15) | |
| ≥3 | 50 (28) | 867 (18) | |
| N/A, before 2007 | 61 (34) | 2377 (50) | |
| Missing | 2 (1) | 64 (1) | |
| Disease-related | |||
| Etiology of MPN at original diagnosis, n (%) | |||
| PV | 46 (26) | 0 (0.0) | |
| ET | 51 (29) | 0 (0.0) | |
| PMF | 60 (34) | 0 (0.20) | |
| MPN-U | 20 (11) | 0 (0.0) | |
| Time between diagnosis of MPN and AML, n (%), y | |||
| Median (min-max) | 3 (0.0-30) | N/A | |
| <2 | 70 (40) | 0 (0.0) | |
| 2-5 | 28 (16) | 0 (0.0) | |
| >5 | 63 (36) | 0 (0.0) | |
| Missing | 16 (9) | 0 (0.0) | |
| WBC at diagnosis, n (%), ×109/L | .08† | ||
| <30 | 121 (68) | 2864 (60) | |
| 30-100 | 31 (18) | 859 (18) | |
| >100 | 9 (5) | 453 (10) | |
| Missing | 16 (9) | 573 (12) | |
| Cytogenetics, n (%) | <.01† | ||
| Favorable | 1 (<1) | 305 (6) | |
| Intermediate | 96 (54) | 2910 (61) | |
| Poor | 67 (38) | 1073 (23) | |
| Not tested | 5 (3) | 134 (3) | |
| Missing | 8 (5) | 327 (7) | |
| Disease status at HCT, n (%) | <.01† | ||
| In remission‡ | 101 (57) | 3511 (74) | |
| Active leukemia§ | 76 (43) | 1238 (26) | |
| Time from diagnosis of AML to HCT, median (min-max), mo | 5 (<1-321) | 6 (<1-215) | <.01* |
| Time from diagnosis of AML to HCT, n (%), mo | <.01† | ||
| <6 | 105 (59) | 2146 (45) | |
| 6-12 | 38 (21) | 1246 (26) | |
| >12 | 34 (19) | 1357 (29) | |
| Therapy-related, n (%) | |||
| Induction therapy | <.01† | ||
| 7+3 ± other | 100 (56) | 2997 (63) | |
| Cytarabine-based | 18 (10) | 233 (5) | |
| Hypomethylating | 7 (4) | 49 (1) | |
| Other | 3 (2) | 212 (4) | |
| No therapy given | 11 (6) | 21 (<1) | |
| Missing | 38 (21) | 1237 (26) | |
| Transplant-related | |||
| Graft source, n (%) | .05† | ||
| Bone marrow | 22 (12) | 864 (18) | |
| Peripheral blood | 155 (88) | 3885 (82) | |
| Donor type, n (%) | .25† | ||
| HLA-identical sibling | 54 (31) | 1774 (37) | |
| Other related | 13 (7) | 282 (6) | |
| Well-matched URD | 83 (47) | 1952 (41) | |
| Partially matched/mismatched URD | 27 (15) | 741 (16) | |
| D-R sex match, n (%) | .05† | ||
| M-M | 76 (43) | 1643 (35) | |
| M-F | 36 (20) | 1257 (26) | |
| F-M | 39 (22) | 891 (19) | |
| F-F | 25 (14) | 922 (19) | |
| Missing | 1 (<1) | 36 (<1) | |
| D-R CMV status, n (%) | .06† | ||
| +/+ | 49 (28) | 1566 (33) | |
| +/− | 13 (7) | 468 (10) | |
| −/+ | 58 (33) | 1489 (31) | |
| −/− | 53 (30) | 1038 (22) | |
| Missing | 4 (2) | 188 (4) | |
| Conditioning regimen, n (%) | .01† | ||
| TBI-MAC | 21 (12) | 971 (20) | |
| Chemo-MAC | 72 (41) | 1912 (40) | |
| RIC/NST | 84 (47) | 1866 (39) | |
| GVHD prophylaxis, n (%) | .39† | ||
| CNI + MMF ± others | 47 (27) | 1091 (23) | |
| CNI + MTX ± others | 99 (56) | 2833 (60) | |
| CNI ± others | 19 (11) | 565 (12) | |
| Post-CY | 10 (6) | 157 (3) | |
| Other | 1 (<1) | 42 (<1) | |
| Missing | 1 (<1) | 61 (1) | |
| In vivo T-cell depletion, n (%) | .64† | ||
| No | 131 (74) | 3440 (72) | |
| Yes | 46 (26) | 1309 (28) | |
| Planned G-CSF/GM-CSF within 7 d post-HCT, n (%) | .81† | ||
| No | 129 (73) | 3504 (74) | |
| Yes | 46 (26) | 1167 (25) | |
| Missing | 2 (1) | 78 (2) | |
| Year of transplant, n (%) | <.01† | ||
| 2001-2005 | 39 (22) | 1600 (34) | |
| 2006-2010 | 72 (41) | 1958 (41) | |
| 2011-2015 | 66 (37) | 1191 (25) | |
| Follow-up, median (min-max), mo | 71 (14-169) | 78 (1-198) |
| Characteristic . | MPN-BP . | De novo AML . | P . |
|---|---|---|---|
| No. of patients | 177 | 4749 | |
| No. of centers | 73 | 221 | |
| Patient-related | |||
| Age at HCT, median (range), y | <.01* | ||
| Median (min-max) | 59 (40-82) | 54 (40-78) | |
| Age at HCT, n (%), y | <.01† | ||
| 40-49 | 30 (17) | 1658 (35) | |
| 50-59 | 73 (41) | 1894 (40) | |
| 60-69 | 59 (33) | 1077 (23) | |
| ≥70 | 15 (8) | 120 (3) | |
| Sex, n (%) | <.01† | ||
| Male | 116 (66) | 2552 (54) | |
| Female | 61 (34) | 2197 (46) | |
| Karnofsky score, n (%) | <.01† | ||
| 90-100 | 88 (50) | 2888 (61) | |
| <90 | 84 (47) | 1664 (35) | |
| Missing | 5 (3) | 197 (4) | |
| HCT-CI, n (%) | <.01† | ||
| 0 | 33 (19) | 705 (15) | |
| 1-2 | 31 (18) | 736 (15) | |
| ≥3 | 50 (28) | 867 (18) | |
| N/A, before 2007 | 61 (34) | 2377 (50) | |
| Missing | 2 (1) | 64 (1) | |
| Disease-related | |||
| Etiology of MPN at original diagnosis, n (%) | |||
| PV | 46 (26) | 0 (0.0) | |
| ET | 51 (29) | 0 (0.0) | |
| PMF | 60 (34) | 0 (0.20) | |
| MPN-U | 20 (11) | 0 (0.0) | |
| Time between diagnosis of MPN and AML, n (%), y | |||
| Median (min-max) | 3 (0.0-30) | N/A | |
| <2 | 70 (40) | 0 (0.0) | |
| 2-5 | 28 (16) | 0 (0.0) | |
| >5 | 63 (36) | 0 (0.0) | |
| Missing | 16 (9) | 0 (0.0) | |
| WBC at diagnosis, n (%), ×109/L | .08† | ||
| <30 | 121 (68) | 2864 (60) | |
| 30-100 | 31 (18) | 859 (18) | |
| >100 | 9 (5) | 453 (10) | |
| Missing | 16 (9) | 573 (12) | |
| Cytogenetics, n (%) | <.01† | ||
| Favorable | 1 (<1) | 305 (6) | |
| Intermediate | 96 (54) | 2910 (61) | |
| Poor | 67 (38) | 1073 (23) | |
| Not tested | 5 (3) | 134 (3) | |
| Missing | 8 (5) | 327 (7) | |
| Disease status at HCT, n (%) | <.01† | ||
| In remission‡ | 101 (57) | 3511 (74) | |
| Active leukemia§ | 76 (43) | 1238 (26) | |
| Time from diagnosis of AML to HCT, median (min-max), mo | 5 (<1-321) | 6 (<1-215) | <.01* |
| Time from diagnosis of AML to HCT, n (%), mo | <.01† | ||
| <6 | 105 (59) | 2146 (45) | |
| 6-12 | 38 (21) | 1246 (26) | |
| >12 | 34 (19) | 1357 (29) | |
| Therapy-related, n (%) | |||
| Induction therapy | <.01† | ||
| 7+3 ± other | 100 (56) | 2997 (63) | |
| Cytarabine-based | 18 (10) | 233 (5) | |
| Hypomethylating | 7 (4) | 49 (1) | |
| Other | 3 (2) | 212 (4) | |
| No therapy given | 11 (6) | 21 (<1) | |
| Missing | 38 (21) | 1237 (26) | |
| Transplant-related | |||
| Graft source, n (%) | .05† | ||
| Bone marrow | 22 (12) | 864 (18) | |
| Peripheral blood | 155 (88) | 3885 (82) | |
| Donor type, n (%) | .25† | ||
| HLA-identical sibling | 54 (31) | 1774 (37) | |
| Other related | 13 (7) | 282 (6) | |
| Well-matched URD | 83 (47) | 1952 (41) | |
| Partially matched/mismatched URD | 27 (15) | 741 (16) | |
| D-R sex match, n (%) | .05† | ||
| M-M | 76 (43) | 1643 (35) | |
| M-F | 36 (20) | 1257 (26) | |
| F-M | 39 (22) | 891 (19) | |
| F-F | 25 (14) | 922 (19) | |
| Missing | 1 (<1) | 36 (<1) | |
| D-R CMV status, n (%) | .06† | ||
| +/+ | 49 (28) | 1566 (33) | |
| +/− | 13 (7) | 468 (10) | |
| −/+ | 58 (33) | 1489 (31) | |
| −/− | 53 (30) | 1038 (22) | |
| Missing | 4 (2) | 188 (4) | |
| Conditioning regimen, n (%) | .01† | ||
| TBI-MAC | 21 (12) | 971 (20) | |
| Chemo-MAC | 72 (41) | 1912 (40) | |
| RIC/NST | 84 (47) | 1866 (39) | |
| GVHD prophylaxis, n (%) | .39† | ||
| CNI + MMF ± others | 47 (27) | 1091 (23) | |
| CNI + MTX ± others | 99 (56) | 2833 (60) | |
| CNI ± others | 19 (11) | 565 (12) | |
| Post-CY | 10 (6) | 157 (3) | |
| Other | 1 (<1) | 42 (<1) | |
| Missing | 1 (<1) | 61 (1) | |
| In vivo T-cell depletion, n (%) | .64† | ||
| No | 131 (74) | 3440 (72) | |
| Yes | 46 (26) | 1309 (28) | |
| Planned G-CSF/GM-CSF within 7 d post-HCT, n (%) | .81† | ||
| No | 129 (73) | 3504 (74) | |
| Yes | 46 (26) | 1167 (25) | |
| Missing | 2 (1) | 78 (2) | |
| Year of transplant, n (%) | <.01† | ||
| 2001-2005 | 39 (22) | 1600 (34) | |
| 2006-2010 | 72 (41) | 1958 (41) | |
| 2011-2015 | 66 (37) | 1191 (25) | |
| Follow-up, median (min-max), mo | 71 (14-169) | 78 (1-198) |
CNI, calcineurin inhibitor; Chemo-MAC, chemotherapy-based MAC; CMV, cytomegalovirus; D-R, donor-recipient; F, female; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HCT-CI, Hematopoietic Cell Transplantation-specific Comorbidity Index; M, male; MAC, myeloablative conditioning; max, maximum; min, minimum; MTX, methotrexate; N/A, not applicable; NST, nonmyeloablative allogeneic stem cell transplantation; RIC, reduced-intensity conditioning; TBI-MAC, TBI-based MAC; WBC, white blood cell.
Hypothesis testing: Kruskal-Wallis test.
Hypothesis testing: Pearson χ2 test.
In remission, CR1/CR2 for de novo AML and blasts <5% for MPN-BP and post-MDS AML.
Active leukemia, blasts in bone marrow or peripheral blood ≥5%.
Baseline characteristics of study cohorts with MPN-BP and post-MDS AML
| Characteristic . | MPN-BP . | Post-MDS AML . | P . |
|---|---|---|---|
| No. of patients | 177 | 1104 | |
| No. of centers | 73 | 165 | |
| Patient-related | |||
| Age at HCT, y | .78* | ||
| Median (min-max) | 59 (40-82) | 59 (40-76) | |
| Age at HCT, n (%), y | .31† | ||
| 40-49 | 30 (17) | 196 (18) | |
| 50-59 | 73 (41) | 429 (39) | |
| 60-69 | 59 (33) | 419 (38) | |
| ≥70 | 15 (8) | 60 (5) | |
| Sex, n (%) | .30† | ||
| Male | 116 (66) | 679 (62) | |
| Female | 61 (34) | 425 (38) | |
| Karnofsky score, n (%) | .53† | ||
| 90-100 | 88 (50) | 579 (52) | |
| <90 | 84 (47) | 481 (44) | |
| Missing | 5 (3) | 44 (4) | |
| HCT-CI, n (%) | .14† | ||
| 0 | 33 (19) | 175 (16) | |
| 1-2 | 31 (18) | 195 (18) | |
| ≥3 | 50 (28) | 240 (22) | |
| N/A, before 2007 | 61 (34) | 469 (42) | |
| Missing | 2 (1) | 25 (2) | |
| Disease-related | |||
| Etiology of MPN at original diagnosis, n (%) | |||
| PV | 46 (26) | 0 (0.0) | |
| ET | 51 (29) | 0 (0.0) | |
| PMF | 60 (34) | 0 (0.0) | |
| MPN-U | 20 (11) | 0 (0.0) | |
| N/A | 0 (0.0) | 1104 (100) | |
| Time between diagnosis of MPN/MDS and AML, n (%), y | |||
| Median (min-max) | 3 (0.0-30) | 0.3 (0.0-23) | |
| <2 | 70 (40) | 764 (69) | |
| 2-5 | 28 (16) | 71 (6) | |
| >5 | 63 (36) | 29 (3) | |
| Missing | 16 (9) | 240 (22) | |
| WBC at diagnosis, n (%), ×109/L | <.01† | ||
| <30 | 121 (68) | 894 (81) | |
| 30-100 | 31 (18) | 95 (9) | |
| >100 | 9 (5) | 21 (2) | |
| Missing | 16 (9) | 94 (9) | |
| Cytogenetics, n (%) | .76† | ||
| Favorable | 1 (<1) | 14 (1) | |
| Intermediate | 96 (54) | 642 (58) | |
| Poor | 67 (38) | 371 (34) | |
| Not tested | 5 (3) | 29 (3) | |
| Missing | 8 (5) | 48 (4) | |
| Disease status at HCT, n (%) | .21† | ||
| In remission‡ | 101 (57) | 685 (62) | |
| Active leukemia§ | 76 (43) | 419 (38) | |
| Time from diagnosis to HCT, median (min-max) | 5 (0.3-321) | 5 (0.2-108) | .13* |
| Time from diagnosis of AML to HCT, n (%), mo | .43† | ||
| <6 | 105 (59) | 681 (62) | |
| 6-12 | 38 (21) | 253 (23) | |
| >2 | 34 (19) | 170 (15) | |
| Therapy-related, n (%) | |||
| Induction therapy | .30† | ||
| 7+3 ± other | 100 (56) | 568 (51) | |
| Cytarabine based | 18 (10) | 99 (9) | |
| Hypomethylating | 7 (4) | 41 (4) | |
| Other | 3 (2) | 62 (6) | |
| No therapy given | 11 (6) | 69 (6) | |
| Missing | 38 (21) | 265 (24) | |
| Transplant-related | |||
| Graft source, n (%) | .15† | ||
| Bone marrow | 22 (12) | 185 (17) | |
| Peripheral blood | 155 (88) | 919 (83) | |
| Donor type, n (%) | .47† | ||
| HLA-identical sibling | 54 (31) | 344 (31) | |
| Other related | 13 (7) | 51 (5) | |
| Well-matched URD | 83 (47) | 522 (47) | |
| Partially matched/mismatched URD | 27 (15) | 187 (17) | |
| D-R sex match, n (%) | .84† | ||
| M-M | 76 (43) | 464 (42) | |
| M-F | 36 (20) | 250 (23) | |
| F-M | 39 (22) | 212 (19) | |
| F-F | 25 (14) | 174 (16) | |
| Missing | 1 (<1) | 4 (<1) | |
| D-R CMV status, n (%) | .12† | ||
| +/+ | 49 (28) | 344 (31) | |
| +/− | 13 (7) | 104 (9) | |
| −/+ | 58 (33) | 382 (35) | |
| −/− | 53 (30) | 235 (21) | |
| Missing | 4 (2) | 39 (4) | |
| Conditioning regimen, n (%) | .54† | ||
| TBI-MAC | 21 (12) | 157 (14) | |
| Chemo-MAC | 72 (41) | 408 (37) | |
| RIC/NST | 84 (47) | 539 (49) | |
| GVHD prophylaxis, n (%) | .38† | ||
| CNI + MMF ± others | 47 (27) | 313 (28) | |
| CNI + MTX ± others | 99 (56) | 619 (56) | |
| CNI ± others | 19 (11) | 118 (11) | |
| Post-CY | 10 (6) | 30 (3) | |
| Other | 1 (<1) | 16 (1) | |
| Missing | 1 (<1) | 8 (<1) | |
| In vivo T-cell depletion, n (%) | .05† | ||
| No | 131 (74) | 735 (67) | |
| Yes | 46 (26) | 369 (33) | |
| Planned G-CSF/GM-CSF within 7 d post-HCT, n (%) | .18† | ||
| No | 129 (73) | 844 (76) | |
| Yes | 46 (26) | 231 (21) | |
| Missing | 2 (1) | 29 (3) | |
| Year of transplant, n (%) | <.01† | ||
| 2001-2005 | 39 (22) | 347 (31) | |
| 2006-2010 | 72 (41) | 489 (44) | |
| 2011-2015 | 66 (37) | 268 (24) | |
| Follow-up, median (min-max) | 71 (14-169) | 94 (3-193) |
| Characteristic . | MPN-BP . | Post-MDS AML . | P . |
|---|---|---|---|
| No. of patients | 177 | 1104 | |
| No. of centers | 73 | 165 | |
| Patient-related | |||
| Age at HCT, y | .78* | ||
| Median (min-max) | 59 (40-82) | 59 (40-76) | |
| Age at HCT, n (%), y | .31† | ||
| 40-49 | 30 (17) | 196 (18) | |
| 50-59 | 73 (41) | 429 (39) | |
| 60-69 | 59 (33) | 419 (38) | |
| ≥70 | 15 (8) | 60 (5) | |
| Sex, n (%) | .30† | ||
| Male | 116 (66) | 679 (62) | |
| Female | 61 (34) | 425 (38) | |
| Karnofsky score, n (%) | .53† | ||
| 90-100 | 88 (50) | 579 (52) | |
| <90 | 84 (47) | 481 (44) | |
| Missing | 5 (3) | 44 (4) | |
| HCT-CI, n (%) | .14† | ||
| 0 | 33 (19) | 175 (16) | |
| 1-2 | 31 (18) | 195 (18) | |
| ≥3 | 50 (28) | 240 (22) | |
| N/A, before 2007 | 61 (34) | 469 (42) | |
| Missing | 2 (1) | 25 (2) | |
| Disease-related | |||
| Etiology of MPN at original diagnosis, n (%) | |||
| PV | 46 (26) | 0 (0.0) | |
| ET | 51 (29) | 0 (0.0) | |
| PMF | 60 (34) | 0 (0.0) | |
| MPN-U | 20 (11) | 0 (0.0) | |
| N/A | 0 (0.0) | 1104 (100) | |
| Time between diagnosis of MPN/MDS and AML, n (%), y | |||
| Median (min-max) | 3 (0.0-30) | 0.3 (0.0-23) | |
| <2 | 70 (40) | 764 (69) | |
| 2-5 | 28 (16) | 71 (6) | |
| >5 | 63 (36) | 29 (3) | |
| Missing | 16 (9) | 240 (22) | |
| WBC at diagnosis, n (%), ×109/L | <.01† | ||
| <30 | 121 (68) | 894 (81) | |
| 30-100 | 31 (18) | 95 (9) | |
| >100 | 9 (5) | 21 (2) | |
| Missing | 16 (9) | 94 (9) | |
| Cytogenetics, n (%) | .76† | ||
| Favorable | 1 (<1) | 14 (1) | |
| Intermediate | 96 (54) | 642 (58) | |
| Poor | 67 (38) | 371 (34) | |
| Not tested | 5 (3) | 29 (3) | |
| Missing | 8 (5) | 48 (4) | |
| Disease status at HCT, n (%) | .21† | ||
| In remission‡ | 101 (57) | 685 (62) | |
| Active leukemia§ | 76 (43) | 419 (38) | |
| Time from diagnosis to HCT, median (min-max) | 5 (0.3-321) | 5 (0.2-108) | .13* |
| Time from diagnosis of AML to HCT, n (%), mo | .43† | ||
| <6 | 105 (59) | 681 (62) | |
| 6-12 | 38 (21) | 253 (23) | |
| >2 | 34 (19) | 170 (15) | |
| Therapy-related, n (%) | |||
| Induction therapy | .30† | ||
| 7+3 ± other | 100 (56) | 568 (51) | |
| Cytarabine based | 18 (10) | 99 (9) | |
| Hypomethylating | 7 (4) | 41 (4) | |
| Other | 3 (2) | 62 (6) | |
| No therapy given | 11 (6) | 69 (6) | |
| Missing | 38 (21) | 265 (24) | |
| Transplant-related | |||
| Graft source, n (%) | .15† | ||
| Bone marrow | 22 (12) | 185 (17) | |
| Peripheral blood | 155 (88) | 919 (83) | |
| Donor type, n (%) | .47† | ||
| HLA-identical sibling | 54 (31) | 344 (31) | |
| Other related | 13 (7) | 51 (5) | |
| Well-matched URD | 83 (47) | 522 (47) | |
| Partially matched/mismatched URD | 27 (15) | 187 (17) | |
| D-R sex match, n (%) | .84† | ||
| M-M | 76 (43) | 464 (42) | |
| M-F | 36 (20) | 250 (23) | |
| F-M | 39 (22) | 212 (19) | |
| F-F | 25 (14) | 174 (16) | |
| Missing | 1 (<1) | 4 (<1) | |
| D-R CMV status, n (%) | .12† | ||
| +/+ | 49 (28) | 344 (31) | |
| +/− | 13 (7) | 104 (9) | |
| −/+ | 58 (33) | 382 (35) | |
| −/− | 53 (30) | 235 (21) | |
| Missing | 4 (2) | 39 (4) | |
| Conditioning regimen, n (%) | .54† | ||
| TBI-MAC | 21 (12) | 157 (14) | |
| Chemo-MAC | 72 (41) | 408 (37) | |
| RIC/NST | 84 (47) | 539 (49) | |
| GVHD prophylaxis, n (%) | .38† | ||
| CNI + MMF ± others | 47 (27) | 313 (28) | |
| CNI + MTX ± others | 99 (56) | 619 (56) | |
| CNI ± others | 19 (11) | 118 (11) | |
| Post-CY | 10 (6) | 30 (3) | |
| Other | 1 (<1) | 16 (1) | |
| Missing | 1 (<1) | 8 (<1) | |
| In vivo T-cell depletion, n (%) | .05† | ||
| No | 131 (74) | 735 (67) | |
| Yes | 46 (26) | 369 (33) | |
| Planned G-CSF/GM-CSF within 7 d post-HCT, n (%) | .18† | ||
| No | 129 (73) | 844 (76) | |
| Yes | 46 (26) | 231 (21) | |
| Missing | 2 (1) | 29 (3) | |
| Year of transplant, n (%) | <.01† | ||
| 2001-2005 | 39 (22) | 347 (31) | |
| 2006-2010 | 72 (41) | 489 (44) | |
| 2011-2015 | 66 (37) | 268 (24) | |
| Follow-up, median (min-max) | 71 (14-169) | 94 (3-193) |
Hypothesis testing: Kruskal-Wallis test.
Hypothesis testing: Pearson χ2 test.
In remission, CR1/CR2 for de novo AML and blasts <5% for MPN-BP and post-MDS AML.
Active leukemia, blasts in bone marrow or peripheral blood ≥5%.
Transplantation outcomes
Comparison of outcomes in MPN-BP vs de novo AML.
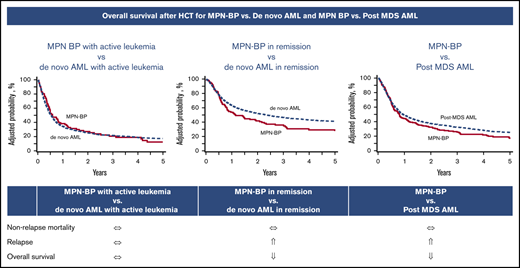
Univariate comparisons for various transplant outcomes between MPN-BP patients vs de novo AML are shown in supplemental Table 1. In a multivariate analysis, there was no difference in grade II-IV aGVHD, grade III-IV aGVHD, and cGVHD between patients undergoing HCT for MPN-BP vs de novo AML (Table 3). In a multivariable analysis comparing MPN-BP with de novo AML, disease status at HCT had a significant interaction with the main variable (Table 3). The OS of MPN-BP patients in remission was significantly inferior to de novo AML patients in remission (hazard ratio [HR], 1.40 [95% confidence interval [CI], 1.12-1.76]; P = .003) (Figure 1A), whereas OS was similar in MPN-BP patients with active leukemia compared with de novo AML patients (HR, 0.93 [95% CI, 0.72-1.20]; P = .59) (Figure 1B). The relapse rate in MPN-BP patients in remission was significantly higher compared with de novo AML in remission (HR, 2.18 [95% CI, 1.69-2.8]; P < .0001) (Figure 1C), whereas no difference in relapse rate was seen in MPN-BP patients with active leukemia vs de novo AML patients with active leukemia (HR, 1.16 [95% CI, 0.88-1.54]; P = .30) (Figure 1D). No difference in NRM was observed between the 2 cohorts (Table 3). Other factors associated with transplant outcomes in this comparison are summarized in supplemental Table 3. Of note, older age and male sex were associated with inferior OS due to higher NRM. Poor performance status and poor-risk cytogenetics were associated with inferior OS, higher relapse risk, and higher NRM. Due to higher NRM, TBI-based myeloablative conditioning (MAC; TBI-MAC) had inferior survival in comparison with chemotherapy-based MAC (Chemo-MAC) or reduced-intensity conditioning (RIC) (supplemental Table 2). NRM was also higher in patients using cyclosporine (CSA) and MMF (CSA-MMF)-based GVHD prophylaxis compared with CSA and methotrexate (supplemental Table 2). Patients using CSA-MMF had a higher risk of grade II-IV and grade III-IV aGVHD (data not shown). In vivo T-cell depletion was associated with lower NRM (supplemental Table 2).
Multivariate Cox model for study cohorts undergoing HCT for MPN-BP vs de novo AML
| . | n . | HR (95% CI) . | P . |
|---|---|---|---|
| aGVHD II-IV | |||
| De novo AML | 4693 | 1.00 (Reference) | .32 |
| MPN-BP | 176 | 1.12 (0.90-1.40) | |
| aGVHD III-IV | |||
| De novo AML | 4677 | 1.00 (Reference) | .44 |
| MPN-BP | 174 | 1.14 (0.81-1.60) | |
| cGVHD | |||
| De novo AML | 4693 | 1.00 (Reference) | .47 |
| MPN-BP | 176 | 1.05 (0.93-1.18) | |
| OS | |||
| In remission* | |||
| De novo AML | 3511 | 1.00 (Reference) | .003 |
| MPN-BP | 101 | 1.40 (1.12 - 1.76) | |
| Active leukemia† | |||
| De novo AML | 1238 | 1.00 (Reference) | .59 |
| MPN-BP | 76 | 0.93 (0.72 - 1.20) | |
| Relapse | |||
| In remission* | |||
| De novo AML | 3461 | 1.00 (Reference) | <.0001 |
| MPN-BP | 101 | 2.18 (1.69-2.80) | |
| Active leukemia† | |||
| De novo AML | 1208 | 1.00 (Reference) | .30 |
| MPN-BP | 76 | 1.16 (0.88 - 1.54) | |
| NRM | |||
| De novo AML | 4669 | 1.00 (Reference) | .92 |
| MPN-BP | 177 | 1.02 (0.74-1.41) | |
| PFS | |||
| De novo AML | 4669 | 1.00 (Reference) | .0003 |
| MPN-BP | 177 | 1.35 (1.15-1.59) |
| . | n . | HR (95% CI) . | P . |
|---|---|---|---|
| aGVHD II-IV | |||
| De novo AML | 4693 | 1.00 (Reference) | .32 |
| MPN-BP | 176 | 1.12 (0.90-1.40) | |
| aGVHD III-IV | |||
| De novo AML | 4677 | 1.00 (Reference) | .44 |
| MPN-BP | 174 | 1.14 (0.81-1.60) | |
| cGVHD | |||
| De novo AML | 4693 | 1.00 (Reference) | .47 |
| MPN-BP | 176 | 1.05 (0.93-1.18) | |
| OS | |||
| In remission* | |||
| De novo AML | 3511 | 1.00 (Reference) | .003 |
| MPN-BP | 101 | 1.40 (1.12 - 1.76) | |
| Active leukemia† | |||
| De novo AML | 1238 | 1.00 (Reference) | .59 |
| MPN-BP | 76 | 0.93 (0.72 - 1.20) | |
| Relapse | |||
| In remission* | |||
| De novo AML | 3461 | 1.00 (Reference) | <.0001 |
| MPN-BP | 101 | 2.18 (1.69-2.80) | |
| Active leukemia† | |||
| De novo AML | 1208 | 1.00 (Reference) | .30 |
| MPN-BP | 76 | 1.16 (0.88 - 1.54) | |
| NRM | |||
| De novo AML | 4669 | 1.00 (Reference) | .92 |
| MPN-BP | 177 | 1.02 (0.74-1.41) | |
| PFS | |||
| De novo AML | 4669 | 1.00 (Reference) | .0003 |
| MPN-BP | 177 | 1.35 (1.15-1.59) |
CI, confidence interval; HR, hazard ratio.
In remission: CR1/CR2 for de novo AML and blasts <5% in peripheral blood and bone marrow for MPN-BP and post-MDS AML.
Active leukemia: blasts in peripheral blood or bone marrow <5%.
Comparative outcomes of MPN-BP vs de novo AML. (A) Adjusted probability of OS of MPN-BP vs de novo AML in remission. The OS of MPN-BP patients in remission was significantly inferior to de novo AML patients in remission (HR, 1.40 [95% CI, 1.12-1.76]; P = .003). (B) Adjusted probability of OS of MPN-BP vs de novo AML with active leukemia. The OS was similar in MPN-BP patients with active leukemia compared with de novo AML patients with active leukemia (HR, 0.93 [95% CI, 0.72-1.20]; P = .59). (C) Adjusted cumulative incidence of relapse of MPN-BP vs de novo AML in remission. The relapse rate in MPN-BP patients in remission was significantly higher compared with de novo AML in remission (HR, 2.18 [95% CI, 1.69-2.8]; P < .0001). (D) Adjusted cumulative incidence of relapse of MPN-BP vs de novo AML with active leukemia. No difference in relapse rate was seen in MPN-BP patients with active leukemia vs de novo AML patients with active leukemia (HR, 1.16 [95% CI, 0.88-1.54]; P = .30).
Comparative outcomes of MPN-BP vs de novo AML. (A) Adjusted probability of OS of MPN-BP vs de novo AML in remission. The OS of MPN-BP patients in remission was significantly inferior to de novo AML patients in remission (HR, 1.40 [95% CI, 1.12-1.76]; P = .003). (B) Adjusted probability of OS of MPN-BP vs de novo AML with active leukemia. The OS was similar in MPN-BP patients with active leukemia compared with de novo AML patients with active leukemia (HR, 0.93 [95% CI, 0.72-1.20]; P = .59). (C) Adjusted cumulative incidence of relapse of MPN-BP vs de novo AML in remission. The relapse rate in MPN-BP patients in remission was significantly higher compared with de novo AML in remission (HR, 2.18 [95% CI, 1.69-2.8]; P < .0001). (D) Adjusted cumulative incidence of relapse of MPN-BP vs de novo AML with active leukemia. No difference in relapse rate was seen in MPN-BP patients with active leukemia vs de novo AML patients with active leukemia (HR, 1.16 [95% CI, 0.88-1.54]; P = .30).
Comparison of outcomes in MPN-BP vs post-MDS AML.
Univariate comparisons for various transplant outcomes between MPN-BP patients vs post-MDS AML according to disease status are shown in supplemental Table 3. In a multivariate analysis, there was no difference in grade II-IV aGVHD, grade II-IV aGVHD, and cGVHD between patients undergoing HCT for MPN-BP vs de novo AML (Table 4). In a multivariable analysis comparing MPN-BP with post-MDS AML, OS of MPN-BP patients was inferior to post-MDS AML patients (HR, 1.19 [95% CI, 1.00-1.43]; P = .05) (Figure 2A) due to increased relapse rate in MPN-BP (HR, 1.60 [95% CI, 1.31-1.96]; P < .0001) (Figure 2B). No difference in NRM was seen in MPN-BP patients compared with those with post-MDS AML (HR, 1.16 [95% CI, 0.88-1.54]; P = .30) (Table 3). Other factors associated with transplant outcomes in this comparison are summarized in supplemental Table 4. As expected, older age was associated with inferior OS due to higher NRM. Poor performance status and poor-risk cytogenetics were associated with inferior OS due to higher relapse risk. TBI-MAC was associated with inferior survival in comparison with Chemo-MAC or RIC due to higher NRM. Peripheral blood as the graft source was associated with better survival due to lower relapse rate (supplemental Table 4). Patients using CSA-MMF–based GVHD prophylaxis had higher NRM compared with CSAand methotrexate (supplemental Table 4). In vivo T-cell depletion was associated with lower NRM.
Multivariate Cox model for study cohorts undergoing HCT for MPN-BP vs post-MDS AML
| . | n . | HR (95% CI) . | P . |
|---|---|---|---|
| aGVHD II-IV | |||
| MDS-AML | 1090 | 1.00 (Reference) | .98 |
| MPN-BP | 176 | 1.00 (0.79-1.27) | |
| aGVHD III-IV | |||
| MDS-AML | 1086 | 1.00 (Reference) | .94 |
| MPN-BP | 174 | 0.99 (0.69-1.41) | |
| cGVHD | |||
| MDS-AML | 1091 | 1.00 (Reference) | .32 |
| MPN-BP | 176 | 0.88 (0.67-1.14) | |
| OS | |||
| Post-MDS AML | 1104 | 1.00 (Reference) | .05 |
| MPN-BP | 177 | 1.19 (1.00-1.43) | |
| Relapse | |||
| Post-MDS AML | 1085 | 1.00 (Reference) | <.0001 |
| MPN-BP | 177 | 1.60 (1.31-1.96) | |
| NRM | |||
| Post-MDS AML | 1085 | 1.00 (Reference) | .81 |
| MPN-BP | 177 | 0.96 (0.69-1.34) | |
| PFS | |||
| Post-MDS AML | 1085 | 1.00 (Reference) | .0003 |
| MPN-BP | 177 | 1.38 (1.16-1.63) |
| . | n . | HR (95% CI) . | P . |
|---|---|---|---|
| aGVHD II-IV | |||
| MDS-AML | 1090 | 1.00 (Reference) | .98 |
| MPN-BP | 176 | 1.00 (0.79-1.27) | |
| aGVHD III-IV | |||
| MDS-AML | 1086 | 1.00 (Reference) | .94 |
| MPN-BP | 174 | 0.99 (0.69-1.41) | |
| cGVHD | |||
| MDS-AML | 1091 | 1.00 (Reference) | .32 |
| MPN-BP | 176 | 0.88 (0.67-1.14) | |
| OS | |||
| Post-MDS AML | 1104 | 1.00 (Reference) | .05 |
| MPN-BP | 177 | 1.19 (1.00-1.43) | |
| Relapse | |||
| Post-MDS AML | 1085 | 1.00 (Reference) | <.0001 |
| MPN-BP | 177 | 1.60 (1.31-1.96) | |
| NRM | |||
| Post-MDS AML | 1085 | 1.00 (Reference) | .81 |
| MPN-BP | 177 | 0.96 (0.69-1.34) | |
| PFS | |||
| Post-MDS AML | 1085 | 1.00 (Reference) | .0003 |
| MPN-BP | 177 | 1.38 (1.16-1.63) |
Comparative outcomes of MPN-BP vs post-MDS AML. (A) Adjusted probability of OS of MPN-BP vs post-MDS AML. The OS of MPN-BP patients was inferior to post-MDS AML patients (HR, 1.19 [95% CI, 1.00-1.43]; P = .05). (B) Adjusted cumulative incidence of relapse of MPN-BP vs post-MDS AML. The relapse rate in MPN-BP patients is significantly higher as compared with post-MDS AML (HR, 1.60 [95% CI, 1.31-1.96]; P < .0001).
Comparative outcomes of MPN-BP vs post-MDS AML. (A) Adjusted probability of OS of MPN-BP vs post-MDS AML. The OS of MPN-BP patients was inferior to post-MDS AML patients (HR, 1.19 [95% CI, 1.00-1.43]; P = .05). (B) Adjusted cumulative incidence of relapse of MPN-BP vs post-MDS AML. The relapse rate in MPN-BP patients is significantly higher as compared with post-MDS AML (HR, 1.60 [95% CI, 1.31-1.96]; P < .0001).
Discussion
In the current study, we evaluated the outcomes of HCT in MPN-BP with de novo AML and with post-MDS AML in 2 separate comparisons. Although NRM was similar between these cohorts, the relapse rate was significantly higher in MPN-BP in remission compared with de novo AML in remission, and MPN-BP vs post-MDS AML. Similar outcomes were observed in patients with MPN-BP with active leukemia compared with de novo AML with active leukemia. We also observed that there is consistent reduction in the transplant activity for patients with active leukemia at the time of HCT for MPN-BP, de novo AML, and post-MDS AML cohorts, respectively.
Biologically, patients with MPN-BP are significantly distinct from de novo AML.8,15,16 Patients with MPN-BP have a complex genetic profile with a higher frequency of adverse-risk cytogenetic abnormalities as well as mutations known to affect the outcomes of AML patients such as TP53, RAS-signaling pathway mutations, and ASXL1 and SRSF2 mutations. A very high rate of relapse seen in the early posttransplant period in patients with MPN-BP is likely related to these adverse biological factors. In addition, remission in MPN-BP is very different compared with the remission in de novo AML and mainly conversion of BP to chronic phase of the disease. We have previously reported that, at the molecular level, there was no difference in molecular profiling of MPN-BP patients in remission vs MPN-BP with active leukemia, and mutation spectrum, variant allele frequency, and residual burden of mutations was similar in these 2 cohorts.17 In comparison with post-MDS AML patients, MPN-BP patients also had significantly longer duration of the underlying hematologic disorder. Longer duration of the underlying hematologic disorder can potentially add to comorbid illnesses, and indeed in the MPN-BP cohort, burden of comorbidities, as measured by HCT-CI, was significantly higher. Underlying comorbid conditions perhaps led to the higher proportion of patients with poor performance status in the MPN-BP cohort.
This study has several limitations as inherent to any retrospective registry-based studies, and caution needs to be exercised in the interpretation of the results. Remission status was based on basic morphological criteria, and there was no central review of bone marrow. Detailed granular data needed for response assessment using international working group criteria were not collected in the CIBMTR database. Comorbidity data were missing in one-third of the patients with MPN-BP, therefore we were unable to adjust for this in the multivariate analysis. Nevertheless, this study significantly adds to the understanding of outcomes of HCT in MPN-BP. Previous reported studies were limited by small patient numbers, short follow-up, and primarily descriptive nature.18-20 Furthermore, multivariate analysis was either not done18 or had insufficient power19,20 and the median follow-up was shorter. The current study has a significantly larger number of patients with mature follow-up.
Several noteworthy findings in this study may guide clinical practice and patient counseling and optimize transplant protocols. The relapse rate was lower with peripheral blood grafts in patients with MPN-BP and post-MDS AML. NRM was similar between MPN-BP vs de novo AML, and MPN-BP vs post-MDS AML. We also demonstrate that a TBI-MAC regimen is associated with higher NRM in both comparisons, and therefore should be avoided in these patients. No significant difference in OS was observed with Chemo-MAC vs RIC. An EBMT study recommended MAC for AML secondary to MPN/MDS as increased relapse was observed after 6 months in patients undergoing RIC.6 However, the EBMT study did not differentiate between TBI-MAC vs Chemo-MAC. In our study, higher NRM was associated with CSA-MMF–based GVHD prophylaxis and lower NRM with T-cell depletion strategies in both comparisons. These findings suggest the need for optimization of GVHD-prevention strategies.
In summary, we demonstrate that HCT outcomes are similar in MPN-BP patients with active leukemia compared with de novo AML with active leukemia. Due to increased relapse risk, the survival of MPN-BP patients in remission is significantly inferior to de novo AML in remission and in comparison with post-MDS AML. Relapse-prevention strategies are required to improve the outcomes of MPN-BP patients undergoing HCT.
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
Acknowledgments
The CIBMTR was supported primarily by Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health; U24HL138660 from the National Heart, Lung, and Blood Institute and National Cancer Institute; R21HL140314 and U01HL128568 from the National Heart, Lung, and Blood Institute; HHSH250201700006C, SC1MC31881-01-00, and HHSH250201700007C from the Health Resources and Services Administration; and N00014-18-1-2850, N00014-18-1-2888, and N00014-20-1-2705 from the Office of Naval Research. Additional federal support was provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, and R01CA231141 from the National Cancer Institute; R01HL126589 from the National Heart, Lung, and Blood Institute; R01AI128775 from the National Institute of Allergy and Infectious Diseases; R01HL129472, R01HL130388, and R01HL131731 from the National Heart, Lung, and Blood Institute; U01AI069197 and U01AI126612 from the National Institute of Allergy and Infectious Diseases; and Biomedical Advanced Research and Development Authority. Support was also provided by Be the Match Foundation, Boston Children’s Hospital, Dana-Farber, Japan Hematopoietic Cell Transplantation Data Center, St. Baldrick’s Foundation, the National Marrow Donor Program, the Medical College of Wisconsin, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptive Biotechnologies; Adienne SA; Allovir, Inc; Amgen, Inc; Anthem, Inc; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc; bluebird bio, Inc; Bristol Myers Squibb; Celgene Corp; Chimerix, Inc; CSL Behring; CytoSen Therapeutics, Inc; Daiichi Sankyo Co Ltd; Gamida-Cell, Ltd; Genzyme; GlaxoSmithKline (GSK); HistoGenetics, Inc; Incyte Corporation; Janssen Biotech, Inc; Janssen Pharmaceuticals, Inc; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc; Kiadis Pharma; Kite Pharma; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt LLC; Medac GmbH; Merck & Company, Inc; Merck Sharp & Dohme Corp; Mesoblast; Millennium, the Takeda Oncology Co; Miltenyi Biotec, Inc; Novartis Oncology; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc; Orca Biosystems, Inc; Pfizer, Inc; Phamacyclics, LLC; Regeneron Pharmaceuticals, Inc; REGiMMUNE Corp; Sanofi Genzyme; Seattle Genetics; Sobi, Inc; Takeda Oncology; Takeda Pharma; Terumo BCT; Viracor Eurofins; and Xenikos BV.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US government.
Authorship
Contribution: V.G., Z.-H.H., and W.S. conceived and designed the study; W.S. provided study material or patients, on behalf of the Chronic Leukemia Working Committee of the CIBMTR; V.G., Z.-H.H., S.K. and W.S. collected, assembled, analyzed, and interpreted data; and all authors wrote the manuscript, approved the final version of the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: V.G. provided consultancy services to Novartis and Celgene; received research funding from Novartis and Incyte; and was on advisory boards for Novartis, Celgene, and Sierra Oncology. R.M.S. was on an advisory board for CareDx. B.L.S. held advisory roles with Celgene, Alexion, Novartis, and Incyte. R.N. reports grants from Miyarisan and Helocyte, and personal fees from Celgene, Merck, and Kyowa-Kirin, from null, outside of the submitted work. G.C.H. reports participating on an advisory board for Incyte; receiving research funding from Phamacyclics, Takeda, Jazz Pharmaceuticals, Incyte, and Astellas Pharma; and receiving travel, accommodations, and expenses from Incyte, The Falk Foundation, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Vikas Gupta, Princess Margaret Cancer Centre, 700 University Ave, 6-326, Toronto, ON M5G 1Z5, Canada; e-mail: vikas.gupta@uhn.ca.
References
Author notes
The full-text version of this article contains a data supplement.

![Comparative outcomes of MPN-BP vs de novo AML. (A) Adjusted probability of OS of MPN-BP vs de novo AML in remission. The OS of MPN-BP patients in remission was significantly inferior to de novo AML patients in remission (HR, 1.40 [95% CI, 1.12-1.76]; P = .003). (B) Adjusted probability of OS of MPN-BP vs de novo AML with active leukemia. The OS was similar in MPN-BP patients with active leukemia compared with de novo AML patients with active leukemia (HR, 0.93 [95% CI, 0.72-1.20]; P = .59). (C) Adjusted cumulative incidence of relapse of MPN-BP vs de novo AML in remission. The relapse rate in MPN-BP patients in remission was significantly higher compared with de novo AML in remission (HR, 2.18 [95% CI, 1.69-2.8]; P < .0001). (D) Adjusted cumulative incidence of relapse of MPN-BP vs de novo AML with active leukemia. No difference in relapse rate was seen in MPN-BP patients with active leukemia vs de novo AML patients with active leukemia (HR, 1.16 [95% CI, 0.88-1.54]; P = .30).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/19/10.1182_bloodadvances.2020002621/5/m_advancesadv2020002621f1.png?Expires=1768714735&Signature=wSkQdY4bWKF8z3ZeC6n8sN8gxpmeSy2HqUBBrPpgO0UNmgd8JCSu5GzE6EROLGVKJ-nYK6ZKt0p26eIwsS9Xr07pmdrI29TyygQQR1hkAknaDTz~nkdJ7S3pb1clqLU7vqWwNrCo6qBruK3UqFdCNUA9LHi9VSXQbgKUlh1gMvQs2SEYgUOQH7wc2ZK3tsKM5hdWqbS2ggAxJVdDPK4ePeb-2AbhYJbY5idUmoXbNni~TSEL6tuZJGFAYSbjkB959JWDogZkNKO18e89qgd60Lqw8sGGoFRrTya0ZpPTnSoOaic4g4iPS3Ys5DqoCVDQGu0iizV1XlNOaSbbnIT4JA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Comparative outcomes of MPN-BP vs post-MDS AML. (A) Adjusted probability of OS of MPN-BP vs post-MDS AML. The OS of MPN-BP patients was inferior to post-MDS AML patients (HR, 1.19 [95% CI, 1.00-1.43]; P = .05). (B) Adjusted cumulative incidence of relapse of MPN-BP vs post-MDS AML. The relapse rate in MPN-BP patients is significantly higher as compared with post-MDS AML (HR, 1.60 [95% CI, 1.31-1.96]; P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/19/10.1182_bloodadvances.2020002621/5/m_advancesadv2020002621f2.png?Expires=1768714735&Signature=JmGbXrICe0a4cYmL-vv5~wJpN~VSH4LZtlPKYuhjOU7I1Aso~abDMn8s2k1Ltas6ZYCWqd-FcMbNlzpVKxnkz7CzW8GnQdeJ~rqLP7DgbOuFILq0sPfpKJRdrLSfsCyjzxuCQVjhlMZxuoc4Zzv5znyuubfQMTeJ55BBxaDSbdPngBtcymNsh4aYTqjC3tuhlNzSTt95paW1OWycBnThL2IFhz2ITuE7dXwxSw3a~OxkEAcHh-K-T2S-QXOC3T4idaXH5ekrQxufzn2sLVJXGf3YYuzTex8X1gsIku5atnXf84N5lvwqepIaFIhLgDiJ0uYsiNWIcqyoYbQpaRPa7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)