Key Points
The intake of probiotics shortened the duration of oral mucositis and diarrhea, and reduced the incidence and severity of aGVHD.
The microbial diversity, population of butyrate producers, and butyrate concentration were maintained in patients who consumed prebiotics.
Abstract
Acute graft-versus-host disease (aGVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Therefore, management of aGVHD is important for successful transplantation. Mucosal damage and alteration of the gut microbiota after allo-HSCT are key factors in the development of aGVHD. We conducted a prospective study to evaluate the ability of prebiotics, which can alleviate mucosal damage and manipulate the gut microbiota, to mitigate posttransplantation complications, including aGVHD. Resistant starch (RS) and a commercially available prebiotics mixture, GFO, were administered to allo-HSCT recipients from pretransplantation conditioning to day 28 after allo-HSCT. Prebiotic intake mitigated mucosal injury and reduced the incidence of all aGVHD grades combined and of aGVHD grades 2 to 4. The cumulative incidence of skin aGVHD was markedly decreased by prebiotics intake. Furthermore, the gut microbial diversity was well maintained and butyrate-producing bacterial population were preserved by prebiotics intake. In addition, the posttransplantation fecal butyrate concentration was maintained or increased more frequently in the prebiotics group. These observations indicate that prebiotic intake may be an effective strategy for preventing aGVHD in allo-HSCT, thereby improving treatment outcomes and the clinical utility of stem cell transplantation approaches. This study was registered on the University Hospital Medical Information Network (UMIN) clinical trials registry (https://www.umin.ac.jp/ctr/index.htm) as #UMIN000027563.
Introduction
Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a viable treatment of various hematological diseases, acute graft-versus-host disease (aGVHD) represents a major cause of morbidity and mortality.1 The standard first-line treatment of aGVHD is glucocorticoid (steroid), but a third to a half of patients do not respond to steroid therapy, and the long-term outlook for patients with steroid-refractory aGVHD is poor.2 Hence, management of aGVHD with alternative therapies is critical for a large proportion of allo-HSCT recipients.
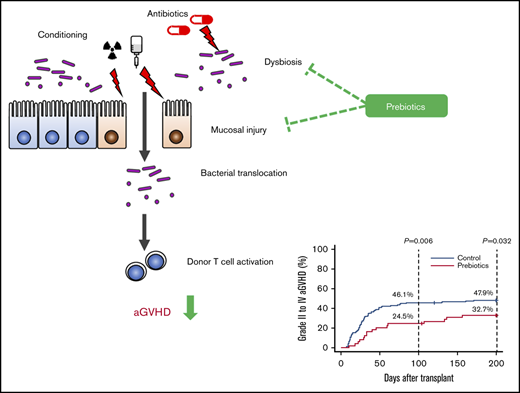
An increasing body of evidence indicates that gut microbiota and their metabolites are closely associated with aGVHD. Translocation of bacterial components such as lipopolysaccharide is thought to be a driver of aGVHD, by stimulating innate pattern-recognition receptors, such as Toll- and Nod-like receptors, of host- and/or donor-derived antigen-presenting cells that produce proinflammatory cytokines, as well as by priming donor T cells.3,4 Furthermore, as shown by some clinical studies, dysbiosis (imbalance of the gut microbiota) increases aGVHD-related mortality.5,6 Therefore, therapeutic strategies that target the gut microbiota could constitute promising treatment options for the management of aGVHD.
Food is one of the most important factors affecting the composition of gut microbiota. The superiority of enteral nutrition over parenteral nutrition in impeding the development of aGVHD, decreasing transplantation-related mortality, and increasing overall survival has been reported.7,8 It is thought that oral food intake and enteral nutrition encourage maintenance of gut mucosal integrity and support the gastrointestinal environment, including gut microbiota, which may be beneficial to allo-HSCT recipients.9 Dietary supplementation that mimics the effects of enteral nutrition can exert similarly beneficial effects. A retrospective single-center clinical study reported that a nutritional supplement comprising glutamine, fiber, and oligosaccharide (GFO) alleviates the mucosal injury associated with allo-HSCT.10 Another prebiotic supplement, resistant starch (RS),11 increases the amount of short-chain fatty acids,12 including butyrate and the abundance of butyrate-producing bacteria in the intestine.13 Notably, both butyrate and butyrate-producing bacteria mitigated aGVHD in a mouse model.14
Based on these observations, we conducted a prospective study to investigate the hypothesis that GFO and RS alleviate mucosal injury and help maintain intestinal microbial diversity, ultimately mitigating aGVHD in allo-HSCT recipients.
Methods
Study design
A prospective study was conducted to evaluate the clinical effects and microbial changes in prebiotic intake in allo-HSCT recipients. Forty-nine individuals who underwent allo-HSCT at Tokyo Metropolitan Komagome Hospital from July 2017 through December 2017 were enrolled in the study (the prebiotics group). After providing written informed consent, the patients ingested RS and GFO from the start of the pretransplantation conditioning regimen until day 28 after the transplantation. Most of the patients provided further informed consent for the analysis of gut microbiota, and fecal samples were collected before the pretransplantation conditioning regimen (the pretransplantation phase) and on day 28 ± 3 (day 28 phase). Forty-three patients consented to fecal sample analysis, and both pretransplantation and day 28 samples were obtained from 30 patients. Fecal samples of 151 patients who did not receive prebiotics and underwent allo-HSCT from April 2013 through February 2015 and consented to fecal sample analysis were previously sequentially collected at Komagome Hospital.15 The clinical data for 142 of those patients were used as the control (the historical control group). Nine patients were excluded from the historical control group because of the lack of clinical data. Of the 142 historical control group patients, microbial analysis of fecal samples collected both at the pretransplantation phase and day 28 phase was possible for 72. The current study was approved by the Ethics Committee of Tokyo Metropolitan Komagome Hospital.
The study schema is shown in supplemental Figure 1. RS (Amylofiber SH; J-Oil Mills Inc, Tokyo, Japan) and the prebiotic mixture GFO (Otsuka Pharmaceutical Factory Inc, Tokushima, Japan) were used. Amylofiber SH is cornstarch containing 70% RS. One pack of GFO contains 3 g glutamine, 5 g polydextrose, and 1.45 g lactosucrose. RS-rich dishes, containing 8 g of RS, were provided to patients for lunch and dinner, and 1 pack of GFO was provided at breakfast from conditioning to day 28. RS was mixed with soup, cocoa, or pudding, and GFO was dissolved in water (supplemental Figure 1). These were served as side dishes, the proportion of consumed RS and GFO was determined, and the median percentage was calculated for each patient. This calculation was made because not all patients were consistently able to consume the entire prebiotic portion provided.
The primary outcome was the incidence and duration of mucositis (oral mucositis and diarrhea), and the secondary key outcomes were the incidence of aGVHD and duration of total parenteral nutrition (TPN). Oral mucositis was assessed in detail using the Eilers’ Oral Assessment Guide (OAG),16 which is considered a key oral evaluation guide recognized by the scientific community for the purpose of evaluating changes in the oral mucosa resulting from antineoplastic treatment.17,18 The OAG score is defined as a total score of 8 items (the voice, swallowing, lips, tongue, saliva, mucous membranes, gingiva, and teeth), each graded from 1 (normal) to 3 (severe). Total scores of 9 to 11 were defined as mild, 12 to 18 as moderate, and 19 to 24 as severe mucositis. Diarrhea was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0. The OAG score and diarrhea were evaluated from allo-HSCT (day 0) to neutrophil engraftment. aGVHD was diagnosed and graded according to previously established criteria.19
Transplantation procedures
Transplantation procedures are described in detail elsewhere.20 Patients with myeloid malignancies received nontotal body irradiation (TBI) regimens, including IV busulfan (3.2 mg/kg for 4 days) and cyclophosphamide (60 mg/kg for 2 days), as myeloablative conditioning. Patients with lymphoid malignancies received 12 Gy TBI and cyclophosphamide (60 mg/kg for 2 days) as myeloablative conditioning. Reduced-intensity conditioning consisted of fludarabine (30 mg/m2 for 6 days), IV busulfan (3.2 mg/kg for 2 days), or melphalan (40 mg/m2 for 2 days), and TBI (4 Gy). Prophylaxis for aGVHD consisted of a short course of methotrexate and cyclosporine A or tacrolimus (FK). FK was used in cases of either unrelated or HLA-mismatched HSCT. In cases of haploidentical transplantation, either a combination of FK, mycophenolate mofetil, and posttransplantation cyclophosphamide or a combination of FK, methylprednisolone, and antithymocyte globulin was used for aGVHD prophylaxis. All patients except for the ones who had already received antibiotic treatments were given oral quinolone (levofloxacin or tosufloxacin) from conditioning at least until neutrophil engraftment or the start of IV antibiotics for febrile neutropenia. The transplantation procedures, including the prophylactic antibiotics strategy, were the same for the historical control and prebiotics groups.
Analysis of fecal samples
Sequencing of the 16S ribosomal RNA gene from fecal samples was performed as previously described.21,22 The hypervariable V1-2 region of the 16S rRNA gene was amplified by polymerase chain reaction (PCR) using barcoded 27Fmod and 338R primers. DNA extracted from Escherichia coli DH5α–competent cells (TaKaRa Bio Inc, Shiga, Japan) was used as the positive control template, whereas a DNA-free sample was used as the negative control. PCR amplicons and negative reaction (for the negative control) were confirmed by electrophoresis. Then, an equal amount of purified PCR amplicons was sequenced on a MiSeq platform (Illumina, San Diego, CA). Next, 3000 high-quality reads were randomly selected per sample and analyzed to minimize the overestimation of species richness during clustering associated with the intrinsic sequencing error.21 Good’s coverage index23 for the 3000 reads per sample in the current study was 0.984, indicating a high degree of coverage and a sufficient read number for the fecal microbiome analysis. The reads were sorted and grouped into operational taxonomic units (OTUs) by using the UCLUST algorithm, at a sequence identity threshold of 97%. Taxonomic assignments of each OTU were made by similarity-searching against the publicly available 16S (RDP v10.27 and CORE) and National Center for Biotechnology Information (NCBI) genome database, using GLSEARCH. OTU-based microbial diversity was estimated by using the Shannon index (SI) with scikit-bio (v0.5.1).
Sixty-one butyrate-producing bacteria have been isolated from humans (supplemental Table 1).24,25 Sequences of 59 of the 61 species were obtained (exceptions were Treponema phagedenis and T vincentii) from the 16S RefSeq release 89. To evaluate the abundance of butyrate-producing bacteria, we analyzed the taxonomic assignment of each OTU, using the aforementioned 16S and NCBI genome database merged into the RefSeq sequence, with a sequence identity threshold of 97%. Singletons were discarded. For the fecal butyrate measurements, 30 mg of frozen stool samples was used, and the butyrate was measured by liquid chromatography-tandem mass spectrometry (see the supplemental Method for details of the protocol).
Statistical analysis
Fisher’s exact test was used for categorical variable analysis and the Mann-Whitney U test or Welch’s test was used for analysis of continuous variables. The cumulative incidences of aGVHD and relapse and nonrelapse mortality were determined using the cumulative incidence function method and compared using Gray’s test. Prebiotic intake, sex, age (<55 vs ≥55 years), disease risk at allo-HSCT, number of transplantations, conditioning regimen (myeloablative vs reduced intensity), donor-recipient sex match (female-male vs others), HLA disparity, type of transplantation, and use of antibiotics with a relative high antianaerobic activity were selected as the potential risk factors for the occurrence of aGVHD and subjected to a multivariate analysis, using backward stepwise Fine and Gray proportional-hazard modeling. Overall survival was determined by the Kaplan-Meier method and compared by using the log-rank test. In all analyses, statistical significance was defined as P < .05, based on a 2-sided test. Statistical analyses were performed with R 3.6.0 software (The R Foundation for Statistical Computing).
Results
Patient characteristics
The characteristics of all patients are summarized in Table 1. The ratio of female-to-male patients was significantly higher in the historical control group than in the prebiotics group. The ratio of patients who received 2 lines of antianaerobic antibiotics was higher in the prebiotics group than in the historical control group (20% vs 4%).
Patient characteristics
| . | Prebiotics group (n = 49) . | Historical control group (n = 142) . | P . |
|---|---|---|---|
| Sex | .179 | ||
| Male | 24 (49) | 87 (61) | |
| Female | 25 (51) | 55 (39) | |
| Age, y | .235 | ||
| <55 | 34 (69) | 84 (59) | |
| ≥55 | 15 (31) | 58 (41) | |
| Primary disease | .217 | ||
| AML | 25 (51) | 70 (49) | |
| ALL | 10 (20) | 25 (18) | |
| MDS | 4 (8) | 28 (20) | |
| Others | 10 (20) | 19 (13) | |
| Disease risk* | .250 | ||
| Low risk | 28 (57) | 67 (47) | |
| High risk | 21 (43 | 75 (53) | |
| No. of transplantations | .803 | ||
| 1 | 44 (90) | 123 (87) | |
| ≥2 | 5 (10) | 19 (13) | |
| Donor sex | .216 | ||
| Male | 37 (76) | 92 (65) | |
| Female | 12 (24) | 50 (35) | |
| Donor-recipient sex | .028 | ||
| Female to male | 2 (4) | 24 (17) | |
| Others | 47 (96) | 118 (83) | |
| HLA disparity (X/6) | .295 | ||
| 0 | 19 (39) | 66 (46) | |
| 1 | 14 (29) | 40 (28) | |
| 2 | 9 (18) | 12 (8) | |
| ≥3 | 7 (14) | 24 (17) | |
| Allo-HSCT type | .309 | ||
| rPBSCT | 6 (12) | 18 (13) | |
| rBMT | 2 (4) | 3 (2) | |
| uPBSCT | 0 (0) | 2 (1) | |
| uBMT | 21 (43) | 77 (54) | |
| CBT | 8 (16) | 15 (11) | |
| Haplo (PT-CY) | 6 (12) | 6 (4) | |
| Haplo (ATG+steroid) | 6 (12) | 21 (15) | |
| Conditioning | .095 | ||
| MAC | 33 (67) | 75 (53) | |
| RIC | 16 (33) | 67 (47) | |
| ATG | .819 | ||
| Yes | 8 (16) | 21 (15) | |
| No | 41 (84) | 121 (85) | |
| TBI | .522 | ||
| Yes | 42 (86) | 115 (81) | |
| No | 7 (14) | 27 (19) | |
| GVHD prophylaxis | .665 | ||
| CsA-based | 10 (20) | 24 (17) | |
| FK-based | 39 (80) | 118 (83) | |
| Use of antibiotics with relatively high anti-anaerobe activity (from conditioning to day 28)† | <.001 | ||
| None | 13 (27) | 25 (18) | |
| 1 line | 26 (53) | 110 (73) | |
| 2 lines | 10 (20) | 6 (4) |
| . | Prebiotics group (n = 49) . | Historical control group (n = 142) . | P . |
|---|---|---|---|
| Sex | .179 | ||
| Male | 24 (49) | 87 (61) | |
| Female | 25 (51) | 55 (39) | |
| Age, y | .235 | ||
| <55 | 34 (69) | 84 (59) | |
| ≥55 | 15 (31) | 58 (41) | |
| Primary disease | .217 | ||
| AML | 25 (51) | 70 (49) | |
| ALL | 10 (20) | 25 (18) | |
| MDS | 4 (8) | 28 (20) | |
| Others | 10 (20) | 19 (13) | |
| Disease risk* | .250 | ||
| Low risk | 28 (57) | 67 (47) | |
| High risk | 21 (43 | 75 (53) | |
| No. of transplantations | .803 | ||
| 1 | 44 (90) | 123 (87) | |
| ≥2 | 5 (10) | 19 (13) | |
| Donor sex | .216 | ||
| Male | 37 (76) | 92 (65) | |
| Female | 12 (24) | 50 (35) | |
| Donor-recipient sex | .028 | ||
| Female to male | 2 (4) | 24 (17) | |
| Others | 47 (96) | 118 (83) | |
| HLA disparity (X/6) | .295 | ||
| 0 | 19 (39) | 66 (46) | |
| 1 | 14 (29) | 40 (28) | |
| 2 | 9 (18) | 12 (8) | |
| ≥3 | 7 (14) | 24 (17) | |
| Allo-HSCT type | .309 | ||
| rPBSCT | 6 (12) | 18 (13) | |
| rBMT | 2 (4) | 3 (2) | |
| uPBSCT | 0 (0) | 2 (1) | |
| uBMT | 21 (43) | 77 (54) | |
| CBT | 8 (16) | 15 (11) | |
| Haplo (PT-CY) | 6 (12) | 6 (4) | |
| Haplo (ATG+steroid) | 6 (12) | 21 (15) | |
| Conditioning | .095 | ||
| MAC | 33 (67) | 75 (53) | |
| RIC | 16 (33) | 67 (47) | |
| ATG | .819 | ||
| Yes | 8 (16) | 21 (15) | |
| No | 41 (84) | 121 (85) | |
| TBI | .522 | ||
| Yes | 42 (86) | 115 (81) | |
| No | 7 (14) | 27 (19) | |
| GVHD prophylaxis | .665 | ||
| CsA-based | 10 (20) | 24 (17) | |
| FK-based | 39 (80) | 118 (83) | |
| Use of antibiotics with relatively high anti-anaerobe activity (from conditioning to day 28)† | <.001 | ||
| None | 13 (27) | 25 (18) | |
| 1 line | 26 (53) | 110 (73) | |
| 2 lines | 10 (20) | 6 (4) |
Data are expressed as number of patients (percentage of subgroup).
ALL, acute lymphoblastic leukemia; ATG, antithymocyte globulin; BMT, bone marrow transplantation; CBT, cord blood transplantation; CsA, cyclosporine; Haplo, haploidentical transplantation; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; PT-CY, posttransplantation cyclophosphamide; RIC, reduced-intensity conditioning; rPBSCT, related peripheral blood stem cell transplantation; uPBSCT, unrelated PBSCT.
Low-risk disease included acute leukemia in the first complete remission (CR), ronic myeloid leukemia (CML) in the first chronic phase, MDS in refractory anemia, malignant lymphoma in CR, and nonmalignant hematologic diseases. All other diagnoses and second allo-HSCT were included in high-risk disease.
Antibiotics with relatively high antianaerobic activity: meropenem, imipenem-cilastatin, and piperacillin-tazobactam. The other antibiotics were third- and fourth-generation cephems, polypeptides, quinolones, sulfamethoxazole-trimethoprim, aminoglycosides, and aztreonam.
Prebiotics alleviated mucosal injury and reduced aGVHD
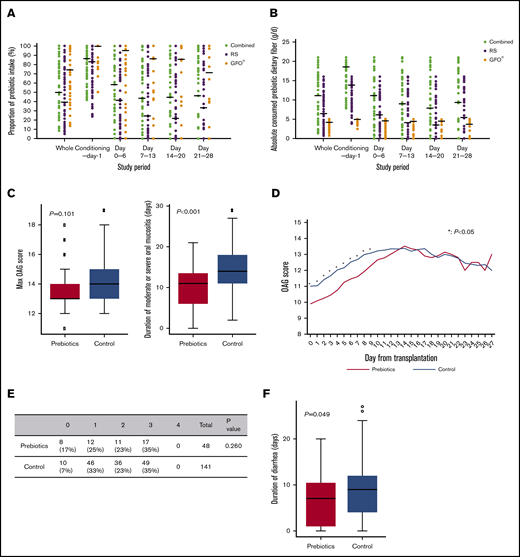
The median intake of prebiotics was 50%: 39% for RS and 74.3% for GFO. The median intake of dietary fiber from prebiotics was 10.7 g/d: 6.6 g/d from RS and 3.7 g/d from GFO. The prebiotic intake was highest during the conditioning period and rapidly decreased after allo-HSCT, slightly improving in week 4 (Figure 1A-B). The main cause of intake reduction was transplantation-related toxicity, such as nausea, vomiting, general fatigue, or painful oral mucositis. No adverse events were obviously attributable to prebiotic intake.
Prebiotics mitigate mucosal injury. (A) The proportion of combined RS and GFO and RS and GFO prebiotic intake during the study period. (B) The absolute amount of consumed dietary fiber derived from the combined and separate RS and GFO prebiotics. The horizontal lines represent median values. (C) Oral mucositis was assessed from day 0 to engraftment according to the OAG score. No significant difference between the maximum OAG scores in the historical control and prebiotics groups was observed (median, 13 vs 14; P = .101). The duration of moderate or severe (ie, OAG score ≥12) oral mucositis in the prebiotics group was significantly shorter than that in the historical control group (median, 11 d vs 14 days; P < .001). (D) Prebiotics reduce the severity of oral mucositis early after allo-HSCT. OAG score from allo-HSCT (day 0) to neutrophil engraftment. Average OAG scores in the 2 groups are depicted. The OAG scores in the prebiotics group were significantly lower than those in the historical control group from days 0 to 9 (*P < .05). (E-F) Diarrhea from day 0 to engraftment was assessed using CTCAE v4.0. (E) The maximum grade of diarrhea was assessed in both groups. The ratio of grade 0 diarrhea was higher in the prebiotics group than in the historical control group (17% and 7%, respectively), although the difference between the groups in the ratio of maximum grade of diarrhea was not significant (P = .260; Fisher’s exact test). (F) The duration of diarrhea in the prebiotics group was significantly shorter than that in the historical control group (median, 7 days vs 9 days; P = .049).
Prebiotics mitigate mucosal injury. (A) The proportion of combined RS and GFO and RS and GFO prebiotic intake during the study period. (B) The absolute amount of consumed dietary fiber derived from the combined and separate RS and GFO prebiotics. The horizontal lines represent median values. (C) Oral mucositis was assessed from day 0 to engraftment according to the OAG score. No significant difference between the maximum OAG scores in the historical control and prebiotics groups was observed (median, 13 vs 14; P = .101). The duration of moderate or severe (ie, OAG score ≥12) oral mucositis in the prebiotics group was significantly shorter than that in the historical control group (median, 11 d vs 14 days; P < .001). (D) Prebiotics reduce the severity of oral mucositis early after allo-HSCT. OAG score from allo-HSCT (day 0) to neutrophil engraftment. Average OAG scores in the 2 groups are depicted. The OAG scores in the prebiotics group were significantly lower than those in the historical control group from days 0 to 9 (*P < .05). (E-F) Diarrhea from day 0 to engraftment was assessed using CTCAE v4.0. (E) The maximum grade of diarrhea was assessed in both groups. The ratio of grade 0 diarrhea was higher in the prebiotics group than in the historical control group (17% and 7%, respectively), although the difference between the groups in the ratio of maximum grade of diarrhea was not significant (P = .260; Fisher’s exact test). (F) The duration of diarrhea in the prebiotics group was significantly shorter than that in the historical control group (median, 7 days vs 9 days; P = .049).
Oral mucositis was observed in all patients, and the maximum OAG score did not differ significantly between the 2 groups. However, the duration of moderate or severe oral mucositis in the prebiotics group was significantly shorter than that in the historical control group (median of 11 and 14 days, respectively; Figure 1C). Furthermore, the OAG scores in the prebiotics group were significantly lower than those in the historical control group early (days 0-9) after allo-HSCT (Figure 1D). The proportion of patients who developed severe (grade 3 or higher) diarrhea was comparable in the 2 groups. However, the proportion of patients without diarrhea was higher in the prebiotics group (17%) than in the historical control group (7%), and the duration of diarrhea in the prebiotics group was shorter than that in the historical control group (median of 7 and 9 days, respectively; Figure 1E-F). No significant difference in the duration of TPN was observed in the 2 groups (data not shown). However, the duration of opioid use until engraftment in the prebiotics group was significantly shorter than in the historical control group (supplemental Figure 2), and the proportion of patients who did not receive opioid therapy was also higher in the prebiotics group (14.5% in the historical control group vs 34.1% in the prebiotics group).
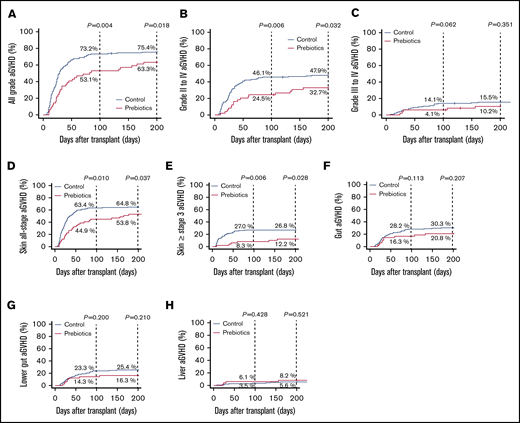
By day 100, the cumulative incidence of aGVHD of all grades was significantly lower in the prebiotics group than in the historical control group. The incidence of grade 2 to 4 aGVHD was also significantly lower in the prebiotics group (Figure 2A-B). Notably, more patients in the prebiotics group developed aGVHD after day 100 than patients in the historical control group. The cumulative incidence of skin aGVHD in the prebiotics group was significantly lower than that in the historical control group (Figure 2D-E). Similarly, the cumulative incidence of gastrointestinal aGVHD was ∼10% lower in the prebiotics group, although the differences were not statistically significant (Figure 2F). Because the historical control group and the prebiotics group were separated in time, the incidences of aGVHD in patients who did not receive prebiotics and underwent transplantation from January 2017 through June 2017 (n = 48) were also evaluated. The cumulative incidences of aGVHD in early 2017 were comparable with those in the historical control group, not the prebiotics group (supplemental Figure 3). In addition, the use of antibiotics with relatively high antianaerobic activity would severely disrupt the gut microbiome and may have reduced the effect of the prebiotics. However, the use of such antibiotics did not significantly increase the cumulative incidences of aGVHD in the 2 groups (supplemental Figure 4). Multivariate analysis revealed that prebiotic intake was the only independent factor that negatively affected the development of aGVHD (Table 2). The overall survival tended to be higher in the prebiotics group than in the historical control group, although the difference was not statistically significant (supplemental Figure 5A). No significant differences in nonrelapse mortality (supplemental Figure 5B) or in the cumulative incidences of relapse (supplemental Figure 5C) were observed between the groups.
Prebiotics decrease the cumulative incidence of aGVHD. (A) The cumulative incidence of all grades of aGVHD was significantly lower in the prebiotics group than in the historical control group. (B) The cumulative incidence of grades 2 to 4 aGVHD was significantly lower in the prebiotics group than in the historical control group. (C) The cumulative incidence of grades 3 and 4 aGVHD tended to be lower in the prebiotics group than in the historical control group although the difference was not significant. (D-E) The cumulative incidence of all-stage skin aGVHD, and stage 3 and higher aGVHD was significantly lower in the prebiotics group than in the historical control group. (F-G) The cumulative incidence of the gut and lower gut aGVHD tended to be lower in the prebiotics group than in the historical control group, although the difference was not significant. (H) The cumulative incidence of the liver aGVHD did not differ between the prebiotics and historical control groups.
Prebiotics decrease the cumulative incidence of aGVHD. (A) The cumulative incidence of all grades of aGVHD was significantly lower in the prebiotics group than in the historical control group. (B) The cumulative incidence of grades 2 to 4 aGVHD was significantly lower in the prebiotics group than in the historical control group. (C) The cumulative incidence of grades 3 and 4 aGVHD tended to be lower in the prebiotics group than in the historical control group although the difference was not significant. (D-E) The cumulative incidence of all-stage skin aGVHD, and stage 3 and higher aGVHD was significantly lower in the prebiotics group than in the historical control group. (F-G) The cumulative incidence of the gut and lower gut aGVHD tended to be lower in the prebiotics group than in the historical control group, although the difference was not significant. (H) The cumulative incidence of the liver aGVHD did not differ between the prebiotics and historical control groups.
Factors for grade II-IV aGVHD (N = 191)
| . | n (%) . | P, multivariate . | HR (95% CI) . |
|---|---|---|---|
| Sex | .460 | 0.830 (0.504-1.367) | |
| Male | 111 (58) | ||
| Female | 80 (42) | ||
| Age, y | .360 | 0.763 (0.428-1.359) | |
| ≥55 | 73 (38) | ||
| <55 | 118 (62) | ||
| Disease risk* | .120 | 1.463 (0.906-2.361) | |
| High | 96 (50) | ||
| Low | 95 (50) | ||
| No. of transplantations | .870 | 0.950 (0.503-1.793) | |
| >1 | 24 (13) | ||
| 1 | 167 (87) | ||
| Conditioning | .250 | 1.403 (0.790-2.493) | |
| MAC | 108 (57) | ||
| RIC | 83 (43) | ||
| Donor-recipient sex | .120 | 0.580 (0.291-1.154) | |
| Female to male | 26 (14) | ||
| Others | 165 (86) | ||
| HLA disparity | .380 | 1.230 (0.773-1.957) | |
| 0 | 85 (45) | ||
| ≥1 | 106 (55) | ||
| Type of transplantation | .100 | 0.491 (0.208-1.157) | |
| rPBSCT | 24 (13) | ||
| Others | 167 (87) | ||
| Use of antibiotics with relatively high antianaerobic activity (conditioning to day 28)† | .470 | 1.275 (0.660-2.462) | |
| Yes | 155 (81) | ||
| No | 36 (19) | ||
| Prebiotics | .009 | 0.495 (0.293-0.836) | |
| Yes | 49 (26) | ||
| No | 142 (74) |
| . | n (%) . | P, multivariate . | HR (95% CI) . |
|---|---|---|---|
| Sex | .460 | 0.830 (0.504-1.367) | |
| Male | 111 (58) | ||
| Female | 80 (42) | ||
| Age, y | .360 | 0.763 (0.428-1.359) | |
| ≥55 | 73 (38) | ||
| <55 | 118 (62) | ||
| Disease risk* | .120 | 1.463 (0.906-2.361) | |
| High | 96 (50) | ||
| Low | 95 (50) | ||
| No. of transplantations | .870 | 0.950 (0.503-1.793) | |
| >1 | 24 (13) | ||
| 1 | 167 (87) | ||
| Conditioning | .250 | 1.403 (0.790-2.493) | |
| MAC | 108 (57) | ||
| RIC | 83 (43) | ||
| Donor-recipient sex | .120 | 0.580 (0.291-1.154) | |
| Female to male | 26 (14) | ||
| Others | 165 (86) | ||
| HLA disparity | .380 | 1.230 (0.773-1.957) | |
| 0 | 85 (45) | ||
| ≥1 | 106 (55) | ||
| Type of transplantation | .100 | 0.491 (0.208-1.157) | |
| rPBSCT | 24 (13) | ||
| Others | 167 (87) | ||
| Use of antibiotics with relatively high antianaerobic activity (conditioning to day 28)† | .470 | 1.275 (0.660-2.462) | |
| Yes | 155 (81) | ||
| No | 36 (19) | ||
| Prebiotics | .009 | 0.495 (0.293-0.836) | |
| Yes | 49 (26) | ||
| No | 142 (74) |
CI, confidence interval; HR, hazard ratio.
Low-risk disease included acute leukemia in the first CR, CML in first chronic phase, MDS in refractory anemia, malignant lymphoma in CR, and nonmalignant hematologic diseases. All other diagnoses and second allo-HSCT were included in high-risk disease.
Antibiotics with relatively high antianaerobic activity: meropenem, imipenem-cilastatin, and piperacillin-tazobactam. The other antibiotics were third- and fourth-generation cephems, polypeptides, quinolones, sulfamethoxazole-trimethoprim, aminoglycosides, and aztreonam.
Prebiotics preserved microbial diversity and the number of butyrate-producing bacteria
For the study, 30 patients from the prebiotics group provided fecal samples in the pretransplantation phase and on day 28 after transplantation. In the historical control group, both pretransplantation and day 28 fecal samples were obtained from 72 patients. The characteristics of these patients are summarized in supplemental Table 2. There were no significant differences between the 2 groups. The SIs of samples collected on day 28 were not significantly different between the 2 groups, but were higher in the historical control group than in the prebiotics group in the pretransplantation phase (Figure 3A). The SIs in the prebiotics group were well maintained compared with those in the historical control group on day 28 (Figure 3B). Notably, the SIs of 6 patients (20%) in the prebiotics group increased on day 28, whereas the same was observed for only 2 (2.8%) patients in the historical control group. At the time of pretransplantation feces collection, 21 patients (29%) in the historical control group and only 1 patient (3%) in the prebiotics group had not yet received any antibiotics. Exclusion of samples from these patients did not meaningfully alter the SIs of the groups (Figure 3A). Populations of butyrate-producing bacteria were preserved in samples collected from the prebiotics group on day 28 and compared with those in the historical control group (Figure 3C). Fecal butyrate concentrations in both pretransplantation and day 28 samples were analyzed for 69 patients in the historical control group and 30 patients in the prebiotics group. Butyrate concentrations in pretransplantation samples, but not in day 28 samples, were significantly higher in the historical control group than in the prebiotics group (Figure 3C). Although butyrate concentration decreased after allo-HSCT in most patients, it was maintained or increased (ie, the ratio of pretransplantation to day 28 butyrate concentrations was ≥0.8) in some patients. This result was more frequently noted in the prebiotics group (6 of 30 patients; 20%) than in the historical control group (3 of 69 patients; 4.4%; Figure 3D).
Prebiotics preserve the microbial diversity and maintain the population of butyrate-producing bacteria and butyrate concentration after all-HSCT. (A) The SI in the gut microbiota during the pretransplantation phase and on day 28. The SI during the pretransplantation phase was significantly higher in the historical control group than in the prebiotics group (P = .011), whereas there was no significant difference between the groups on day 28 (P = .444) (left). Exclusion of patients who did not receive any antibiotics at the time of pretransplantation feces collection did not affect the outcome (right). (B) The microbial diversity before and on day 28 after allo-HSCT were evaluated by SI. Patients were classified into 3 groups according to the dynamics of the SI (deteriorated [≤−0.5], invariant [from −0.5 to 0.5], and improved [≥0.5]). Compared with the historical control group, the microbial diversity in the prebiotics group was well maintained or even improved in some cases (deteriorated, 57.7% vs 76.4%; invariant, 23.3% vs 20.8%; improved, 20% vs 2.8%; P = .004). (C) Butyrate-producing bacteria were quantified at preconditioning and on day 28 after allo-HSCT. Compared with the historical control group, the butyrate-producing bacteria counts in the prebiotics group were sustained (P = .027; prebiotics, n = 30; historical control, n = 72). Fecal butyrate concentration was quantified at preconditioning and on day 28 after allo-HSCT. Butyrate levels at preconditioning in the historical control group were significantly higher than those in the prebiotics group (P = .013). No difference was observed between the 2 groups on day 28 (P = .331), and butyrate levels were below the detection limit in many samples in both groups. (D) Changes in fecal butyrate concentration in the 2 groups. Butyrate concentration was maintained or increased (ie, the ratio of concentrations at preconditioning to those on day 28 was ≥0.8, as indicated by the red lines) in some patients. This was more frequently observed in the prebiotics group (6 of 30; 20%) than in the historical control group (3 of 68; 4.4%). (E) Patients in the prebiotics group were divided into 2 subgroups. Subgroup 1 (n = 14) included patients with SI before allo-HSCT of >2 and whose prebiotic intake was >50%. The other patients were included in subgroup 2 (n = 16). The cumulative incidence of grade 2 to 4 aGVHD on day 100 tended to be lower in subgroup 1 than in subgroup 2 (14.3% and 43.8%, respectively; P = .093).
Prebiotics preserve the microbial diversity and maintain the population of butyrate-producing bacteria and butyrate concentration after all-HSCT. (A) The SI in the gut microbiota during the pretransplantation phase and on day 28. The SI during the pretransplantation phase was significantly higher in the historical control group than in the prebiotics group (P = .011), whereas there was no significant difference between the groups on day 28 (P = .444) (left). Exclusion of patients who did not receive any antibiotics at the time of pretransplantation feces collection did not affect the outcome (right). (B) The microbial diversity before and on day 28 after allo-HSCT were evaluated by SI. Patients were classified into 3 groups according to the dynamics of the SI (deteriorated [≤−0.5], invariant [from −0.5 to 0.5], and improved [≥0.5]). Compared with the historical control group, the microbial diversity in the prebiotics group was well maintained or even improved in some cases (deteriorated, 57.7% vs 76.4%; invariant, 23.3% vs 20.8%; improved, 20% vs 2.8%; P = .004). (C) Butyrate-producing bacteria were quantified at preconditioning and on day 28 after allo-HSCT. Compared with the historical control group, the butyrate-producing bacteria counts in the prebiotics group were sustained (P = .027; prebiotics, n = 30; historical control, n = 72). Fecal butyrate concentration was quantified at preconditioning and on day 28 after allo-HSCT. Butyrate levels at preconditioning in the historical control group were significantly higher than those in the prebiotics group (P = .013). No difference was observed between the 2 groups on day 28 (P = .331), and butyrate levels were below the detection limit in many samples in both groups. (D) Changes in fecal butyrate concentration in the 2 groups. Butyrate concentration was maintained or increased (ie, the ratio of concentrations at preconditioning to those on day 28 was ≥0.8, as indicated by the red lines) in some patients. This was more frequently observed in the prebiotics group (6 of 30; 20%) than in the historical control group (3 of 68; 4.4%). (E) Patients in the prebiotics group were divided into 2 subgroups. Subgroup 1 (n = 14) included patients with SI before allo-HSCT of >2 and whose prebiotic intake was >50%. The other patients were included in subgroup 2 (n = 16). The cumulative incidence of grade 2 to 4 aGVHD on day 100 tended to be lower in subgroup 1 than in subgroup 2 (14.3% and 43.8%, respectively; P = .093).
Low microbial diversity before allo-HSCT may compromise the beneficial effect of prebiotics
In the current study, neither the cumulative incidence of aGVHD nor the SI on day 28 in patients with the amount of prebiotics intake above the median (>50%) differed significantly from that of those in patients with the below-median intake (data not shown). Indeed, some patients in the prebiotics group experienced severe aGVHD even when they consumed a higher-than-average amount of prebiotics. Thus, the larger amount of intake alone was not thought to affect the development of aGVHD. Because prebiotics are fermented by the intestinal microbiota and selectively stimulate the growth and/or activity of intestinal bacteria,26 prebiotic intake may be most effective in patients with a high intestinal microbial diversity. Hence, we conducted an additional subgroup analysis. Patients in the prebiotics group were divided into 2 subgroups: those whose SI before allo-HSCT was >2 and whose median prebiotic intake was >50% (n = 14; subgroup 1) and others (n = 16; subgroup 2). Although not statistically significant, the cumulative incidence of grades 2 to 4 aGVHD on day 100 in subgroup 1 tended to be lower than that in subgroup 2 (Figure 3E).
Discussion
In the current study, we examined the effects of the prebiotics GFO and RS on aGVHD in allo-HSCT recipients. Oral mucositis in the early phase after allo-HSCT was mild, the duration of moderate to severe oral mucositis and diarrhea was shortened, and the incidence and severity of aGVHD were decreased in the prebiotics group.
RS reportedly stimulates intestinal butyrate production, which may strengthen the mucosal barrier. Oral administration of glutamine protects the mucosal barrier in humans.27,28 Polydextrose (a dietary fiber) and lactosucrose (an oligosaccharide) also attenuate intestinal inflammation.29 Because mucosal damage is a well-known initial trigger of aGVHD, it is likely that the reduced incidence and severity of aGVHD observed in patients ingesting both GFO and RS in the current study were associated with reduced mucosal damage. The incidence of grade 2 to 4 aGVHD was significantly lower in the prebiotics group than in the historical control group (Figure 2B), suggesting that prebiotic intake may lower the number of patients who would otherwise require systemic steroid therapy. Indeed, fewer patients received systemic steroid therapy before day 200 in the prebiotics group than in the historical control group (39% vs 58%, respectively).
The incidence of skin aGVHD in the prebiotics group was significantly lower than in the historical control group (Figure 2D-E). That result is not surprising because the gastrointestinal tract also acts as a critical amplifier of systemic aGVHD severity.30 Although not statistically significant, the incidence of gut aGVHD in the prebiotics group was ∼10% lower than in the historical control group in the current study (Figure 2F). The lack of statistical significance may be associated with the small number of patients analyzed.
Interestingly, the incidence of late aGVHD was higher in the prebiotics group than in the historical control group (Figure 2). In the current study, the duration of prebiotic intake was relatively short, ending on day 28. Early cessation of prebiotic intake may elicit late inflammation and lead to the development of late aGVHD in some patients. Hence, a longer period of prebiotic intake (eg, until day 100 or beyond) may mitigate late inflammation, reducing the incidence of late aGVHD. On the other hand, it is possible that classic and late aGVHD are biologically different and that prebiotics may negatively affect (or at least have little effect on) the development of the latter. Further evaluation of a larger cohort and longer prebiotic administration than in the current study are necessary to verify the impact of prebiotic intake on gut, liver, and late aGVHD.
In the current study, prebiotic intake did not influence overall survival, nonrelapse mortality, and cumulative incidence of relapse. The maximum OAG score and the incidence of severe diarrhea did not differ significantly between the 2 groups and may be one of the reasons for the lack of obvious clinical advantages in the prebiotics group. In addition, the short duration of prebiotic intake in the current study may have had little impact on survival and mortality.
Microbial analysis revealed that the SI in the prebiotics group was well maintained and even increased in 6 (20%) patients (Figure 3B). In addition, the number of butyrate-producing bacteria was preserved in the prebiotics group compared with the historical control group, and the fecal butyrate concentration tended to be maintained or increased in the prebiotics group, unlike that in the historical control group (Figure 3C-D). These effects may be associated with prebiotic intake. However, the fecal butyrate concentration was below the detection limit on day 28 in many patients. Therefore, factors other than butyrate may account for the protection against aGVHD elicited by prebiotics. Alternatively, intratissue or early-phase (eg, within 2 weeks after allo-HSCT) butyrate may be more suitable samples for evaluating the association between short-chain fatty acids and aGVHD.14,31
Data obtained in the current study also suggest that patients with a relatively high microbial gut diversity before allo-HSCT may be primed for the beneficial effects of prebiotics (Figure 3E). This conclusion is not unreasonable, because the bioactivity of prebiotics is associated with bacterial fermentation. It may also suggest that, to reconstitute gut microbiota,32,33 combining pretransplantation fecal microbiota transplantation with prebiotics may be an ideal treatment strategy for highly dysbiotic patients.
There are some limitations to the current study. First, the diversity of gut microbiota in the pretransplantation phase was different between the 2 analyzed groups. These differences were significant even when patients who had not received antibiotics at the time of feces collection were excluded from analysis. The clinical characteristics (supplemental Table 2) of the feces contributors were similar. Consequently, these clinical characteristics did not appear to greatly influence the microbial composition. The factors that contribute to the differences in gut biodiversity remain unclear. In the current study, the pretransplantation microbial diversity was higher in the historical control group than in the prebiotics group. High microbial diversity before transplantation is a predictor of better overall survival and reduced treatment-related mortality.34 In addition, fewer patients in the historical control group received 2 lines of antianaerobic antibiotics, which have been reported to increase aGVHD.35,36 Therefore, high microbial diversity before transplantation and less frequent use of antianaerobic antibiotics should predispose the historical control group to better outcomes after allo-HSCT. Second, the difference in the transplantation period between the prebiotics group and the historical control group may have played a considerable role. Because of the lack of fecal samples for patients who underwent transplantation from March 2015 through June 2017, the groups were separated in time, which may have influenced the clinical outcome. According to a previous report, the severity of aGVHD decreases over time.37 However, transplantation procedures, including empiric antibiotic strategy, have not changed from 2013 to 2017. Furthermore, the cumulative incidence of aGVHD in early 2017 was comparable with that in the historical control group, not the prebiotics group (supplemental Figure 1). Hence, it is unlikely that the reduced incidence of aGVHD in the prebiotics group was associated with the different transplantation period. Third, prebiotics used in the current study supplied 21 g of dietary fiber (5 g of polydextrose from GFO and 8 g of RS per dish). The prebiotic dosage used was determined based on the target intake for healthy Japanese adults (>20 g/d for men), not for ill individuals. In addition, the median intake of prebiotics by the patients was relatively low (50%). Moreover, the amount of dietary fiber intake from normal diet was not assessed in this study, which may influence the finding of no obvious dose response with prebiotics. Therefore, it is unclear whether the set prebiotic dose was suitable for allo-HSCT recipients; further evaluation is warranted.
In summary, in this study prebiotic intake reduced posttransplantation complications, such as oral mucositis in the early phase of allo-HSCT, the duration of diarrhea, and aGVHD, possibly by manipulating the gut microbiota. The observations presented herein demonstrate the important role of diet in allo-HSCT (ie, a useful tool for the management of posttransplantation complications). In addition, the findings indicate that therapies seeking to manage the microbiota of allo-HSCT recipients may constitute a new treatment approach for aGVHD. However, in view of the comparison with a historical control group, a prospective, randomized study should be conducted to verify the effects observed in this study.
For original data, please contact the corresponding author Kazuhiko Kakihana (0705kky@gmail.com).
Acknowledgments
The authors thank the patients who participated in the study; Junko Ohta, Yuma Noguchi, and Atsushi Wada for technical assistance; and Editage (www.editage.jp) for English language editing.
This work was supported by Agency for Research and Development-Advanced Research & Development Programs for Medical Innovation (AMED LEAP) grant JP19gm0010003 (K.H. and M.H.); AMED grant JP19ek0510021 and Friends of Leukemia Research Fund (K.K.); and Clinical Research Fund of Tokyo Metropolitan Government grant H290303005 (Y.S.).
Authorship
Contribution: Y.Y., Y.S., Y. Nisaka, K.I., and K.K. designed the study; Y.S., Y.O., and R.T. provided resistant starch-containing foods; K.Y., K.I., Y.Y., Y. Nisaka, T.T., A.I., Y. Najima, N.D., T.K., K.O., and K.K. collected the samples and clinical data; K.Y., K.K., K.I., K.T., Y.K., W.S., S.S., A.S., K.A., M.H., and K.H. performed the research and analyzed the data; and K.Y., Y.K., K.T., K.A., and K.K. wrote the manuscript.
Conflict-of-interest disclosure: K.H. is a scientific advisory board member of Vedanta Biosciences and 4BIO CAPITAL. Y.K. is an employee of JSR Corporation. The remaining authors declare no competing financial interests.
Correspondence: Kazuhiko Kakihana, Hematology Division, Tokyo Metropolitan Komagome Hospital, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113-8677, Japan; e-mail: 0705kky@gmail.com.
References
Author notes
K.Y., K.I., Y.K., and K.K. contributed equally to this study.
The full-text version of this article contains a data supplement.

![Prebiotics preserve the microbial diversity and maintain the population of butyrate-producing bacteria and butyrate concentration after all-HSCT. (A) The SI in the gut microbiota during the pretransplantation phase and on day 28. The SI during the pretransplantation phase was significantly higher in the historical control group than in the prebiotics group (P = .011), whereas there was no significant difference between the groups on day 28 (P = .444) (left). Exclusion of patients who did not receive any antibiotics at the time of pretransplantation feces collection did not affect the outcome (right). (B) The microbial diversity before and on day 28 after allo-HSCT were evaluated by SI. Patients were classified into 3 groups according to the dynamics of the SI (deteriorated [≤−0.5], invariant [from −0.5 to 0.5], and improved [≥0.5]). Compared with the historical control group, the microbial diversity in the prebiotics group was well maintained or even improved in some cases (deteriorated, 57.7% vs 76.4%; invariant, 23.3% vs 20.8%; improved, 20% vs 2.8%; P = .004). (C) Butyrate-producing bacteria were quantified at preconditioning and on day 28 after allo-HSCT. Compared with the historical control group, the butyrate-producing bacteria counts in the prebiotics group were sustained (P = .027; prebiotics, n = 30; historical control, n = 72). Fecal butyrate concentration was quantified at preconditioning and on day 28 after allo-HSCT. Butyrate levels at preconditioning in the historical control group were significantly higher than those in the prebiotics group (P = .013). No difference was observed between the 2 groups on day 28 (P = .331), and butyrate levels were below the detection limit in many samples in both groups. (D) Changes in fecal butyrate concentration in the 2 groups. Butyrate concentration was maintained or increased (ie, the ratio of concentrations at preconditioning to those on day 28 was ≥0.8, as indicated by the red lines) in some patients. This was more frequently observed in the prebiotics group (6 of 30; 20%) than in the historical control group (3 of 68; 4.4%). (E) Patients in the prebiotics group were divided into 2 subgroups. Subgroup 1 (n = 14) included patients with SI before allo-HSCT of >2 and whose prebiotic intake was >50%. The other patients were included in subgroup 2 (n = 16). The cumulative incidence of grade 2 to 4 aGVHD on day 100 tended to be lower in subgroup 1 than in subgroup 2 (14.3% and 43.8%, respectively; P = .093).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/19/10.1182_bloodadvances.2020002604/2/m_advancesadv2020002604f3.png?Expires=1770816028&Signature=YhvzjjPb8fOJRmVrZ4sUoWeJRNHZX9S22UwNYFZC5OZb29e-CUd--fUwl3GADroGejK6ZO9zGP0lbEptE2yjyw-3d6ExzbCvLdVih7Sa5FTbO7BP~wm6mNoiWbVqXZxyf9aL1haCPPvzXzFUBNlpxI3LstIND0-sRYDUbdaua-49Rv2gjbW4bUDcVNLDLoli8R90wN1a~x0nNxGZ-UWlP7H1mpbbvXNkWciJytfc4pKId7vMyw1NHOF7uJUkjhCvx-J~o70t9e1AAzemt3GgRztcI4sTbfHIvvBlOwrwn2V0mSuXl~PH93D8~whFL0eIXg1-axaUDJgEM3NX-f1fRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)