Key Points
Nanomolar concentrations of drag-reducing polymer (DRP) reduce vaso-occlusion in the liver of sickle cell disease (SCD) mice.
The potential for DRP as a rheology-based treatment/therapy for SCD warrants further study.
Introduction
Sickle cell disease (SCD) is an autosomal-recessive blood disorder affecting over 100 000 individuals in the United States.1 Mutant sickle hemoglobin polymerizes within red blood cells (RBCs) under deoxygenated conditions resulting in cell rigidity, hemolysis, and vaso-occlusion.2 These events catalyze an inflammatory response, which can lead to acute painful vaso-occlusive episodes.2,3 Acute pain is the predominant cause for seeking medical treatment, resulting in estimated medical care costs of over $1.1 billion dollars annually for those with SCD.4,5 The role of impaired hemorheology in SCD and its contribution toward vaso-occlusion is well documented.6,7 Poor blood rheology including elevated whole-blood viscosity, plasma viscosity, RBC membrane rigidity, and cytosolic viscoelasticity impedes microvascular blood flow by increasing intravascular resistance and decreasing RBC velocity.8,9 Abnormal cellular adhesion markers presented on sickle RBCs due to membrane damage and on prematurely released reticulocytes further contribute to impaired flow and the precipitation of vaso-occlusion.3,10,11
Recently, blood additives known as drag-reducing polymers (DRPs) have been shown to alter blood rheology beneficially and to improve the hemodynamics and outcomes of animals following hemorrhagic shock,12 liver ischemia/reperfusion injury,13 and traumatic brain injury significantly,14 while simultaneously providing increased capillary recruitment, perfusion, and oxygenation.15 However, the effects of DRP are unknown in the context of SCD, where a rheology-based therapy to improve microcirculatory blood flow has the potential to greatly reduce the incidence of vaso-occlusion. In this study, we have used quantitative liver intravital microscopy (qLIM) to show that the administration of nanomolar concentrations of DRP reduces vaso-occlusion within liver sinusoids of transgenic SCD mice following inflammatory stimulus via lipopolysaccharide (LPS).
Methods
DRP preparation
Polyethylene oxide (molecular weight, 4000 kDa; Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline to 0.2% (2000 parts per million [ppm]) concentration and gently mixed for 4 to 6 hours. Before injection, polyethylene oxide was diluted with sterile saline to 50 ppm and slowly rocked.
Mice
Male and female (12-16 weeks old) Townes SCD mice (SS, homozygous for Hbatm1(HBA)Tow, homozygous for Hbbtm2(HBG1,HBB*)Tow) were used in this study.16 Experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Surgical preparation and qLIM imaging
Details of the surgical preparation and qLIM methodology have been described in detail elsewhere.17,18 Briefly, SCD mice were injected with 0.1 µg/kg LPS from Escherichia coli O111:B4 (Sigma-Aldrich) mixed with 50 µL of either 50 ppm DRP solution or sterile saline into the tail vein. Approximately 1 hour later, mice were anesthetized with an intraperitoneal injection of 100 mg/kg body weight ketamine HCl (100 mg/mL; Henry Shein Animal Health, Dublin, OH) and 20 mg/kg body weight xylazine (20 mg/mL; Lloyd Laboratories, Shenandoah, IA). A tracheotomy was performed for mechanical ventilation with 95% O2 and supply maintenance anesthesia (1% to 1.5% isoflurane). The right lobe of the liver was exposed and immobilized using a micromachined liver window for imaging and held through the use of light suction. The right carotid artery was cannulated for delivery of intravascular dyes including Texas Red–dextran (molecular weight, 70 kDa; Thermo Fisher Scientific, Waltham, MA) used to visualize blood flow through the sinusoids, and Alexa Fluor 546 rat anti-mouse Ly6G monoclonal antibody clone 1A8 (BioLegend, San Diego, CA) used to visualize neutrophils. qLIM movies (supplemental Data) were captured using a Nikon MPE multiphoton excitation microscope (Center for Biologic Imaging, University of Pittsburgh) at ∼15 frames per second over a field of view (FOV) measuring 263 µm by 263 µm with 10 to 15 different FOVs recorded for each mouse.
Image analysis
All movies were processed using FIJI software (ImageJ v2.0).19 Image time series were processed using image subtraction, a 3 × 3 median filter, and adjustment-of-intensity histograms. All image-processing operations were performed uniformly on every image frame over the entire FOV. Blood vessel identification and segmentation was performed using the Weka segmentation tool (v3.2.33)20 on FIJI. Only vessels identified with clearly defined (in focus) borders and having both borders of the vessel wall within the FOV were subject to quantitative analysis. Vaso-occlusion was defined as the complete and permanent cessation of all cellular traffic being identified during movie capture. Liver microcirculation in mice was observed using qLIM for a maximum of 30 minutes. During this time, we did not see any resolution of sinusoidal vaso-occlusion. This finding is identical to the findings reported in our previous study.18 Areas of vaso-occlusion were defined as far as the cellular blockage was clearly visible within the vessel, and vessels without cellular traffic during image capture were not considered to be occluded. The percentage of flowing vessel area was quantified in addition to the count (quantity) of flowing vessels per FOV. Neutrophil-containing vaso-occlusions were defined as 2 or more neutrophils found within a vaso-occlusive region and within the lumen of the blood vessel(s). Multiple neutrophil-containing regions of vaso-occlusion were counted within a FOV only when occluded areas were within separate regions of microvascular branches or were separated by areas of flowing microvessels.
Statistical analysis
The paired 2-tailed Student t test was used to evaluate differences between SCD mice treated with LPS and SCD mice treated with both LPS and DRP. A value of P < .05 was assumed to indicate statistical significance.
Results and discussion
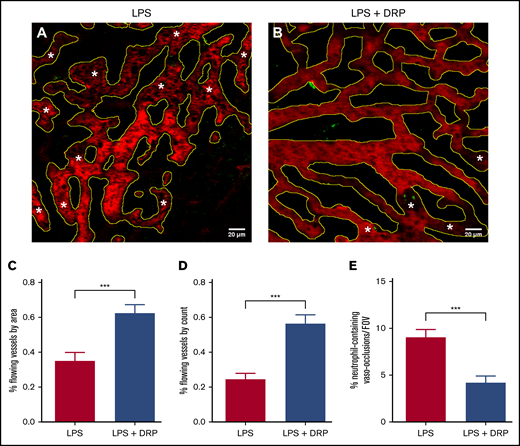
Vaso-occlusion within the liver sinusoids of SCD mice was successfully observed using qLIM. Given the recently documented transient liver ischemia and damage in SCD mice under basal conditions,18 all mice were observed to present some degree of vaso-occlusion following LPS inflammatory stimulus. The geometry or tortuosity of vessels did not appear to influence the likelihood of vessel vaso-occlusion. As shown in Figure 1A-B, increased levels of vaso-occlusion were observed in SCD mice administered IV LPS compared with SCD mice administered both LPS and DRPs. The administration of DRPs resulted in a significant increase in both the percent area as well as the percentage of flowing vessels per FOV (Figure 1C-D). Furthermore, DRPs significantly reduced the number of vaso-occlusions containing 2 or more neutrophils (Figure 1E).
DRP reduces incidence of vaso-occlusion in the liver of transgenic SCD mice. (A-B) Still images obtained using qLIM techniques. Texas Red–dextran and Ly6G green were used to stain the blood plasma and neutrophils, respectively. RBCs can be discerned as dark shapes within the vessels and were not stained. Microscopy was performed using a Nikon MPE multiphoton excitation microscope in collaboration with the Center for Biologic Imaging (CBI), University of Pittsburgh. Yellow outlines denote areas identified as vessels. *Regions of vaso-occlusion where loss of blood flow was detected. Scale bars represent 20 µm. Three SCD mice treated with LPS (39 FOVs) and 3 SCD mice treated with both LPS and DRPs (30 FOVs) were used in this study. Blood flow in ∼30 blood vessels was assessed in each FOV. Quantification of the percentage of total flowing vessel area per FOV (C), and quantification of the percentage of total flowing vessels per FOV (D) by their individual count. (E) Number of neutrophil-containing vaso-occlusions per FOV in SCD mice. Data presented as mean ± standard error; ***P < .001.
DRP reduces incidence of vaso-occlusion in the liver of transgenic SCD mice. (A-B) Still images obtained using qLIM techniques. Texas Red–dextran and Ly6G green were used to stain the blood plasma and neutrophils, respectively. RBCs can be discerned as dark shapes within the vessels and were not stained. Microscopy was performed using a Nikon MPE multiphoton excitation microscope in collaboration with the Center for Biologic Imaging (CBI), University of Pittsburgh. Yellow outlines denote areas identified as vessels. *Regions of vaso-occlusion where loss of blood flow was detected. Scale bars represent 20 µm. Three SCD mice treated with LPS (39 FOVs) and 3 SCD mice treated with both LPS and DRPs (30 FOVs) were used in this study. Blood flow in ∼30 blood vessels was assessed in each FOV. Quantification of the percentage of total flowing vessel area per FOV (C), and quantification of the percentage of total flowing vessels per FOV (D) by their individual count. (E) Number of neutrophil-containing vaso-occlusions per FOV in SCD mice. Data presented as mean ± standard error; ***P < .001.
Although the rheological mechanisms of DRPs are not fully understood, it is currently hypothesized21 that their ability to increase precapillary pressures and improve capillary recruitment and perfusion is a direct result of their reduction in flow separations and small vortices within blood vessels.22,23 We hypothesized that these mechanisms may help to reduce SCD vaso-occlusion by ameliorating flow stagnation, thereby reducing the probability that a single transient vaso-occlusive event would occur and/or propagate into the occlusion of a larger area. Furthermore, the ability of DRP to diminish the width of the microvasculature near-wall “cell-free layer” has been shown to reduce plasma skimming into capillaries,23 increase near-wall shear stress and RBC velocity,15 and prevent the peripheral margination of platelets.24 The demargination of immune-surveillance cells accompanied by increased microvascular near-wall shear rates may potentially disrupt adhesion molecule interactions and possibly attenuate the risk of neutrophil-induced vaso-occlusion. Comparable observation of this effect has previously been documented in vivo, where the administration of DRPs demonstrated reduced neutrophil extracellular trap formation and platelet microthrombi,13 as well as inhibition of human breast cancer cell extravasation.25 Previously, we have shown that DRPs prevent margination of rigid particles within microchannels.24 In the current study, we show that IV administration of DRPs prevents IV LPS-triggered vaso-occlusion in the liver sinusoids. Taken together, these findings suggest a potential role for sickle RBCs in sinusoidal vaso-occlusion. The relative contribution of adhesion vs increased stiffness of RBCs to sinusoidal vaso-occlusion as well as the efficacy of DRPs in preventing vaso-occlusion in lung, spleen, and other organs will be investigated in future studies. In conclusion, our findings suggest that DRPs can be beneficial in preventing ischemic liver injury of SCD. These findings also warrant the need for further clinical studies to assess the efficacy of DRPs as a rheology-based prophylactic therapy to prevent ischemic organ injury in SCD patients.
Data-sharing requests may be e-mailed to the corresponding author, Marina V. Kameneva, at kamenevamv@upmc.edu.
Acknowledgments
This work was supported in part by the following grants from the National Institutes of Health, National Heart, Lung, and Blood Institute: T32-HL07612412 (D.C.), 1R01HL128297 (P.S.), and 1R01HL141080 (P.S.). This work was also supported in part by funds from the Hemophilia Center of Western Pennsylvania and Vitalant (P.S.). T.P.-S. was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant 1K01DK125617-01, a Community Liver Alliance award, and a Pittsburgh Liver Research Center pilot and feasibility grant. R.V. was supported by American Heart Association award 19PRE34430188.
Authorship
Contribution: R.V. conducted the qLIM experiments; D.C. performed the data analysis and, with consultation and contribution from all coauthors, wrote the manuscript; T.P.-S. was involved in the design of qLIM experiments; and P.S. and M.V.K. were responsible for experimental design and project supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina V. Kameneva, McGowan Institute for Regenerative Medicine, 450 Technology Dr, Suite 300, Pittsburgh, PA 15219; e-mail: kamenevamv@upmc.edu.
References
Author notes
The full-text version of this article contains a data supplement.