Key Points
VTE developed in 11% of lymphoma patients after CAR T-cell therapy and was managed safely with anticoagulation.
Coagulation abnormalities after CAR T-cell therapy occur but do not commonly lead to bleeding events.
Introduction
Cancer-associated venous thromboembolism (VTE) constitutes a major cause of morbidity and mortality in patients with hematologic malignancies.1 VTE can occur after chimeric antigen receptor (CAR) T-cell therapy, whereas coagulopathy in the form of low fibrinogen and bleeding can be complications of cytokine release syndrome (CRS).2-5 Therefore, there is the potential that the use of anticoagulation to treat or prevent VTE might increase bleeding risks after CAR T-cell therapy. However, data are limited about the incidence, characteristics, and management of VTE and coagulopathy, which we report in a series of relapsed/refractory large B-cell lymphoma (LBCL) patients receiving CAR T-cell therapy.
Methods
We performed a single-center retrospective study of 148 consecutive patients receiving CD19 CAR T-cell therapy for LBCL between May 2015 and September 2019, excluding patients on unpublished clinical trials. VTE events were classified as “prior recent” if occurring within 6 months before CAR T-cell infusion and “new” if occurring between day 0 and day 100 after CAR T-cell infusion. Serum fibrinogen levels were measured at the treating physician’s discretion in 31 patients in the first 100 days after CAR T-cell infusion (21% of patients). Hypofibrinogenemia was defined as nadir <200 mg/dL. CRS was graded by using the modified Lee grading system,6 and neurotoxicity was graded by using the CAR-T cell Toxicity Management grading system4 or individual terms for Common Terminology Criteria for Adverse Events version 4.03 neurotoxicity. Thromboprophylaxis was not routinely used, including for patients admitted to intensive care. Diagnosis of VTE was based on imaging and was deemed “symptomatic” or “incidental” based on clinical signs in conjunction with imaging. For catheter-associated VTE, the catheters were not removed except per standard procedures at approximately day +30. Univariate comparison of baseline characteristics in patients with a new VTE was by χ2 or Fisher’s exact test. The study was approved by the Institutional Review Board at the H. Lee Moffitt Cancer and Research Institute.
Results and discussion
Baseline characteristics and clinical outcomes are provided in supplemental Table 1. In the full cohort of 148 patients, 10 (7%) had baseline platelet counts <75 × 103/μL. Among baseline characteristics, bulky disease >10 cm, use of bridging therapy, and Eastern Cooperative Oncology Group performance status 2 to 4 were most significantly associated with a new VTE event after CAR T-cell therapy (P < .01). Among clinical outcomes, new VTEs were more common in patients experiencing severe CRS (P = .027) or neurotoxicity (P = .014).
For the analysis of prior recent VTE, we did not include patients treated on clinical trials (n = 27) in which it was an exclusion criterion. Among remaining patients, 23% (28 of 121) had a prior recent VTE event within the previous 6 months, and 42% (12 of 28) of these patients continued anticoagulation after CAR T-cell infusion. For those who continued anticoagulation, one-half (6 of 12) had anticoagulation held later due to treatment-induced thrombocytopenia at a median of 5 days (range, 2-7 days) after CAR T-cell infusion. For those who held anticoagulation before CAR T-cell infusion (n = 16), 2 of 16 (13%) developed a new VTE event between day 0 and day 100. No patient who continued anticoagulation after CAR T-cell infusion (n = 12) developed a new VTE.
A new episode of VTE between day 0 and day 100 after CAR T-cell infusion occurred in 11% (16 of 148) of the patients, including the aforementioned 2 cases with known VTE in which anticoagulation was held before infusion. New VTE episodes after CAR T-cell infusion were treated initially with anticoagulation in most patients (12 of 16 [75%]), although it was subsequently held in some patients due to thrombocytopenia (5 of 12 [42%]) at a median of 5 days (range, 3-10 days) after initiation of anticoagulation. No patient had a major bleeding event or died of VTE or anticoagulation. VTE and their management are summarized in Table 1, with details about the 16 patients with new VTE given in supplemental Table 2. Of the 16 patients with new VTE, 10 died due to lymphoma or toxicity, and 5 of the 6 patients with durable remissions remained on anticoagulation for a minimum of 3 months.
VTE and coagulation abnormalities in CAR T-cell patients
| Summary of VTE and coagulation abnormalities and management . | Value . |
|---|---|
| Prior recent VTE (within 6 mo) before CAR T-cell infusion* | 28/121 (23) |
| DVT alone | 17 |
| PE alone | 1 |
| DVT + PE | 7 |
| Other VTE (splenic/mesenteric) | 3 (2/1) |
| Patients who had AC held after CAR T-cell infusion | 16/28 (57) |
| Median platelet count when AC held after CAR T-cell infusion (range), ×103/μL | 54 (39-116) |
| Median days after infusion when AC held (range) | 5 (2-7) |
| Median platelet count when AC resumed after initial interruption (range), ×103/μL | 66 (54-213) |
| Patients with recurrent thrombosis when AC was held | 2/16 |
| New diagnosis of VTE between day 0 and day 100 after CAR T-cell infusion | 16/148 (11) |
| DVT | 8/16 |
| Upper extremity (all catheter-associated)† | 4/8 |
| Lower extremity | 4/8 |
| PE | 4/16 |
| Others (mesenteric/cerebral/renal) thrombosis | 4/16 |
| Asymptomatic/incidental new VTE | 5/16 (31) |
| Patients who had AC held after initiation/patients initiating AC‡ | 5/12 (42) |
| Median platelet count when AC was held (range), ×103/μL | 53 (39-202) |
| Median days after initiation when AC was held (range) | 5 (3-10) |
| Patients who had AC resumed after initial interruption§ | 2/5 |
| Patients with serum fibrinogen measured between day 0 and day 100‖ | 31 |
| Nadir serum fibrinogen level <100 mg/dL | 9/31 |
| Nadir serum fibrinogen level 100-200 mg/dL | 6/31 |
| Nadir serum fibrinogen level >200 mg/dL | 16/31 |
| Days to reach nadir serum fibrinogen after infusion, median (range)¶ | 11 (5-27) |
| Abnormal PT or PTT in patients with hypofibrinogenemia# | 6/15 |
| PT only | 4 |
| PTT + PT | 2 |
| Patients receiving cryoprecipitate** | 8 |
| Median no. of prepooled units used per patient (range) | 2 (1-5) |
| Median days to first cryoprecipitate use after infusion (range) | 9 (6-18) |
| Median days to last cryoprecipitate use after infusion (range) | 14 (9-29) |
| Patients receiving fresh frozen plasma | 1 |
| No. of fresh frozen plasma units used | 3 |
| Summary of VTE and coagulation abnormalities and management . | Value . |
|---|---|
| Prior recent VTE (within 6 mo) before CAR T-cell infusion* | 28/121 (23) |
| DVT alone | 17 |
| PE alone | 1 |
| DVT + PE | 7 |
| Other VTE (splenic/mesenteric) | 3 (2/1) |
| Patients who had AC held after CAR T-cell infusion | 16/28 (57) |
| Median platelet count when AC held after CAR T-cell infusion (range), ×103/μL | 54 (39-116) |
| Median days after infusion when AC held (range) | 5 (2-7) |
| Median platelet count when AC resumed after initial interruption (range), ×103/μL | 66 (54-213) |
| Patients with recurrent thrombosis when AC was held | 2/16 |
| New diagnosis of VTE between day 0 and day 100 after CAR T-cell infusion | 16/148 (11) |
| DVT | 8/16 |
| Upper extremity (all catheter-associated)† | 4/8 |
| Lower extremity | 4/8 |
| PE | 4/16 |
| Others (mesenteric/cerebral/renal) thrombosis | 4/16 |
| Asymptomatic/incidental new VTE | 5/16 (31) |
| Patients who had AC held after initiation/patients initiating AC‡ | 5/12 (42) |
| Median platelet count when AC was held (range), ×103/μL | 53 (39-202) |
| Median days after initiation when AC was held (range) | 5 (3-10) |
| Patients who had AC resumed after initial interruption§ | 2/5 |
| Patients with serum fibrinogen measured between day 0 and day 100‖ | 31 |
| Nadir serum fibrinogen level <100 mg/dL | 9/31 |
| Nadir serum fibrinogen level 100-200 mg/dL | 6/31 |
| Nadir serum fibrinogen level >200 mg/dL | 16/31 |
| Days to reach nadir serum fibrinogen after infusion, median (range)¶ | 11 (5-27) |
| Abnormal PT or PTT in patients with hypofibrinogenemia# | 6/15 |
| PT only | 4 |
| PTT + PT | 2 |
| Patients receiving cryoprecipitate** | 8 |
| Median no. of prepooled units used per patient (range) | 2 (1-5) |
| Median days to first cryoprecipitate use after infusion (range) | 9 (6-18) |
| Median days to last cryoprecipitate use after infusion (range) | 14 (9-29) |
| Patients receiving fresh frozen plasma | 1 |
| No. of fresh frozen plasma units used | 3 |
Values are n or n/N (%), unless otherwise noted. AC, anticoagulation; DVT, deep vein thrombosis; PE, pulmonary embolism; PT, prothrombin time; PTT, partial thromboplastin time.
Patients who received CAR T-cell therapy on clinical trial (N = 27) were excluded for “prior recent” VTE as this was a trial exclusion criterion.
None of the catheter-associated VTEs was associated with local or bloodstream infections.
Initial anticoagulation for new VTE was low-molecular-weight heparin (n = 6), unfractionated heparin (n = 2), rivaroxaban (n = 2), dabigatran (n = 1), and apixaban (n = 1).
Platelet count (×103/μL) when AC resumed was 55 and 61, respectively, in these 2 patients.
Reasons for checking serum fibrinogen levels included minor bleeding (n = 4), workup for anemia and/or thrombocytopenia (n = 10), workup for elevated international normalized ratio and/or PTT (n = 2), workup for possible hemophagocytic lymphohistiocytosis (n = 4), workup during severe CRS or neurotoxicity (n = 4), patient on anticoagulation (n = 3), screening workup for postrelapse clinical trial (n = 1), and unknown (n = 3).
Days postinfusion the fibrinogen level was first measured: median (range), 7 (0-45). Number of days the level was measured between day 0 and day 100: median (range), 3 (1-32).
Patients with PT or PTT levels that were above the normal range at the time of nadir fibrinogen in patients experiencing a fibrinogen level <200 mg/dL.
Reasons for giving cryoprecipitate (all patients had serum fibrinogen levels <100 mg/dL) included concomitant severe CRS (n = 1), minor bleeding (n = 1), transient decrease in hemoglobin without an identifiable bleeding source (n = 1), elevated PT/PTT without overt DIC (n = 1), and low fibrinogen level without other reason(s) (n = 4).
We did not identify any cases with overt disseminated intravascular coagulation (DIC) as the cause of VTE or major bleeding. In 1 case, the low fibrinogen level was found to be concurrent with the detection of VTE, and in 6 cases, the hypofibrinogenemia was concurrent to an elevated international normalized ratio and/or partial thromboplastin time. However, no patient met criteria for overt DIC, as peripheral blood schistocytosis, VTE, and/or bleeding did not cooccur.7,8 Cryoprecipitate was used to correct hypofibrinogenemia in 8 cases, and in the single case of concurrent VTE, it was identified before cryoprecipitate use (Table 1; supplemental Table 3).
Discussion
In our series, 22% of patients undergoing CAR T-cell therapy had a prior recent VTE event, and new VTE events occurred in 11% of patients after CAR T-cell infusion. Univariate characteristics that associated with a new VTE were bulky disease, use of bridging therapy, poor performance status, severe CRS, and severe neurotoxicity. Due to the low number of spontaneous VTE and bleeding events in these cohorts, we did not attempt multivariate modeling based on baseline characteristics.
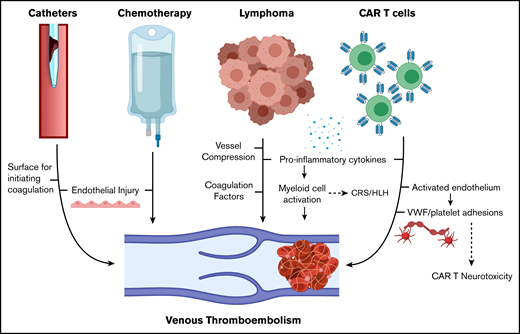
To place our results in context, 10% of patients with newly diagnosed diffuse LBCL develop VTE, and patients undergoing stem cell transplantation (autologous stem cell transplantation [ASCT]) develop VTE at a rate of 1% to 4%.9,10 The preexisting risk of relapsed/refractory lymphoma combined with the inflammation produced by CAR T-cell therapy is proposed to contribute to a hypercoagulable state (Figure 1).11-13 In addition, our institution uses tunneled central venous catheters from the start of lymphodepleting chemotherapy, and one-quarter of the new VTE events in our series were catheter-related thrombosis (CRT) (overall incidence, 4 of 148 [2.6%]), similar to catheter-related thrombosis after ASCT.14
Mechanisms of VTE in patients with lymphoma receiving CAR T-cell therapy. HLH, hemophagocytic lymphohistiocytosis. Created with BioRender.com and adapted from elsewhere.11-13
Mechanisms of VTE in patients with lymphoma receiving CAR T-cell therapy. HLH, hemophagocytic lymphohistiocytosis. Created with BioRender.com and adapted from elsewhere.11-13
Most VTE events were managed with anticoagulation, and no major bleeding events were caused by anticoagulation. More than 40% of the patients who initially started anticoagulation for a new VTE had it held later when the platelet count was <50 × 103/μL. None developed recurrent or worsening VTE events by day 100 after CAR T-cell therapy. We incidentally identified 5 of the 16 new VTE cases, but additional new or recurrent incidental VTE may have gone undetected.
We identified only one case with concurrent hypofibrinogenemia and VTE, and no cases of overt DIC were seen. For patients with low fibrinogen levels, there were no major bleeding events, although some patients were treated with cryoprecipitate to correct fibrinogen levels. Further study is needed to determine if fibrinogen replacement after CAR T-cell therapy provides therapeutic benefit.
American Society of Clinical Oncology guidelines recommend against routine thromboprophylaxis in patients admitted for the sole purpose of chemotherapy or ASCT.15 Further study is needed in the CAR T-cell population, particularly in patients with bulky disease or other higher risk characteristics. Although almost all patients in our cohort had adequate starting platelet counts, prophylactic strategies may be limited by the development of thrombocytopenia, which is reported to occur below 50 × 103/μL in 38% of patients.16 In the visual abstract, we provide management strategies for CAR T cell–associated VTE based on our institutional experience.
In conclusion, there is a significant incidence of new VTE in LBCL patients undergoing CD19-directed CAR T-cell therapy (11%). Most patients can be managed with therapeutic anticoagulation without bleeding or recurrent thrombotic complications. Hypofibrinogenemia after CAR T-cell therapy may be associated with other coagulation abnormalities but was not associated with major bleeding events.
Requests by other investigators for data sharing may be submitted to the corresponding author (Michael D. Jain; e-mail: michael.jain@moffitt.org).
Authorship
Contribution: H.H., F.L.L., T.N., and M.D.J. conceived and designed the study; H.H., A.-S.M., A.D., C.L., F.G., A.K., and R.S.M. performed the chart review and data analysis; C.B., J.C.C., B.S., J.P.-I., F.K., A.L., H.L., and M.L.D. provided patients, collected data, and reviewed and revised the manuscript; H.H., F.L.L., T.N., and M.D.J. wrote the manuscript; and all authors reviewed and approved the final version of the manuscript before submission.
Conflict-of-interest disclosure: C.B. is on the advisory board for Kite/Gilead; and serves on the speaker bureau for Novartis. J.C.C. is on the advisory board for Kite/Gilead, Novartis, Bayer, Genentech, MorphoSys, Karyopharm, and AbbVie; and serves on the speaker bureau for AstraZeneca and BeiGene. B.S. reports consultancy for Celgene/Juno, Adaptive, Kite/Gilead, Novartis, Pharmacyclics, Spectrum/Acroteca, and AstraZeneca; and research funding from Jazz and Incyte. J.P.-I. reports consultancy for Takeda, AbbVie, Janssen, Novartis, Gilead, and Teva. A.L. serves on the scientific advisory board for EUSA Pharma. M.L.D. reports research funding from Celgene, Novartis, and Atara; other financial support from Novartis, Precision Biosciences, Celyad, Bellicum, and GlaxoSmithKline; and stock options from Precision Biosciences, Adaptive Biotechnologies, and Anixa Biosciences. F.L.L. has a scientific advisory role with Kite, a Gilead Company, Novartis, Celgene/Bristol-Myers Squibb, GammaDelta Therapeutics, Wugen, Amgen, Calibr, and Allogene; is a consultant with grant options for Cellular Biomedicine Group, Inc.; and receives research support from Kite, a Gilead Company. T.N. receives research support to the institution from Novartis and Karyopharm. M.D.J. serves a consultancy/advisory role for Kite/Gilead and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Michael D. Jain, Department of Blood & Marrow Transplant and Cellular Immunotherapy, Moffitt Cancer Center, 12902 USF Magnolia Dr, CSB 7114, Tampa, FL 33612; e-mail: michael.jain@moffitt.org.
References
Author notes
T.N. and M.D.J. contributed equally to this work.
The full-text version of this article contains a data supplement.